Abstract
Anthracyclines including doxorubicin and daunorubicin are commonly used for the treatment of both hematologic and solid tumors. Dose related adverse effects often limit the effectiveness of anthracyclines in chemotherapy. Drug-related systemic inflammation mediated by interleukin-1beta (IL-1β) has been implicated in contributing to these adverse effects. The molecular mechanisms underlying anthracycline-mediated expression and IL-1β release are not understood. Elucidating the molecular basis by which anthracyclines upregulate IL-1β activity may present opportunities to decrease the inflammatory consequences of these drugs. Here we demonstrate that doxorubicin induces a systemic increase in IL-1β and other inflammatory cytokines, chemokines and growth factors including TNFα, IL-6, Gro-α/CXCL1, CCL2/MCP-1, granulocyte colony stimulating factor (GCSF) and CXCL10/IP-10. Studies with IL-1R-deficient mice demonstrate that IL-1 signaling plays a role in doxorubicin-induced increases in IL-6 and GCSF. In vitro studies with doxorubicin and daunorubicin failed to induce expression of pro-IL-1β in unprimed murine bone marrow-derived macrophages (BMDM) but enhanced the expression of pro-IL-1β in BMDM that had previously been primed with LPS. Furthermore, doxorubicin and daunorubicin induced the processing and release of IL-1β from LPS-primed BMDM by providing danger signals that lead to assembly and activation of the inflammasome. The release of IL-1β required the expression of ASC, caspase-1 and NLRP3, demonstrating that doxorubicin and daunorubicin-induced inflammation is mediated by the NLRP3 inflammasome. As with other agents that induce activation of the NLRP3 inflammasome, the ability of doxorubicin to provide proinflammatory danger signals was inhibited by co-treatment of cells with ROS inhibitors or by incubating cells in high extracellular potassium. These studies suggest that proinflammatory responses to anthracycline chemotherapeutic agents are mediated, at least in part, by promoting the processing and release of IL-1β, and that some of the adverse inflammatory consequences that complicate chemotherapy with anthracyclines may be reduced by suppressing the actions of IL-1β.
Key words: doxorubicin, daunorubicin, anthracycline, interleukin-1, cancer therapy, inflammation, quality of life, inflammasome
Introduction
Doxorubicin (adriamycin) and daunorubicin are anthracycline drugs that are employed as anticancer agents for the treatment of both hematologic and solid tumors.1 It is well known that administration of anthracyclines is associated with acute and late cardiac toxicity, leading to increased risk of heart failure.2 In addition to cardiotoxicity, cancer patients treated with these drugs often experience a cluster of symptoms including fatigue, lethargy, decreased appetite, sleep disturbance, difficulty thinking and pain.3 While these symptoms are not life-threatening they have a profound negative effect on physical functioning and quality of life (QOL).4–16 There is growing evidence that the pro-inflammatory cytokine IL-1β may play an important role in the symptoms associated with anthracycline therapy. First, in a recent study serum levels of IL-1β were increased in doxorubicin-treated mice relative to their untreated counterparts.17 Pre-treatment of mice with recombinant human IL-1 receptor antagonist (IL-1Ra) prior to doxorubicin administration protected mice from doxorubicin-induced mortality, heart damage, cardiomyocyte apoptosis and loss of cardiac function. Second, it has long been recognized that fatigue, lethargy, decreased appetite, sleep disturbance, difficulty thinking and pain experienced by cancer patients undergoing treatment with anthracyclines are remarkably similar to those associated with sickness behavior, a normal physiological response to activation of the innate immune system in which IL-1β plays a central role (reviewed in ref. 18). In a recent study we demonstrated that a doxorubicin-based chemotherapy regimen could induce systemic increases in IL-1β production and fatigue in mice (manuscript in review). Blood levels of several other inflammatory cytokines and chemokines were also increased by doxorubicin treatment and were significantly correlated to level of fatigue, including CXCL1/Gro-α, CCL2/MCP-1, granulocyte colony stimulating factor (GCSF) and CXCL10/IP-10. Taken together, this evidence demonstrates that anthracycline therapies can trigger a systemic inflammatory response characterized by the production and release of IL-1β and suggests that suppression of IL-1β expression and release may present an opportunity to decrease symptom burden in cancer patients treated with these agents. Yet, to date the mechanism that underlies anthracycline-mediated expression and release of IL-1β is not understood and is the focus of the present study.
IL-1β is an initiator cytokine that plays a central role in the regulation of immune and inflammatory responses.18 IL-1β is produced by activated macrophages and epithelial cells and requires two distinct signals for its synthesis, processing and secretion. The first signal, which induces the expression of the 35 kDa pro-IL-1β, is mediated by the activation of NFκB and the stress-activated protein kinases, JNK and p38.19 The second signal induces the processing of pro-IL-1β to mature 17 kDa IL-1β by assembly of a multiprotein complex called the inflammasome.20–23 The inflammasome is fundamental for microbial detection20 and for sensing stress or endogenous danger signals such as extracellular ATP, hypotonic stress or toxins associated with cell injury.24,25 Upon sensing a danger signal, the inflammasome complex is formed by assembly of at least three critical components: (1) a member of a family of NOD-like receptors, containing PYD domains, such as AIM2, NLRP1, NLRP2 or NLRP3; (2) the adaptor protein ASC that forms a scaffold; and (3) IL-1β-converting enzyme (ICE) or caspase-1.26–28
Here we demonstrate that doxorubicin induced a systemic increase in IL-1β and other inflammatory cytokines, chemokines and growth factors including TNFα, IL-6, CXCL1/Gro-α, CCL2/MCP-1, GCSF and CXCL10/IP-10. Drug-induced increases in IL-6 and GCSF were dependent on IL-1 signaling, since doxorubicin failed to cause an increase in the levels of IL-6 and GCSF in IL-1 receptor-deficient mice. In vitro studies demonstrated that although doxorubicin and daunorubicin were unable to induce the expression of 35 kDa pro-IL-1β in naive murine bone marrow-derived macrophages (BMDM), these agents were capable of inducing the secretion of 17 kDa IL-1β from cells that had previously been primed by LPS to express pro-IL-1β. The release of IL-1β required the expression of ASC, caspase-1 and NLRP3, demonstrating that doxorubicin and daunorubicin induced the release of IL-1β by activating the NLRP3 inflammasome. As with other agents that induce activation of the NLRP3 inflammasome, the ability of doxorubicin to provide proinflammatory danger signals was inhibited by co-treatment of cells with ROS inhibitors or by incubating cells in high extracellular potassium. These results support the idea that proinflammatory responses to anthracycline chemotherapeutic agents are mediated, at least in part, by promoting the processing and release of IL-1β, and that some of the adverse inflammatory consequences that complicate chemotherapy with anthracyclines may be reduced by suppressing the anthracycline-mediated release of IL-1β.
Results
Effect of IL-1 signaling on doxorubicin-induced inflammatory response in mice.
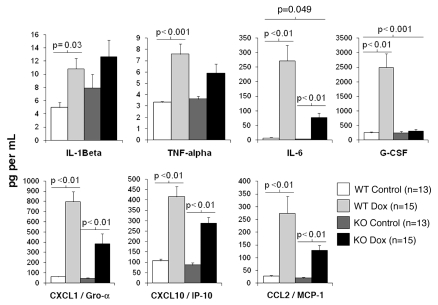
Mature IL-1β released from activated immune cells in response to a harmful stimulus induces the production of several inflammatory cytokines and chemokines via binding to its IL-1 receptor on target cells. To determine whether IL-1β signaling is required for this inflammatory response to doxorubicin treatment, serum levels of IL-1β, TNFα, IL-6, CXCL10/IP-10, CXCL1/Gro-α, CCL2/MCP-1 and G-CSF were measured in wild-type and IL-1R-deficient doxorubicin treated mice and their sham-injected counterparts.
In wild-type mice, doxorubicin induced an increase in serum levels of IL-1β (p = 0.04), TNFα (p < 0.01), IL-6 (p < 0.01), CXCL10/IP-10 (p < 0.01), CXCL1/Gro-α (p < 0.01), G-CSF (p < 0.01) and CCL2/MCP-1 (p = 0.01) relative to saline-injected wild-type mice (Fig. 1). A similar increase in serum levels of IL-6 (p < 0.01), CXCL10/IP-10 (p < 0.01), CXCL1/Gro-α (p < 0.01) and CCL2/MCP-1 (p < 0.01) was evident in doxorubicin treated IL-1R-/- mice relative to control mice. In contrast, serum levels of IL-1β, TNFα and G-CSF were not significantly elevated in doxorubicin treated IL-1R-deficient mice relative to their sham-injected counterparts (Fig. 1). To determine whether there was a statistically significant interaction between drug and genotype, the effect of IL-1R deficiency on the doxorubicin induced inflammatory response was determined by 2 × 2 ANOVA. A significant drug × genotype interaction on serum levels of IL-6, (p = 0.049) and G-CSF (p < 0.01) was observed after Bonferroni correction for multiple comparisons but not for the other serum analytes examined (Fig. 1). Although doxorubicin increased serum levels of IL-6 in both wild-type and IL-1R-deficient mice, the magnitude of the response was approximately 2-fold greater in magnitude in wild-type mice than in IL-1R-deficient mice (Fig. 1). The effect of IL-1 deficiency on doxorubicin-induced GCSF levels was particularly striking. While serum levels of G-CSF were 10-fold higher in wild-type doxorubicin-treated mice relative to controls, there was no observable effect of doxorubicin on levels of GCSF in IL-1R-deficient mice (Fig. 1).
Figure 1.
Effect of IL-1 signaling on doxorubicin-induced inflammatory response in mice. WT and IL-1R1-deficient (KO) mice were injected intraperitoneally (IP) with doxorubicin or normal saline (NS). Peripheral blood was collected 16 h post-injection and serum levels of IL-1β, TNFα, IL-6, CXCL10/IP-10, CCL2/MCP-1, GCSF and CXCL1/Gro-α were measured. Differences between serum levels of inflammatory analytes by genotype and drug group were analyzed by two-way ANOVA. Data are presented as the mean analyte level (pg/mL) ± standard deviation. p < 0.05 was considered to be statistically significant.
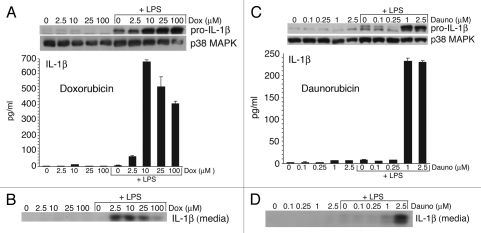
Doxorubicin-induced IL-1β release from primed bone marrow derived macrophages (BMDM).
A variety of agents such as asbestos, silicates and uric acid crystals are unable to induce expression of IL-1β in cultured macrophages. However, when these agents are added to macrophages that have been previously primed by LPS to express pro-IL-1β, the pro-IL-1β is processed by caspase-1 and secreted as active IL-1β through activation of the inflammasome.25,29,30
To determine whether doxorubicin would induce the expression and release of IL-1β, LPS-primed and unprimed BMDM were exposed to doxorubicin (2.5, 10, 25 or 100 µM) for 8 h. Cell lysates and media were analyzed for both pro-IL-1β and mature IL-1β, respectively (Fig. 2A and B). In unprimed BMDM, doxorubicin failed to induce expression of pro-IL-1β (Fig. 2A, top) or secretion of IL-1β into the medium (Fig. 2A, bottom and Fig. 2B). In contrast, LPS-primed BMDM exposed to doxorubicin exhibited a dose dependent increase in expression of pro-IL-1β (Fig. 2A, top). LPS-primed macrophages exposed to doxorubicin also showed increased release of IL-1β (Fig. 2B). Measured by ELISA, the release of IL-1β from LPS primed macrophages was maximal (∼700 pg/mL) after exposure to 10 µM of doxorubicin and was decreased slightly in cells exposed to 25 and 100 µM doxorubicin, respectively (Fig. 2A, bottom, and Fig. 2B). These data demonstrate that doxorubicin was unable to induce the expression of pro-IL-1β in naive BMDM, but that doxorubicin was effective in mediating both increased expression of pro-IL-1β and the release of IL-1β when added to LPS-primed BMDM.
Figure 2.
Doxorubicin and daunorubicin stimulate IL-1β release from wild-type BMDM. (A and C) Whole cell lysates (WCLs) were subjected to immunoblot analysis for detection of pro-IL-1β and p38 MAPK (loading control). Secreted IL-1β was measured by ELISA analysis of media supernatants collected from unprimed and LPS-primed wild-type BMDM after 8 h doxorubicin (A), daunorubicin (C) or vehicle exposure. Bars represent the mean ± SD of triplicate wells. (B and D) Media supernatants from corresponding cell cultures were precipitated and subjected to immunoblot analysis for detection of IL-1β p17.
Daunorubicin-induced IL-1β release from primed BMDM.
Daunorubicin is an anthracycline that is believed to act by similar mechanisms as doxorubicin.31 To determine whether daunorubicin would act like doxorubicin in mediating the release of IL-1β, LPS-primed or unprimed BMDM were exposed to daunorubicin (0.1, 0.25, 1 or 2.5 µM) for 8 h. As with doxorubicin, daunorubicin failed to induce pro-IL-1β in unprimed BMDM (Fig. 2C, top) or to release IL-1β into the medium (Fig. 2C, bottom, and Fig. 2D). Exposure of LPS primed BMDM to doses of daunorubicin greater than 0.25 µM resulted in increased expression of pro-IL-1β (Fig. 2C, top). Release of IL-1β from LPS primed macrophages was maximal (230 pg/mL) following exposure to 1 or 2.5 µM daunorubicin (Fig. 2C, bottom). These data demonstrate that daunorubicin, like doxorubicin, mediated the release of IL-1β LPS-primed BMDM.
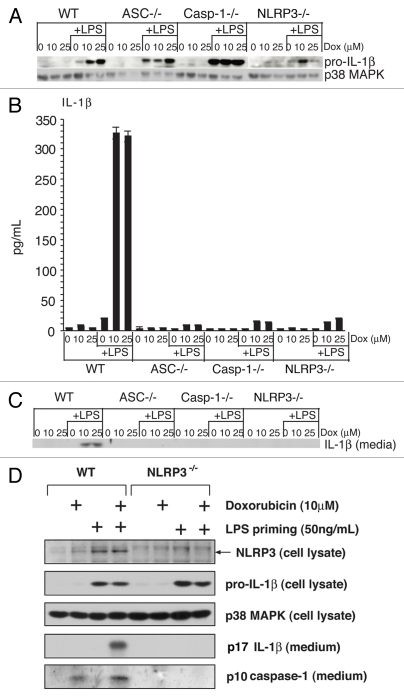
Doxorubicin-induced IL-1β release requires ASC, caspase-1 and NLRP3.
To determine if BMDM respond to doxorubicin through formation of the NLRP3 inflammasome, we tested whether the doxorubicin-induced release of IL-1β from wild-type BMDM would differ quantitatively from BMDM deficient in ASC, caspase-1 or NLRP3. LPS-primed or unprimed BMDM were exposed to doxorubicin (10 or 25 µM) for 8 h, at which time cell lysates and media were analyzed for pro-IL-1β and mature IL-1β, respectively. Doxorubicin failed to induce expression of pro-IL-1β (Fig. 3A) or release of IL-1β from all four strains of unprimed BMDM (Fig. 3B and C). In all four strains of BMDM that had previously been primed by LPS, exposure to doxorubicin increased the expression of pro-IL-1β (Fig. 3A). As expected, doxorubicin induced the release of IL-1β from LPS primed wild-type BMDM (Fig. 3A and C). However, the release of IL-1β from LPS-primed BMDM exposed to doxorubicin was significantly suppressed in each of the mutant strains of BMDM (Fig. 3B and C). These data suggest that ASC, caspase-1 and NLRP3 are each required for doxorubicin to mediate the processing and release of IL-1β from BMDM.
Figure 3.
Doxorubicin-mediated release of IL-1β requires ASC, caspase-1 and NLRP3. Unprimed or LPS-primed BMDM from wild-type mice or mice deficient in ASC, caspase-1 or NLRP3 were exposed to 10 or 25 εM doxorubicin or vehicle for 8 h. (A) WCLs were subjected to immunoblot analysis for detection of pro-IL-1β and p38 MAPK (loading control). Secreted IL-1β was measured by ELISA (B) or immunoblotting (C) analysis of media supernatants. (D) WT or NLRP3-deficient BMDM were either primed or unprimed for 4 h with LPS, as indicated. Following rinsing of cells in LPS-free medium, cells were exposed to doxorubicin or control vehicle for an additional 8 h. Cell lysates or culture media were examined by immunoblotting, as indicated. Bars represent the mean ± SD of triplicate wells.
Activation of the NLRP3 inflammasome leads to the processing and release of caspase-1 from cells.32,33 To further verify that NLRP3 was required for inflammasome activation, primed or unprimed WT and NLRP3-/- BMDM were exposed to 10 µM doxorubicin for 8 h and the release of p10 caspase-1 in the medium was measured. Doxorubicin induced the release of p10 caspase-1 in wt cells, but not in cells deficient in NLRP3 (Fig. 3D), demonstrating that NLRP3 was required for the processing and activation of caspase-1 in doxorubicin-treated cells.
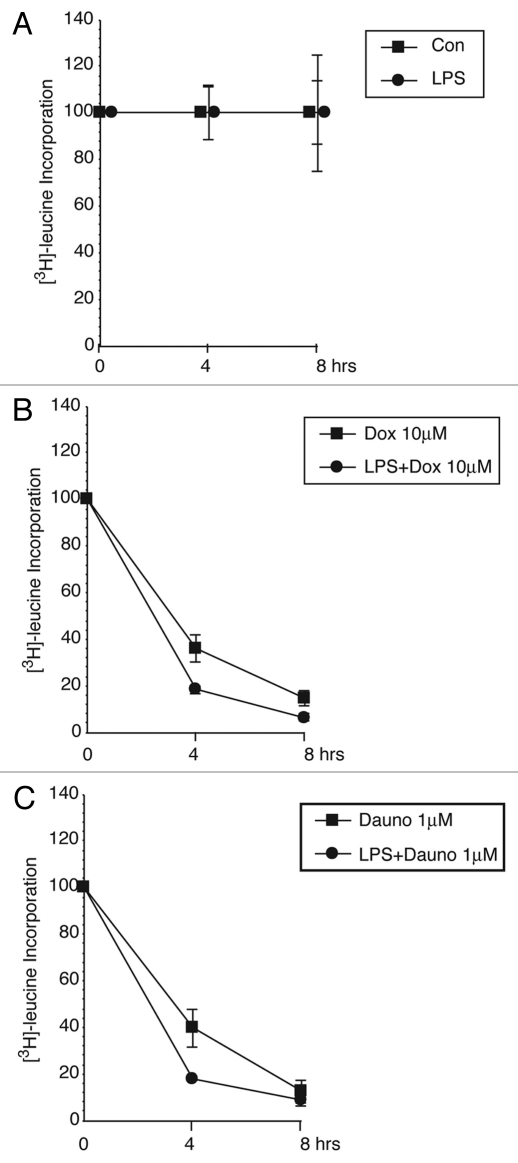
Doxorubicin- and daunorubicin-induced inhibition of protein translation measured by incorporation of [3H]-leucine.
Previous studies from our laboratory established that ricin, a toxin whose primary action includes translational inhibition, is a potent activator of the NLRP3 inflammasome.34 Prior studies had demonstrated that doxorubicin is an inhibitor of protein synthesis.35,36 To determine if doxorubicin and daunorubicin would inhibit protein synthesis at the concentrations employed in the current studies, we exposed unprimed and LPS-primed BMDM to doxorubicin (10 µM) or daunorubicin (1 µM) for 4 or 8 h, at which time cells were exposed to [3H]-leucine for 30 min. Exposure of unprimed and LPS-primed cells to doxorubicin or daunorubicin resulted in a progressive decrease in the incorporation of [3H]-leucine, resulting in 85–90% decrease by 8 h (Fig. 4B and C). Continuous examination of cells by microscopy revealed insignificant cell detachment, even 8 h after exposure to doxorubicin or daunorubicin (not shown).
Figure 4.
Doxorubicin and daunorubicin inhibit protein synthesis. Unprimed or LPS-primed wild-type BMDM were treated in triplicate for 4 or 8 h with vehicle, 10 εM doxorubicin or 1 εM daunorubicin. Cells were pulse-labeled with [3H]-leucine for the final 30 min of treatment.
ROS inhibitors reduce doxorubicin- and daunorubicin-induced secretion of IL-1β from BMDM.
The required presence of ASC, caspase-1 and NLRP3 for doxorubicin-mediated release of IL-1β suggests that doxorubicin acts through formation of the NLRP3 inflammasome.37 Generation of reactive oxygen species (ROS) has been implicated in the activation of the NLRP3 inflammasome, as demonstrated by the ability of ROS inhibitors such as N-acetyl-cysteine (NAC) and diphenyliodonium (DPI) to block activation of the NLRP3 inflammasome.30,33,37 To determine if ROS inhibitors would suppress doxorubicin- and daunorubicin-mediated NLRP3 inflammasome activation, BMDM that had been primed or not with LPS were co-treated with NAC or DPI and doxorubicin (10 µM) or daunorubicin (2.5 µM) for 8 h prior to harvesting of cells and measurement of released IL-1β. Primed BMDM exposed to doxorubicin or daunorubicin demonstrated increased secretion of IL-1β, which was reduced by co-treatment with DPI or NAC (Fig. 5).
Figure 5.
ROS inhibitors, DPI and NAC, prevent doxorubicin- and daunorubicin-mediated release of IL-1β from wild-type BMDM. LPS-primed wild-type cells were treated with vehicle, 10 εM doxorubicin or 2.5 εM daunorubicin in combination with vehicle, DPI or NAC for 8 h. Media supernatants were subjected to ELISA for determination of released IL-1β. Bars represent the mean ± SD of triplicate wells.
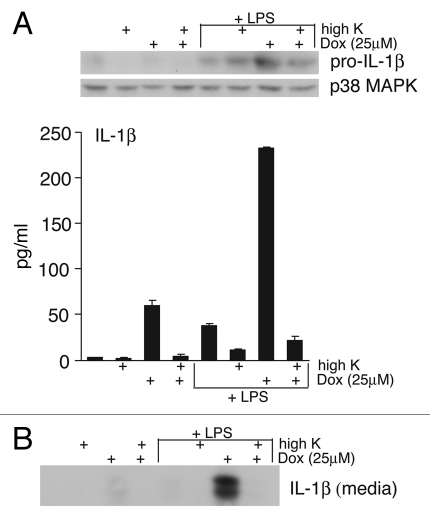
Elevated extracellular potassium reduces doxorubicin-induced secretion of IL-1β from BMDM.
In vitro studies of inflammasome activation suggest that the NLRP3 inflammasome assembly requires a low K+ intracellular environment.33 High extracellular K+ inhibits the IL-1β release caused by a variety of danger signals that activate the NLRP3 inflammasome including asbestos, silica and ATP.37 To determine if high extracellular K+ would block doxorubicin-mediated NLRP3 inflammasome activation, LPS-primed or unprimed BMDM were exposed to doxorubicin (10 µM) in the presence or absence of high K+ media for 8 h, at which time presence of IL-1β was determined. As expected, LPS primed BMDM exposed to doxorubicin demonstrated an increase in pro-IL-1β (Fig. 6A, top) and an increase in release of IL-1β (Fig. 6A and B). LPS-primed BMDM that were treated with doxorubicin in the presence of elevated K+ demonstrated nearly a 10-fold decrease in release of mature IL-1β (Fig. 6A, bottom), demonstrating that elevated extracellular K+ suppressed the ability of doxorubicin to mediate the release of IL-1β.
Figure 6.
Elevated extracellular K+ prevents doxorubicin-mediated release of IL-1β from wild-type BMDM. Unprimed or LPS -primed wild-type cells were treated with vehicle or 25 εM doxorubicin, in combination with either normal media or media with high extracellular K+ for 8 h. (A) WCLs were subjected to immunoblotting for pro-IL-1β and p38 MAPK (loading control). Released IL-1β was measured by ELISA analysis of media supernatants. Bars represent the mean ± SD of triplicate wells. (B) Media supernatants were also precipitated and subjected to immunoblot analysis for detection of IL-1β p17.
Discussion
In the current study we determined that doxorubicin and daunorubicin potently activated the NLRP3 inflammasome. LPS-primed BMDM treated with doxorubicin or daunorubicin displayed increased expression of pro-IL-1β and induced the secretion of mature IL-1β. The release of IL-1β from LPS primed BMDM exposed to doxorubicin was significantly suppressed in BMDM that were deficient in ASC, caspase-1 or NLRP3, suggesting that each of these inflammasome components is required for doxorubicin to mediate the processing and release of IL-1β. As with other agents known to activate the NLRP3 inflammasome, doxorubicin-mediated release of IL-1β was suppressed by the ROS inhibitors, NAC and DPI30,33,37 and by elevated extracellular K+.37 These studies suggest that doxorubicin and daunorubicin share signaling pathways similar to other agents that lead to the processing and secretion of IL-1β through activation of the NLRP3 inflammasome. As with other agents that activate the NLRP3 inflammasome, the mechanism by which priming of macrophages occurs in vivo is not well understood. Macrophage priming in vivo may occur through activation of TLRs by release of cellular macromolecules, including cytoplasmic DNA, that occurs following cell death and tissue destruction.28,38
Prior studies suggest that the ability of these drugs to activate the NLRP3 inflammasome may be related to their ability to produce ribotoxic stress. Ribotoxic stressors are agents that inhibit protein translation and activate JNK and p38.39 The activation of JNK and p38 by ribotoxic stressors requires ZAK, an upstream MAP3K.40 Well-characterized ribotoxic stressors include anisomycin, blasticidin, ricin, Shiga toxin, sarcin and ultraviolet radiation.39,41 Doxorubicin and daunorubicin exhibit the two salient characteristics of ribotoxic stress agents: the inhibition of protein synthesis and the ZAK-mediated activation of JNK and p38.36 Nigericin and valinomycin are potassium ionophores that activate the NLRP3 inflammasome33,42 and are also potent inhibitors of protein synthesis.43–45 We have determined that a broad range of protein synthesis inhibitors lead to activation of the NLRP3 inflammasome in BMDMs in vitro (unpublished). Taken together, these results suggest that inhibition of translation per se may serve as a danger signal that leads to activation of the NLRP3 inflammasome.
Consistent with our data demonstrating that anthracyclines activate the inflammasome in vitro, and we have found that doxorubicin induces increased blood levels of IL-1β when injected into mice. The ability of doxorubicin to increase IL-1β levels in animals has been previously reported by Zhu et al. Once released into the periphery, IL-1β may lead to the production of other inflammatory cytokines and chemokines including IL-6. In the present study we show that in addition to IL-1β, doxorubicin can induce expression of TNFα, IL-6, GCSF, CXCL10/IP-10, CCL2/MCP-1 and CXCL1/Gro-α. Our studies with IL-1R-deficient mice demonstrate the importance of doxorubicin-mediated IL-1 signaling in the induction of some, but not all, of these inflammatory cytokines and chemokines. Mature IL-1β generally acts on target cells in an autocrine and paracrine fashion to stimulate the production of itself as well as other downstream inflammatory targets. There was not a significant drug by genotype interaction in serum IL-1β or TNFα levels. However, it is noteworthy that, while doxorubicin increased serum levels of both cytokines in wild-type mice, in IL-1R deficient mice it did not. Indeed, of all of the inflammatory cytokines and chemokines measured, the magnitude of the response to doxorubicin was generally lower in IL-1R deficient mice compared to their wild-type counterparts. Taken together, these results suggest that a defect in IL-1 signaling leads to an overall dampening of the inflammatory response to doxorubicin administration in mice.
The effect of IL-1R deficiency on doxorubicin-mediated IL-6 levels is of particular interest because we have previously shown that serum IL-6 is an inflammatory marker of cytotoxic chemotherapy-mediated fatigue behavior in mouse studies and is one of the few inflammatory markers examined clinically that is a reasonable marker of persistent cancer treatment related fatigue.46,47 Therefore, blocking IL-6 production by inhibition of components of the inflammasome may decrease symptom burden in cancer patients.
The requirement of doxorubicin-induced IL-1 signaling for expression of GCSF was particularly striking. GCSF is a growth factor and cytokine produced by macrophages, epithelial cells, stromal cells and immune cells which stimulates the bone marrow to produce granulocytes and stem cells and differentiation and survival of precursor and mature neutrophils.48 Because IL-1β is a potent inducer of GCSF expression, the observed increase in serum levels of IL-1β and GCSF in response to doxorubicin is not surprising.49 Similar to other chemotherapeutics, doxorubicin is cytotoxic to hematopoietic progenitor cells and leads to bone marrow suppression during cancer treatment. The ability of doxorubicin to stimulate the production of GCSF has clear clinical benefits, allowing increased mobilization of stem cells and recovery of the bone marrow compartment following injury. Indeed, human recombinant GCSF has been developed specifically to prevent cytotoxic chemotherapy-mediated granulocytopenia in cancer patients undergoing cancer treatment.48 It is unlikely that compensatory pathways exist for the GCSF response to doxorubicin since GCSF production is completely blocked in the absence of the IL-1R. Thus, targeting the inflammasome to decrease symptom burden in cancer patients may have unintended negative consequences. Further pre-clinical experiments, which are currently ongoing, will allow us to determine whether targeting components of the inflammasome would be a feasible approach to managing the negative effects of anthracyclines in the clinical setting.
Materials and Methods
Reagents and antibodies.
Doxorubicin, daunorubicin, LPS (L-2630), N-acetyl cysteine (NAC), diphenyleneiodonium chloride (DPI) and insulin (I6634) were purchased from Sigma-Aldrich (St. Louis, MO). Trichloroacetic acid (TCA) was purchased from Fisher Scientific (Pittsburgh, PA). Antibody against IL-1β was purchased from Abcam (Cambridge, MA) and antibody against p38 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The mouse IL-1β enzyme-linked immunosorbent assay (ELISA) Ready-Set-Go was purchased from eBioscience (San Diego, CA).
Animals and animal procedures.
All animal procedures were performed according to protocols that have been approved by the Institutional Animal Care and Use Committee at Oregon Health and Science University, Portland, OR. C57BL/6J, IL-1R1 deficient and caspase-1 deficient mice were purchased from the Jackson Laboratory (Bar Harbor, ME). ASC- and NLRP3-deficient mice were kindly provided by V. Dixit (Genentech, San Francisco, CA).
Treatment of mice with doxorubicin.
Mice in all experiments were female 12–15-week-old C57BL/6 mice or IL-1R1 deficient mice (on a C57/BL/6 background) housed five to a cage in pathogen-free rooms (12 h light-dark cycle) with ad libitum access to drinking water. Doxorubicin-HCl was purchased as a lyophilized 10 mg tablet from Bedford Labs (Bedford, OH) and was dissolved in sterile, deionized water to attain a stock solution of 1 mg/ml, stored at 4°C. WT and IL-1R1-deficient mice were injected intraperitoneally (IP) with doxorubicin (75 mg/m2) in a volume of 1 mL normal saline (NS). The injection volume was used to improve absorption of the drug and to reduce localized tissue inflammation at the injection site. A similar number of control mice were injected with the same volume of NS without drug. All injections began at 3 pm. After injection, mice were returned to their home cages, at which time mouse chow was removed. Sixteen hours post-injection, mice were terminally sedated using isofluorane according to protocols established at OHSU Department of Comparative Medicine and blood was collected by cardiac puncture.
Measurement of serum inflammatory cytokine and chemokines.
Peripheral blood was allowed to clot at room temperature for 60 min and subjected to centrifugation in a microcentrifuge tube at 10,000 rpm for 2 min. Serum was immediately frozen at −70°C prior to cytokine analysis. IL-1β, TNFα, IL-6, CXCL10/IP-10, CCL2/MCP-1, GCSF and CXCL1/Gro-α in serum were measured in duplicate in two separate experiments, using a bead-based immunofluorescence assay (Luminex Inc., Austin, TX). Cytokine analysis kits were obtained from Millipore Inc., and assays were performed according to the protocol supplied by the manufacturer. Data were collected and analyzed using the Luminex-100 system Version IS (Luminex Inc., Austin, TX). A four- or five-parameter regression formula was used to calculate the sample concentrations from the standard curves.
Analysis.
The distribution of each serum analyte was assessed and transformations applied, if necessary. The types of transformations used for each serum analyte are presented in Table 1. Significant differences between drug groups or control groups for each genotype were determined by one-way analysis of variance (ANOVA) with Bonferroni adjustment for multiple comparisons. To determine whether there was an interaction between drug groups (CAF or Control) and genotype (WT or KO) on serum analyte, we performed a 2 × 2 ANOVA with Bonferroni adjustment for multiple comparisons. All data were analyzed using the SPSS statistical analysis software. Data are presented as the mean ± standard deviation (SD). p < 0.05 was considered to be statistically significant.
Table 1.
List of functional transformations performed on analytes to improve normality of distribution
| Analytes | Transformation |
| IL-1β | 1/Square root |
| TNFα | None |
| IL-6 | Square root |
| G-CSF | None |
| IP-10 | Natural log |
| KC | Natural log |
| MCP-1 | Natural log |
Isolation of bone marrow-derived macrophages (BMDM).
Male mice, 8–10 weeks of age, were used throughout the experiments. Cells were prepared from WT C57BL/6J, ASC-, Caspase-1- and NLRP3-deficent mice. Marrow was flushed from femurs and tibias with PBS and cultured in alpha-Minimum Essential Medium (αMEM, Cellgro, Herndon, VA), supplied with 10% Fetal Bovine Serum (FBS, Cellgro, Herndon, VA), 50 µg/ml gentamicin and 100 ng/ml recombinant mouse Colony Stimulating Factor 1 (CSF-1, R&D Systems, Minneapolis, MN) for 72 h on non-tissue culture treated 10 cm Petri dishes. BMDM were passaged and cultured for an additional 72 h. Each confluent 10 cm dish was transferred into one 6-well or one 12-well tissue culture plate (Sarstedt, Newton, NC) and cultured for 24 h before initiating experimental treatment.
Treating BMDM.
Cells were serum deprived in α-MEM for 30 min followed by treatment with 50 ng/mL LPS for 4 h. Cells were rinsed and fresh media was added followed by exposure to the indicated doses of doxorubicin or daunorubicin for 8 h prior to harvest. Doxorubicin and daunorubicin doses were chosen to reflect peak plasma and hematopoietic tissue levels observed in cancer patients following bolus administration of these drugs.50–52 In experiments involving co-treatment with doxorubicin or daunorubicin, N-acetyl cysteine (NAC; 30 mM), diphenyleneiodonium chloride (DPI; 25 µM) or elevated potassium (130 mM) was added after LPS pretreatment. In experiments employing elevated potassium, sodium was replaced by potassium at an equivalent molar concentration.
Immunoblotting.
BMDM cells were lysed in 2× ESB lysis buffer in preparation for immunoblotting. Equal volumes of the cell lysates were separated on a 10% denaturing polyacrylamide gel in the presence of sodium dodecyl sulfate and were transferred onto polyvinylidene difluoride membranes according to standard laboratory procedures. Proteins from BMDM media supernatants were precipitated using TCA plus 200 µg insulin carrier protein and separated on 13% gels. Membranes were incubated with the indicated antibodies and the corresponding horseradish peroxidase-conjugated secondary antibodies. Signals were detected by using enhanced chemiluminescence.
ELISA.
Media from BMDM were collected and analyzed in triplicate using IL-1β ELISA (eBioscience) according to the manufacturer's protocol.
Measurement of protein synthesis via incorporation of [3H]-leucine.
BMDM were grown in 12-well tissue culture plates. Treatments were performed in leucine-free/serum-free Dulbecco modified Eagle medium, for the indicated times and doses of doxorubicin. For the final 30 min of doxorubicin treatment before harvesting, the cells were pulse-labeled with 1 µCi of [3H]-leucine in 1 ml of DMEM. Ten percent trichloroacetic acid was added to terminate incorporation. Wells were washed in water and 88% formic acid was added to solubilize the trichloroacetic acid-insoluble proteins. The samples were counted in a liquid scintillation counter. In each experiment, three wells were used per experimental point.
Acknowledgments
These studies were supported by grants DK079419 (K.A.D.S.) and AI1059335 (B.E.M.) from the National Institutes of Health and grant RSGPB-05-212-01-CPPB (L.J.W.) from the American Cancer Society.
References
- 1.Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis. 2007;49:330–352. doi: 10.1016/j.pcad.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug Saf. 2000;22:263–302. doi: 10.2165/00002018-200022040-00002. [DOI] [PubMed] [Google Scholar]
- 3.Dodd MJ, Miaskowski C, Lee KA. Occurrence of symptom clusters. J Natl Cancer Inst Monogr. 2004:76–78. doi: 10.1093/jncimonographs/lgh008. [DOI] [PubMed] [Google Scholar]
- 4.Cella D. Factors influencing quality of life in cancer patients: anemia and fatigue. Semin Oncol. 1998;25:43–46. [PubMed] [Google Scholar]
- 5.Cella D, Peterman A, Passik S, Jacobsen P, Breitbart W. Progress toward guidelines for the management of fatigue. Oncology (Williston Park) 1998;12:369–377. [PubMed] [Google Scholar]
- 6.Greene D, Nail LM, Fieler VK, Dudgeon D, Jones LS. A comparison of patient-reported side effects among three chemotherapy regimens for breast cancer. Cancer Pract. 1994;2:57–62. [PubMed] [Google Scholar]
- 7.Schwartz AL, Nail LM, Chen S, Meek P, Barsevick AM, King ME, et al. Fatigue patterns observed in patients receiving chemotherapy and radiotherapy. Cancer Invest. 2000;18:11–19. doi: 10.3109/07357900009023057. [DOI] [PubMed] [Google Scholar]
- 8.Demetri GD, Kris M, Wade J, Degos L, Cella D. Quality-of-life benefit in chemotherapy patients treated with epoetin alfa is independent of disease response or tumor type: results from a prospective community oncology study. Procrit Study Group. J Clin Oncol. 1998;16:3412–3425. doi: 10.1200/JCO.1998.16.10.3412. [DOI] [PubMed] [Google Scholar]
- 9.Glaus A, Crow R, Hammond S. A qualitative study to explore the concept of fatigue/tiredness in cancer patients and in healthy individuals. Eur J Cancer Care (Engl) 1996;5:8–23. doi: 10.1111/j.1365-2354.1996.tb00247.x. [DOI] [PubMed] [Google Scholar]
- 10.Irvine D, Vincent L, Graydon JE, Bubela N, Thompson L. The prevalence and correlates of fatigue in patients receiving treatment with chemotherapy and radiotherapy. A comparison with the fatigue experienced by healthy individuals. Cancer Nurs. 1994;17:367–378. [PubMed] [Google Scholar]
- 11.Jacobsen PB, Hann DM, Azzarello LM, Horton J, Balducci L, Lyman GH. Fatigue in women receiving adjuvant chemotherapy for breast cancer: characteristics, course and correlates. J Pain Symptom Manage. 1999;18:233–242. doi: 10.1016/s0885-3924(99)00082-2. [DOI] [PubMed] [Google Scholar]
- 12.Mock V, Dow KH, Meares CJ, Grimm PM, Dienemann JA, Haisfield-Wolfe ME, et al. Effects of exercise on fatigue, physical functioning and emotional distress during radiation therapy for breast cancer. Oncol Nurs Forum. 1997;24:991–1000. [PubMed] [Google Scholar]
- 13.Sitzia J, Huggins L. Side effects of cyclophosphamide, methotrexate and 5-fluorouracil (CMF) chemotherapy for breast cancer. Cancer Pract. 1998;6:13–21. doi: 10.1046/j.1523-5394.1998.1998006013.x. [DOI] [PubMed] [Google Scholar]
- 14.Ferrell BR, Grant M, Funk B, Garcia N, Otis-Green S, Schaffner ML. Quality of life in breast cancer. Cancer Pract. 1996;4:331–340. [PubMed] [Google Scholar]
- 15.Nail LM, Winningham ML. Fatigue and weakness in cancer patients: the symptoms experience. Semin Oncol Nurs. 1995;11:272–278. doi: 10.1016/s0749-2081(05)80008-7. [DOI] [PubMed] [Google Scholar]
- 16.Vogelzang NJ, Breitbart W, Cella D, Curt GA, Groopman JE, Horning SJ, et al. Patient, caregiver and oncologist perceptions of cancer-related fatigue: results of a tripart assessment survey. The Fatigue Coalition. Semin Hematol. 1997;34:4–12. [PubMed] [Google Scholar]
- 17.Zhu J, Zhang J, Xiang D, Zhang Z, Zhang L, Wu M, et al. Recombinant human interleukin-1 receptor antagonist protects mice against acute doxorubicin-induced cardiotoxicity. Eur J Pharmacol. 2010;643:247–253. doi: 10.1016/j.ejphar.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 18.Dinarello CA. IL-1: discoveries, controversies and future directions. Eur J Immunol. 2010;40:599–606. doi: 10.1002/eji.201040319. [DOI] [PubMed] [Google Scholar]
- 19.Bankers-Fulbright JL, Kalli KR, McKean DJ. Interleukin-1 signal transduction. Life Sci. 1996;59:61–83. doi: 10.1016/0024-3205(96)00135-x. [DOI] [PubMed] [Google Scholar]
- 20.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 21.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 22.Ogura Y, Sutterwala FS, Flavell RA. The inflammasome: first line of the immune response to cell stress. Cell. 2006;126:659–662. doi: 10.1016/j.cell.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Yu HB, Finlay BB. The caspase-1 inflammasome: a pilot of innate immune responses. Cell Host Microbe. 2008;4:198–208. doi: 10.1016/j.chom.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 25.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 26.Burns K, Martinon F, Tschopp J. New insights into the mechanism of IL-1beta maturation. Curr Opin Immunol. 2003;15:26–30. doi: 10.1016/s0952-7915(02)00017-1. [DOI] [PubMed] [Google Scholar]
- 27.Tschopp J, Martinon F, Burns K. NALPs: a novel protein family involved in inflammation. Nat Rev Mol Cell Biol. 2003;4:95–104. doi: 10.1038/nrm1019. [DOI] [PubMed] [Google Scholar]
- 28.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, et al. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci USA. 2008;105:9035–9040. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singal PK, Li T, Kumar D, Danelisen I, Iliskovic N. Adriamycin-induced heart failure: mechanism and modulation. Mol Cell Biochem. 2000;207:77–86. doi: 10.1023/a:1007094214460. [DOI] [PubMed] [Google Scholar]
- 32.Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W, et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14:1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 34.Lindauer ML, Wong J, Magun B. Ricin toxin activates the NALP3 inflammasome. Toxins. 2010;2:1500–1514. doi: 10.3390/toxins2061500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Momparler RL, Karon M, Siegel SE, Avila F. Effect of adriamycin on DNA RNA and protein synthesis in cell-free systems and intact cells. Cancer Res. 1976;36:2891–2895. [PubMed] [Google Scholar]
- 36.Sauter KA, Magun EA, Iordanov MS, Magun BE. ZAK is required for doxorubicin, a novel ribotoxic stressor, to induce SAPK activation and apoptosis in HaCaT cells. Cancer Biol Ther. 2010;10:258–266. doi: 10.4161/cbt.10.3.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 38.Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iordanov MS, Pribnow D, Magun JL, Dinh TH, Pearson JA, Chen SL, et al. Ribotoxic stress response: activation of the stress-activated protein kinase JNK1 by inhibitors of the peptidyl transferase reaction and by sequence-specific RNA damage to the alpha-sarcin/ricin loop in the 28S rRNA. Mol Cell Biol. 1997;17:3373–3381. doi: 10.1128/mcb.17.6.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jandhyala DM, Ahluwalia A, Obrig T, Thorpe CM. ZAK: a MAP3Kinase that transduces Shiga toxin- and ricin-induced proinflammatory cytokine expression. Cell Microbiol. 2008;10:1468–1477. doi: 10.1111/j.1462-5822.2008.01139.x. [DOI] [PubMed] [Google Scholar]
- 41.Iordanov MS, Pribnow D, Magun JL, Dinh TH, Pearson JA, Magun BE. Ultraviolet radiation triggers the ribotoxic stress response in mammalian cells. J Biol Chem. 1998;273:15794–15803. doi: 10.1074/jbc.273.25.15794. [DOI] [PubMed] [Google Scholar]
- 42.Freche B, Reig N, van der Goot FG. The role of the inflammasome in cellular responses to toxins and bacterial effectors. Semin Immunopathol. 2007;29:249–260. doi: 10.1007/s00281-007-0085-0. [DOI] [PubMed] [Google Scholar]
- 43.Breitbart H. Effect of ionophores and metabolic inhibitors on protein synthesis in rabbit reticulocytes. Biochim Biophys Acta. 1981;656:160–166. doi: 10.1016/0005-2787(81)90082-4. [DOI] [PubMed] [Google Scholar]
- 44.Panet R, Atlan H. Coupling between K efflux, ATP metabolism and protein synthesis in reticulocytes. Biochem Biophys Res Commun. 1979;88:619–626. doi: 10.1016/0006-291x(79)92093-x. [DOI] [PubMed] [Google Scholar]
- 45.Smith JV, Falconer IR. Effect of nigericin and varying potassium concentrations on the prolactin-stimulated synthesis of milk fat in explants of mammary alveolar tissue from rabbits. J Endocrinol. 1983;99:261–268. doi: 10.1677/joe.0.0990261. [DOI] [PubMed] [Google Scholar]
- 46.Wood LJ, Nail LM, Perrin NA, Elsea CR, Fischer A, Druker BJ. The cancer chemotherapy drug etoposide (VP-16) induces proinflammatory cytokine production and sickness behavior-like symptoms in a mouse model of cancer chemotherapy-related symptoms. Biol Res Nurs. 2006;8:157–169. doi: 10.1177/1099800406290932. [DOI] [PubMed] [Google Scholar]
- 47.Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav Immun. 2007;21:413–427. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Roberts AW. G-CSF: a key regulator of neutrophil production, but that's not all! Growth Factors. 2005;23:33–41. doi: 10.1080/08977190500055836. [DOI] [PubMed] [Google Scholar]
- 49.Kittler EL, McGrath H, Temeles D, Crittenden RB, Kister VK, Quesenberry PJ. Biologic significance of constitutive and subliminal growth factor production by bone marrow stroma. Blood. 1992;79:3168–3178. [PubMed] [Google Scholar]
- 50.Wurz GT, Soc L, Emshoff VD, Cadman TB, DeGregorio MW. Pharmacokinetic analysis of high-dose toremifene in combination with doxorubicin. Cancer Chemother Pharmacol. 1998;42:363–366. doi: 10.1007/s002800050830. [DOI] [PubMed] [Google Scholar]
- 51.Speth PA, Linssen PC, Boezeman JB, Wessels HM, Haanen C. Leukemic cell and plasma daunomycin concentrations after bolus injection and 72 h infusion. Cancer Chemother Pharmacol. 1987;20:311–315. doi: 10.1007/BF00262582. [DOI] [PubMed] [Google Scholar]
- 52.Speth PA, Linssen PC, Termond EF, Boezeman JB, Wessels HM, Haanen C. In vivo and in vitro pharmacokinetic differences between four structurally closely related anthracyclines in hematopoietic cell subtypes in humans. Drug Metab Dispos. 1989;17:98–105. [PubMed] [Google Scholar]