Abstract
Troglitazone is a ligand for the peroxisome proliferator activated receptor gamma (PPARγ) that decreases growth of human prostate cancer cells in vitro and in vivo. However, the mechanism by which troglitazone reduces prostate cancer cell growth is not fully understood. To understand the signaling pathways involved in troglitazone-induced decreases in prostate cancer growth, we examined the effect of troglitazone on androgen-independent C4-2 human prostate cancer cells. Initial experiments revealed troglitazone inhibited C4-2 cell proliferation by arresting cells in the G0/G1 phase of the cell cycle and inducing apoptosis. Since the proto-oncogene product c-Myc regulates both apoptosis and cell cycle progression, we next examined whether troglitazone altered expression of c-Myc. Troglitazone decreased c-Myc protein levels as well as expression of downstream targets of c-Myc in a dose-dependent manner. In C4-2 cells, troglitazone-induced decreases in c-Myc protein involve proteasome-mediated degradation of c-Myc protein as well as reductions in c-Myc mRNA levels. It appears that troglitazone stimulates degradation of c-Myc by increasing c-Myc phosphorylation, for the level of phosphorylated c-Myc was elevated in prostate cancer cells exposed to troglitazone. While troglitazone dramatically decreased the amount of c-Myc within C4-2 cells, the PPARγ ligands ciglitazone, rosiglitazone and pioglitazone did not reduce c-Myc protein levels. Furthermore the downregulation of c-Myc by troglitazone was not blocked by the PPARγ antagonist GW9662 and siRNA-mediated decreases in PPARγ protein. Thus, our data suggest that troglitazone reduces c-Myc protein independently of PPARγ.
Key words: troglitazone, prostate cancer, c-Myc, cell cycle, PPARγ
Introduction
Other than skin cancer, prostate cancer is the most common form of cancer found in American men. The American Cancer Society reports that there will be 217,730 new cases of prostate cancer in the United States in 2010.1 In addition, it is estimated that this year 32,050 American men will die of this disease.1 Radiation and surgery are common treatments for early stage prostate cancer, while androgen ablation therapy is the standard treatment for advanced stage prostate cancer. Androgen ablation is a hormonal deprivation therapy where the circulating levels of androgen in the body are reduced. Androgen ablation therapy is initially effective at slowing the growth rate of prostate cancer. However, androgen ablation therapy results in the development of more aggressive forms of prostate cancer that do not require androgens for growth. There is currently no effective treatment for these androgen-independent forms of prostate cancer. As a result, there is great interest in identifying more effective treatment options for this form of prostate cancer.
Agonists for the peroxisome proliferator activated receptor gamma (PPARγ) have shown promise as potential therapeutic agents for prostate cancer. Our laboratory and others have shown that one group of synthetic PPAR. ligands, the thiazolidinediones (TZDs), significantly inhibit proliferation of human prostate cancer cells. The TZDs, which were identified as high affinity PPAR. agonists in 1995, include the compounds ciglitazone, troglitazone (Rezulin), rosiglitazone (Avandia) and pioglitazone (Actos).2 The TZDs rosiglitazone and ciglitazone decreased proliferation of the PC-3 and C4-2 human prostate cancer cell lines and primary cell cultures of human prostate cancer cells.3–6 Troglitazone has also been shown to inhibit growth of the PC-3, LNCaP and DU145 cell lines.7 Clinical trials have been performed testing the effect of TZDs as a treatment for prostate cancer. However, the results have not been consistent across these studies. Smith et al. showed that rosiglitazone did not increase prostate specific antigen (PSA) doubling time, a marker of prostate cancer progression, or delay the time to disease progression.8 However, Hisatake et al. demonstrated that the TZD troglitazone stabilized PSA levels in a 75-year-old man with advanced prostate cancer.9 In addition, Mueller et al. conducted a phase II clinical study in patients with advanced prostate cancer using troglitazone. They observed a high number of patients who had stable levels of PSA after treatment with troglitazone.10 Taken together, these clinical trials suggest the effectiveness of TZDs in prostate cancer patients may vary depending on the compound tested. Of the compounds tested, troglitazone appears to be the most effective at slowing clinical progression of prostate cancer. However, it is unclear what factors determine the clinical response to TZDs.
Inhibition of cell proliferation can occur through changes in cell cycle and/or apoptosis. One protein which plays an important role in cell cycle progression and apoptosis is the proto-oncogene product c-Myc. The c-Myc protein is a basic/helix-loop-helix/leucine zipper transcription factor. By dimerizing with its binding partner, Max, c-Myc can bind specific E-box sequences within DNA and regulate gene transcription. c-Myc regulates expression of several gene products that are involved in cell proliferation and apoptosis. Expression of the cyclin dependent kinase inhibitors p21 and p27 is reduced by c-Myc.11,12 Two proteins which promote cell proliferation, cyclin dependent kinase 4 (CDK4) and the phosphatase CDC25A, are also positively regulated by c-Myc.13–16 c-Myc also regulates expression of proteins that control apoptosis. Expression of the pro-apoptotic proteins Bad in the rat frontal cortex and Bax in glioblastomas have been shown to be regulated by c-Myc.17,18 In Caco-2 colon cancer cells, rosiglitazone not only reduced c-Myc protein levels but also induced expression of Bax.19 In addition, work by Kanunfre et al. suggested decreases in c-Myc expression by ciglitazone resulted in an increase in apoptosis within the Raji B lymphoblast-like cell line and the human Jurkat leukemia cells.20 Thus, decreases in c-Myc are associated with TZD-induced inhibition of cell proliferation within colon cancers and leukemic cells.
While ciglitazone and rosiglitazone have been shown to decrease c-Myc protein expression, it is not known if other TZDs are effective in reducing c-Myc levels in cancer cells. In addition, the mechanism by which TZDs suppress c-Myc expression is not fully understood. To define the role of c-Myc in troglitazone-induced decreases in prostate cancer cell proliferation, we examined the ability of troglitazone to regulate c-Myc expression in human prostate cancer cells. In this study we show that troglitazone not only suppresses prostate cancer cell growth but also decreases c-Myc protein expression. In the C4-2 cell line, troglitazone decreases c-Myc protein through proteasome-mediated degradation of c-Myc protein as well as a reduction in c-Myc mRNA levels. Furthermore, our results demonstrate troglitazone reduces c-Myc protein independently of PPARγ activation.
Results
Troglitazone inhibits C4-2 cell growth via cell cycle arrest and apoptosis.
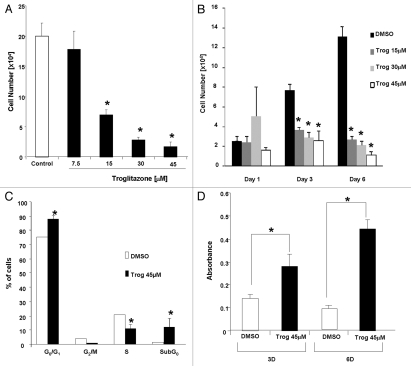
Micromolar concentrations of troglitazone have been shown to inhibit growth of ovarian and colon cancer cells.21–24 To determine whether troglitazone was also effective at suppressing growth of the androgen-independent C4-2 prostate cancer cell line, we exposed the cells to different concentrations (7.5–45 µM) of troglitazone. In these proliferation studies, cell number was measured after the cells were treated for 6 days with troglitazone. Troglitazone produced a dose-dependent decrease in C4-2 cell proliferation, which was indicated by a decrease in cell number (Fig. 1A). A significant decrease in cell proliferation was first detected in C4-2 cells exposed to 15 µM troglitazone. The maximum decrease in proliferation was noted at a concentration of 45 µM troglitazone. Furthermore, the ability of troglitazone to reduce cell proliferation was time-dependent. A significant decrease in C4-2 cell number was detected in cells treated for three days or longer with troglitazone (Fig. 1B).
Figure 1.
Troglitazone inhibits growth of C4-2 cells. (A) C4-2 cells were treated with DMSO vehicle (Control) or troglitazone (7.5–45 εM) for 6 days. Cells were harvested after 6 days and total cell number was measured using a Z1 Coulter Counter. Each bar represents the mean ± SD of three wells. *p < 0.05 when compared to DMSO control. A representative experiment is shown. (B) C4-2 cells were treated with DMSO vehicle or troglitazone (15–45 εM) for the indicated times. Following treatment total cell number was counted using a Z1 Coulter Counter. Each bar represents the mean ± SD of three wells. *p < 0.05 when compared to DMSO control. A representative experiment is shown. (C) Cells were treated with DMSO vehicle or troglitazone 45 εM for 72 h. The percentage of cells within the different phases of the cell cycle was determined using flow cytometry. *p < 0.05 when compared to control. Each bar represents the mean ± SD of three samples. A representative experiment is shown. (D) C4-2 cells were treated with DMSO vehicle or troglitazone 45 εM for 3 or 6 days. Following treatment, the level of DNA fragmentation (a hallmark of apoptosis) was measured using a Cell Death ELISA. *p < 0.05 when compared to DMSO control. A representative experiment is shown.
Decreases in cell number can result from either an alteration in cell cycle progression or an increase in the level of apoptosis. Therefore, we decided to examine whether troglitazone alters C4-2 cell cycle distribution and apoptosis through FACS analysis. Troglitazone treatment for six days produced an increase in the G0/G1 cell population, indicating a G0/G1 cell cycle arrest. Troglitazone did not significantly alter the number of cells in the G2/M population. In addition, we noted a significant decrease in the percentage of C4-2 cells present in the S phase (Fig. 1C). Interestingly, the number of cells in the sub-G0 population was also significantly increased with troglitazone treatment. Since apoptotic cells can be present in the sub-G0 population, these data suggest that troglitazone inhibits C4-2 cell proliferation by inducing apoptosis. To confirm whether troglitazone stimulates apoptosis in C4-2 cells we used a Cell Death ELISA assay to measure the levels of DNA fragmentation following troglitazone exposure. Treatment of C4-2 cells with troglitazone for both three and six days produced a significant increase in DNA fragmentation (Fig. 1D). Taken together, our data indicate that an increase in G0/G1 cell cycle arrest and apoptosis both contribute to troglitazone-induced decreases in C4-2 proliferation.
Troglitazone decreases c-Myc protein expression.
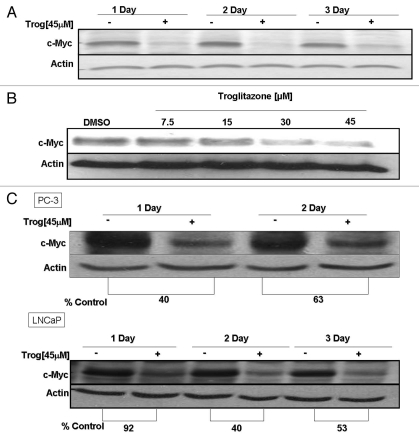
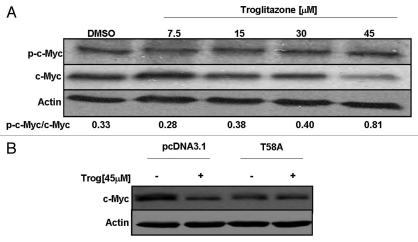
c-Myc is a proto-oncogene that plays roles in both cell cycle progression and apoptosis. To determine whether alterations in c-Myc expression may contribute to the troglitazone-induced decrease in C4-2 cell proliferation, we examined the effect of troglitazone on c-Myc protein levels. Protein gel blot analysis revealed troglitazone induced a time-dependent decrease in c-Myc protein expression within the C4-2 cell line (Fig. 2A). This decrease could be detected 24 h after the addition of troglitazone. Furthermore, c-Myc levels remained low after 3 days of troglitazone exposure. Troglitazone treatment also decreased c-Myc protein levels within C4-2 cells in a dose-dependent manner. The greatest decrease in c-Myc protein expression occurred at 45 µM, the concentration of troglitazone that produced the greatest reduction in C4-2 cell proliferation (Fig. 2B).
Figure 2.
Troglitazone decreases c-Myc protein expression in human prostate cancer cells. (A) C4-2 cells were treated with DMSO vehicle or troglitazone 45 εM for indicated times. Following treatment, cells were harvested and the amount of c-Myc and actin protein in each sample was determined using protein gel blot analysis. (B) C4-2 cells were treated with DMSO vehicle or troglitazone (7.5–45 εM) for 48 h. The amount of c-Myc and actin protein in each sample was determined using protein gel blot analysis. (C) PC-3 or LNCaP cells were treated with DMSO vehicle or troglitazone 45 εM for indicated times. Protein gel blot analysis was then used to determine the level of c-Myc and actin protein in each sample.
Troglitazone-induced decrease of c-Myc protein is not cell line specific.
To determine if the troglitazone-induced decrease in c-Myc protein was unique to the C4-2 cell line, we examined the effect of troglitazone on c-Myc levels in the androgen-dependent LNCaP and the androgen-independent PC-3 prostate cancer cells. Troglitazone at a concentration of 45 µM produced a >40% decrease in c-Myc protein levels in the LNCaP cells (Fig. 2C). In addition, c-Myc protein expression was also dramatically reduced in PC-3 cells following troglitazone treatment (Fig. 2C). The concentrations of c-Myc that suppressed c-Myc protein levels were also effective at reducing proliferation of each cell line (data not shown). Therefore, it appears that a growth-inhibitory concentration of troglitazone is able to reduce c-Myc levels in multiple human prostate cancer cell lines.
Reductions in c-Myc protein inhibit C4-2 cell proliferation.
It has been previously reported that c-Myc promotes the growth of human prostate cancer cells.25,26 To confirm the role of c-Myc in prostate cancer cell proliferation, we tested how reductions in c-Myc influenced proliferation of C4-2 cells. In these studies we used siRNA technology to knock down levels of c-Myc protein in the C4-2 cell line. Cell count assays revealed that proliferation was significantly reduced in C4-2 cells exposed to c-Myc siRNA (Fig. 3). Thus, our data indicate a decrease in c-Myc protein levels can result in reduced proliferation within the C4-2 prostate cancer cell line.
Figure 3.
Knock down of c-Myc protein reduces proliferation of C4-2 cells. C4-2 cells were first transfected with either a non-specific control SMART-pool or c-Myc siRNA. Two days later, cells were detached using trypsin-EDTA and total cell number in each well was measured using a Z1 Coulter Counter. Each bar represents the mean ± SD for three wells. *p < 0.05 when compared to DMSO control. A representative experiment is shown. Protein gel blot analysis was used to determine the levels of c-Myc and actin protein in transfected cells.
Troglitazone regulates expression of downstream targets of c-Myc.
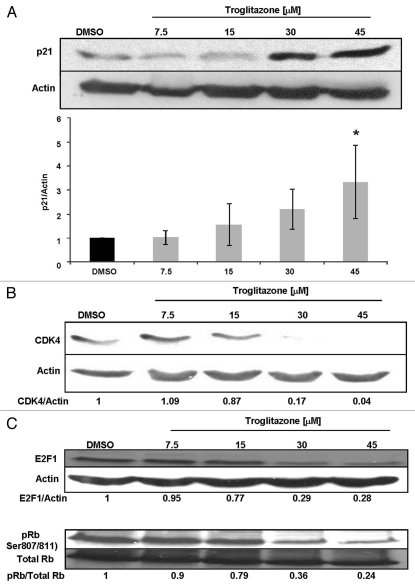
Changes in the expression level of c-Myc within a cell can alter the expression of p21 and CDK4, two proteins that regulate cell cycle progression.15,27–29 While c-Myc is a negative regulator of the cyclin dependent kinase inhibitor p21, it stimulates production of cyclin dependent kinase 4 (CDK4). We performed protein gel blot analysis to determine whether troglitazone also regulates expression of these two downstream targets of c-Myc. In these studies, protein levels were examined after C4-2 cells were treated with troglitazone for 48 h. Troglitazone induced a dose-dependent increase in p21 protein levels. We observed a statistically significant increase in p21 following treatment of C4-2 cells with troglitazone 45 µM (Fig. 4A). Troglitazone also produced a dose-dependent decrease in CDK4 protein expression. The greatest decrease in CDK4 expression was observed at a concentration of 45 µM troglitazone (Fig. 4B). E2F1 and retinoblastoma protein (Rb) control the ability of cells to progress from G1 to S phase of the cell cycle and are positively regulated by c-Myc expression.30,31 We next examined the effect of troglitazone on E2F1 and Rb protein levels. Exposing C4-2 cells to troglitazone resulted in a dose-dependent decrease in E2F1 protein. Furthermore, troglitazone increased the activity of Rb, for the ratio of phospho Rb/total Rb protein within C4-2 cells decreased with troglitazone treatment (Fig. 4C). Taken together, our results indicate that troglitazone not only regulates c-Myc protein but also affects expression of multiple c-Myc regulated gene products.
Figure 4.
Troglitazone regulates downstream targets of c-Myc. (A) C4-2 cells were treated with DMSO vehicle or troglitazone (7.5–45 εM) for 48 h. After treatment cells were harvested and levels of p21 and actin protein in each sample was measured using protein gel blot analysis. Average data from four independent experiments are shown in the bar graph. *p < 0.05 compared to control. (B) C4-2 cells were treated with DMSO vehicle or troglitazone (7.5–45 εM) for 48 h. Protein gel blot analysis was used to measure the CDK4 and actin levels in treated cells. (C) C4-2 cells were treated with DMSO vehicle or troglitazone (7.5–45 εM) for 48 h. Total levels of E2F1, phospho Rb, total Rb and actin were determined using protein gel blot analysis.
Troglitazone reduces c-Myc mRNA and stimulates proteasomal degradation of c-Myc protein.
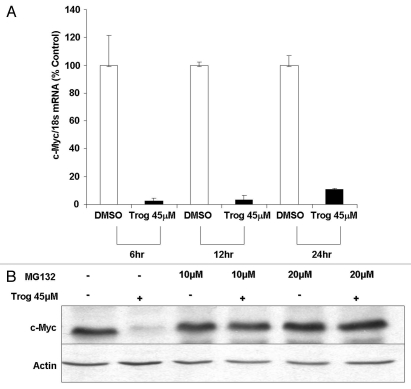
Reductions in protein within cancer cells can occur as a result of decreased synthesis or stability of mRNA. To determine whether alterations in mRNA contribute to troglitazone-induced decreases in c-Myc protein, we used quantitative RT-PCR to measure c-Myc mRNA levels in C4-2 cells exposed to troglitazone. Troglitazone significantly decreased c-Myc mRNA within the C4-2 cell line. A decrease in c-Myc mRNA was initially observed six hours after troglitazone treatment (Fig. 5A). c-Myc mRNA levels remained low after 24 h of troglitazone exposure.
Figure 5.
Troglitazone decreases c-Myc mRNA levels and stimulates degradation of c-Myc protein. (A) C4-2 cells were treated with either DMSO vehicle or troglitazone 45 εM for indicated times. The levels of c-Myc and 18S mRNA was then measured by qRT-PCR. Each bar represents the mean ± SD of three samples. The c-Myc/18S RNA ratio for troglitazone is expressed as a percentage of the time matched DMSO control. (B) C4-2 cells were treated with for 48 h DMSO vehicle or troglitazone 45 εM in the presence or absence of the proteasome inhibitor MG132. Following treatment, protein gel blot analysis was used to measure the levels of c-Myc and actin protein in treated cells.
Rapid decreases in c-Myc protein can also occur through a proteasome-mediated degradation pathway.32,33 Recent reports have noted that troglitazone and the troglitazone derivative STG28 induce proteasomal degradation of the β-catenin and cyclin D1 proteins in the LNCaP prostate cancer cell line.34,35 To determine whether troglitazone regulates degradation of c-Myc protein, we examined whether troglitazone-induced decreases in c-Myc protein were altered in C4-2 cells treated with the proteasome inhibitor MG132. There was no significant alteration in c-Myc protein levels within C4-2 cells that were exposed to MG132 alone. However, the troglitazone-induced decrease of c-Myc protein was blocked by the addition of MG132 (Fig. 5B). Together these data suggest that activation of the proteasome pathway is required for troglitazone-induced decreases of c-Myc protein.
Troglitazone increases phosphorylation of c-Myc protein.
It has been shown that increased phosphorylation of c-Myc protein at threonine-58 (T58) and serine-62 (S62) targets c-Myc for degradation through the proteasomal pathway.32,36 To determine the effect of troglitazone on c-Myc phosphorylation, we examined the level of phosphorylated c-Myc in C4-2 cells exposed to increasing concentrations of troglitazone. Protein gel blots involving an antibody that recognizes c-Myc phosphorylated either at T58 alone or at both T58 and S62 phosphorylation sites revealed troglitazone produces a dose-dependent increase in the ratio of phospho c-Myc/total c-Myc. Over the range of concentrations tested, we saw the greatest increase in the proportion of phospho c-Myc at 45 µM troglitazone (Fig. 6A). To further examine the role of c-Myc phosphorylation, we tested whether mutation of T58 to a phosphorylation-resistant alanine residue would alter the ability of troglitazone to reduce c-Myc. In these studies, troglitazone was added to C4-2 cells transfected with a plasmid construct encoding the mutant T58A c-Myc protein or the pcDNA3.1 parent vector. Troglitazone treatment decreased c-Myc protein levels in C4-2 cells transfected with the parent vector. However, troglitazone did not reduce c-Myc levels in cells transfected with the c-Myc T58A mutant construct (Fig. 6B). Taken together, our data suggest that phosphorylation of c-Myc at T58 is critical for troglitazone-induced decreases in c-Myc protein.
Figure 6.
(A) Troglitazone regulates phosphorylation of c-Myc protein. C4-2 cells were treated with DMSO vehicle or troglitazone (7.5–45 εM) for 48 h. Following treatment, cells were harvested and the amount of phospho c-Myc, total c-Myc and actin protein in each sample was determined using protein gel blot analysis. (B) C4-2 cells were transfected with control pcDNA3.1 vector or c-MycT58A plasmid construct. Two days later, the cells were treated with DMSO vehicle (−) or troglitazone 45 εM (+) for 24 h. Following treatment, protein gel blot analysis was used to measure the levels of c-Myc and actin protein in treated cells.
Inhibition of the PI3Kinase/AKT pathway prevents troglitazone-induced decreases in c-Myc protein levels.
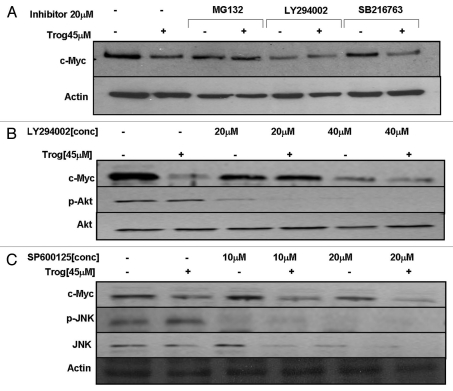
The kinases glycogen synthase kinase 3beta (GSK3β), phosphatidylinositol-3-kinase (PI3K) and extracellular-signal-regulated kinase 1/2 (ERK 1/2) MAP kinase have been shown to promote degradation of c-Myc by inducing phosphorylation of c-Myc at T58 and/or S62. To determine the kinase(s) required for troglitazone-induced decreases in c-Myc protein we exposed C4-2 cells to troglitazone in the presence or absence of various kinase inhibitors. The PI3Kinase inhibitor LY294002 alone blocked Akt phosphorylation and decreased c-Myc protein expression. LY294002 also prevented any further decrease in c-Myc protein in the presence of troglitazone (Fig. 7A and B). Compounds that are known inhibit the action of GSK3β (SB216763) and Erk 1/2 (UO126) had no effect on basal c-Myc levels and did not block troglitazone-induced degradation of c-Myc protein (Fig. 7A and data not shown). We also tested the effect of the JNK inhibitor SP600125 on troglitazone-induced decreases in c-Myc protein expression. While SP600125 was effective at reducing phosphorylation of JNK, it did not block the reduction of c-Myc protein by troglitazone (Fig. 7C). Together these data suggest that, of the kinases tested, the activity of PI3Kinase may influence the ability of troglitazone to degrade c-Myc protein.
Figure 7.
Inhibition of PI3Kinase prevents troglitazone-induced decreases in c-Myc protein. (A) C4-2 cells were treated with DMSO vehicle or troglitazone 45 εM in the presence or absence of MG132 (proteasome inhibitor), LY294002 (PI3Kinase inhibitor) or SB216763 (GSK3β inhibitor) for 24 h. Following treatment, protein gel blot analysis was used to measure the levels of c-Myc and actin protein in treated cells. (B) Cells were treated for 24 h with DMSO vehicle or troglitazone 45 εM in the presence or absence of LY294002 (20–40 εM). Protein gel blot analysis was used to measure levels of c-Myc, p-Akt and Akt in each sample. (C) C4-2 cells were treated for 24 h with DMSO vehicle or troglitazone 45 εM in the presence or absence of SP600125 (10–20 εM) (JNK inhibitor). Protein gel blot analysis was used to measure levels of c-Myc, p-JNK, JNK and actin in treated cells.
Troglitazone decreases c-Myc expression independently of PPARγ.
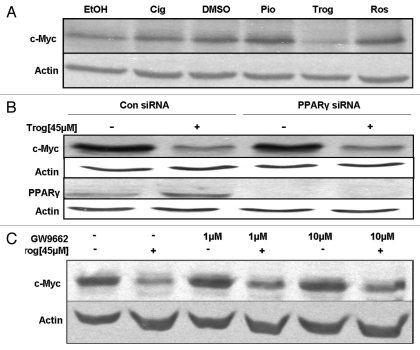
Cellular responses that are due to activation of PPARγ can be produced by multiple PPARγ agonists. To determine if the downregulation of c-Myc was a common response of C4-2 cells to PPARγ agonists, we next tested the effect of additional PPARγ ligands on c-Myc protein expression. Protein gel blot analysis revealed the reduction in c-Myc protein produced by troglitazone was not mimicked by other PPARγ ligands. Ciglitazone showed a slight increase in c-Myc protein expression when compared to its control (EtOH), while the PPARγ ligands pioglitazone and rosiglitazone had no effect on c-Myc protein expression in the C4-2 cells (Fig. 8A).
Figure 8.
Troglitazone decreases c-Myc protein independently of PPARγ. (A) C4-2 cells were treated with ethanol (EtOH) or DMSO vehicle, ciglitazone (Cig, 45 εM), pioglitazone (Pio, 45 εM), troglitazone (Trog, 45 εM) or rosiglitazone (Ros, 45 εM) for 48 h. The amount of c-Myc and actin protein in treated cells was then determined using protein gel blot analysis. (B) C4-2 cells were first transfected with either a non-specific control SMARTpool or PPARγ SMARTpool siRNA. Two days later, the cells were treated with DMSO vehicle or troglitazone 45 εM for 48 h. Protein gel blot analysis was used to determine levels of c-Myc, PPARγ and actin protein in treated cells. (C) C4-2 cells were treated with DMSO vehicle or troglitazone 45 εM in the presence or absence of the PPARγ antagonist GW9662. Following treatment, the levels of c-Myc and actin protein in treated cells was measured using protein gel blot.
Since troglitazone was the only PPARγ ligand that was able to decrease c-Myc protein expression, we hypothesized that this decrease may occur via a PPARγ independent pathway. To test this hypothesis, we used siRNA technology to knock down PPARγ protein levels within the C4-2 cells. In these studies, C4-2 cells were transfected with a nonspecific control siRNA or PPARγ SMARTpool siRNA by electroporation. With this transfection method, we were able to effectively knock down PPARγ protein levels within the C4-2 cells (Fig. 8B). However, reducing the level of PPARγ within C4-2 cells did not inhibit the ability of troglitazone to decrease c-Myc protein expression. To confirm these results we tested the effect of the PPARγ antagonist GW9662 on troglitazone-induced c-Myc downregulation. Concentrations of GW9962 that have been reported to reduce PPARγ activation (1–10 µM) did not block the troglitazone-induced decrease in c-Myc protein expression (Fig. 8C).3,37 Therefore, our studies indicate that activation of PPARγ is not required for the troglitazone-induced decrease in c-Myc protein.
Discussion
Although several reports have shown that TZDs inhibit proliferation of prostate cancer cells, the underlying mechanism by which these compounds reduce prostate cancer cell proliferation have not been fully characterized. The present study indicates the TZD troglitazone reduces proliferation of the C4-2 human prostate cancer cell line by inducing cell cycle arrest and apoptosis. In addition, our data suggest that one mechanism by which the TZD troglitazone reduces prostate cancer cell proliferation is by decreasing levels of the proto-oncogene product c-Myc. To our knowledge, ours is the first report describing downregulation of c-Myc by troglitazone in human prostate cancer cells.
Like other TZDs, troglitazone has been shown to activate human PPARγ in transactivation assays and promote adipogenesis.38,39 However, under our experimental conditions troglitazone was the only TZD that was able to downregulate c-Myc protein expression. Furthermore, this downregulation of c-Myc was not blocked by the PPARγ antagonist GW9662 or siRNA-based knockdown of PPARγ protein. Taken together, these data suggest that PPARγ is not required for troglitazone-induced decreases in c-Myc protein. A previous study by Laidler et al. revealed that the PPARγ ligands ciglitazone and linoleic acid inhibit c-Myc expression in multiple human prostate cancer cell lines.37 However, the decrease in c-Myc mRNA and protein stimulated by ciglitazone and linoleic acid was PPARγ-dependent and could be blocked by GW9662. Other groups have noted that troglitazone can alter protein expression and cell function independently of PPARγ. Data from the Chen laboratory suggest that PPARγ is not required for the troglitazone-induced decreases in β-catenin and cyclin D1 within LNCaP prostate cancer cell line.35,40 In addition, Qiao et al. showed that troglitazone-induced growth inhibition and apoptosis occurred independently of PPARγ in colon cancer cells.22 Furthermore, troglitazone inhibits glioma cell migration and brain invasion in a PPARγ independent manner.41 Therefore it appears that the activation of PPARγ independent signaling pathways is a common mechanism by which troglitazone regulates protein expression and activity across cancer cell types.
In our study, high micromolar concentrations of troglitazone (≥30 µM) were the most effective at inhibiting C4-2 cell proliferation and reducing c-Myc protein levels. These concentrations are higher than the reported serum level of troglitazone achieved in patients receiving oral troglitazone at a dose of 600 mg/day (∼5 mM).42 However, the concentrations used in this study are comparable to (and in some cases lower than) the amount used by other investigators to demonstrate troglitazone suppresses growth and regulates protein expression in other human prostate cancer cell lines.34,40 The two clinical trials involving troglitazone have shown that high oral doses of troglitazone (600–800 mg/day) are effective at reducing growth of human tumors.9,10 Thus, it may be that high levels of troglitazone are necessary in order to regulate intracellular protein levels and ultimately produce the greatest antitumor effect.
The unique chemical structure of troglitazone may contribute to the ability of troglitazone to regulate c-Myc expression. Compared to other TZDs, troglitazone contains a side chain that is similar to the vitamin E isoforms α-tocopherol, γ-tocopherol, α-tocotrienol and γ-tocotrienol.43,44 Multiple vitamin E isoforms have been shown to reduce c-Myc expression in cancer cells. α-tocopherol succinate decreases c-Myc protein levels in MDA-MB-231 human breast cancer cells.45 In addition, Sun et al. demonstrated that γ-tocotrienol downregulates c-Myc protein in the SGC-7901 human gastric cancer cell line.46 γ-tocotrienol also decreased tumor necrosis factor-stimulated increases in c-Myc protein within KBM-5 human myeloid cells.47 Since vitamin E isoforms are effective at reducing growth of other cancer cell types, it is possible that the presence of a vitamin E-like side chain is the reason troglitazone uniquely downregulates c-Myc in prostate cancer cells. However, additional studies are required to confirm the extent to which this side chain contributes to troglitazone-induced downregulation of c-Myc.
Our data indicate that the troglitazone-induced decrease of c-Myc protein levels in C4-2 cells occurs at least in part through proteasome-mediated degradation. Degradation of c-Myc protein by the proteasome has been linked to phosphorylation of c-Myc at threonine 58 and serine 62. Our data suggest that troglitazone-induced degradation of c-Myc also requires phosphorylation of c-Myc at threonine 58. Phosphorylation of c-Myc at threonine 58 and serine 62 can be regulated by GSK3β as well as the kinases ERK, JNK and PI3K/Akt.48,49 In LNCaP cells, Wei et al. demonstrated that troglitazone and the troglitazone derivative STG28 increase the phosphorylation and subsequent degradation of β-catenin via activation of GSK3β.34 However, GSK3β was not required for the STG28-induced phosphorylation and degradation of cyclin D1 within this cell line.40 Our data suggest that, while troglitazone increases the ratio of phospho c-Myc/total c-Myc within C4-2 cells, it does so through a non-GSK3β dependent pathway. It has been previously shown that inhibition of PI3Kinase suppresses c-Myc mRNA and protein expression in non-prostate cancer cell lines.50–52 The PI3Kinase/Akt pathway appears to play some role in our response, for inhibition of PI3Kinase did prevent degradation of c-Myc protein by troglitazone. Phosphorylation of c-Myc at threonine-58 by PI3Kinase has been shown to result in c-Myc degradation.32 At present, our data indicate troglitazone does not directly regulate PI3Kinase activity (as measured by alterations in Akt phosphorylation) in C4-2 cells. However, the PI3Kinase/Akt pathway may influence the activity of another kinase or protein required for troglitazone-induced degradation. Additional studies are required to further characterize the proteins/kinases necessary for troglitazone-induced phosphorylation at threonine-58 and subsequent degradation of c-Myc protein.
Decreases in c-Myc protein levels can occur not only by proteasomal degradation but also through decreases in c-Myc mRNA levels. In this study we observed a decrease in c-Myc mRNA as early as 6 h following troglitazone treatment. This would suggest that troglitazone reduces c-Myc protein within the C4-2 cells by both increasing protein degradation as well as decreasing c-Myc mRNA levels. In human leukemic cell lines, troglitazone has been shown to reduce expression of c-Myc mRNA and protein levels.53,54 Troglitazone induced decreases in c-Myc mRNA within HL-60 leukemic cells were independent of the NFκB signaling pathway, but required suppression of transcriptional factor 4 (Tcf-4) activity.55 We are currently conducting experiments to determine whether troglitazone regulates transcription of c-Myc mRNA in prostate cancer cells and if so which transcription factors are involved in this regulation.
In conclusion, our data demonstrate that troglitazone not only inhibits prostate cancer cell growth but also decreases c-Myc protein expression by a PPARγ-independent mechanism. We believe this decrease in c-Myc protein results in altered expression of c-Myc downstream targets which regulate cell cycle progression (such as E2F1 and CDK4) and ultimately lead to a decrease in cell proliferation. siRNA-mediated knockdown of c-Myc reduces proliferation of established human prostate cancer cells.26,56 Furthermore, decreases in c-Myc inhibit prostate carcinogenesis as well as the development of androgen-independent forms of prostate cancer.13,57 The results of our study suggest that the antitumor effects troglitazone noted in clinical trials and animal studies may be linked to the ability of this compound to decrease expression of c-Myc.9,10
Materials and Methods
Materials.
The compounds troglitazone (#71750), rosiglitazone (#71740), pioglitazone (#71745) and GW9662 (#70785) were obtained from Cayman Chemicals. Stock solutions of troglitazone, rosiglitazone and pioglitazone were prepared by diluting each drug in 100% DMSO and stored at −20°C. Ciglitazone (#100528-860) was obtained from VWR International. Stock solutions of ciglitazone were prepared by diluting the drug in 100% ethanol and stored at −20°C. Z-Leu-Leu-Leu-al (MG132) was purchased from Sigma Aldrich (Cat. #C2211). Stock solutions of MG132 were diluted in DMSO and stored at −20°C. Rabbit anti-mouse IgG secondary antibody was obtained from Zymed Laboratories, Inc. (Cat. #61-6500). Both horseradish peroxidase-conjugated donkey anti-rabbit (Cat. #NA934V) and sheep anti-mouse (Cat. #NXA931) antibodies were from Amersham Biosciences. The DMEM low glucose (Cat. #11885084), Ham F-12 (Cat. #11765054), DMEM/F-12 (1:1) (Cat. #12634010) and RPMI 1640 (Cat. #72400047) tissue culture media were purchased from Invitrogen. Penicillin/streptomycin solution (Cat. #15140122), phosphate buffered saline (PBS) (Cat. #AM9624) and Hank's balanced salt solution (HBSS) (Cat. #14175095) were also purchased from Invitrogen. Fetal bovine serum (FBS) was purchased from HyClone (Cat. #FRB25827). The media additives d-biotin (Cat. #47866), adenine hemisulfate (Cat. #A3159-5G), insulin solution (Cat. #I0516) and apo-transferrin (Cat. #T5886) were purchased from Sigma Aldrich.
Cell lines.
LNCaP cells (ATCC, Cat. #CRL-1740) were grown in RPMI 1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin. The C4-2 cell line (ViroMed Laboratories) is an androgen-independent derivative of the LNCaP cell line that expresses the androgen receptor and a mutated form of PTEN. C4-2 cells were grown in T medium (80% DMEM low glucose medium, 20% Hams' F12 medium, 5% heat inactivated FBS, 1% penicillin/streptomycin, 0.244 µg/ml d-biotin, 25 µg/ml adenine hemisulfate, 5 µg/ml insulin and 5 µg/ml apo-transferrin). The androgen-independent PC-3 cell line (ATCC, Cat. #CRL-1435) was grown in DMEM-F12 medium supplemented with 10% FBS and 1% penicillin/streptomycin. Cell lines were maintained in an incubator at 37°C in a 5% CO2 atmosphere. All experiments were performed using the culture medium described above.
Cell proliferation assay.
C4-2 cells were plated at a density of 20,000 cells per well in a six-well culture plate and treated with DMSO vehicle or the indicated drug concentrations for one, three or six days. Every three days, the medium was changed and fresh drug was added to each well. At the end of the treatment period, the cells were washed in cold Hank's Balanced Salt Solution (HBSS) and harvested using Trypsin-EDTA (0.05%). The total number of cells in each well was then counted using a Z1 Coulter Counter (Beckman Coulter, Cat. #BK6605698).
Analysis of cell cycle distribution.
C4-2 cells were plated at a density of 500,000 cells per 10 cm culture dish and treated with troglitazone (45 µM) or DMSO vehicle for three days. After treatment, the cells were detached from the culture dishes using 0.25% Trypsin-EDTA. Cells were then washed with phosphate buffered saline (PBS) containing 0.1% Bovine Serum Album (BSA) and fixed in 100% ethanol at 4°C for 1 h. The RNA in the fixed cells was digested by incubating the samples with RNase A (final concentration 0.05 µg/ml) for 3 h at 4°C. Cells were next stained with 50 µg/ml propidium iodide solution. The cell cycle distribution of treated cells was then determined by fluorescence-activated cell sorting (FACS) analysis that was performed at the Veterans Affairs Medical Center Flow Cytometry Special Resource Center (Nashville, TN).
Cell death ELISA.
C4-2 cells were plated at a density of 500,000 cells per 10-cm culture dish and treated with troglitazone (45 µM) or DMSO vehicle for three or six days. After treatment, the cells were harvested and re-suspended in 1 ml of culture media. Cells were then centrifuged at 1,500 rpm for 5 min at room temperature. After centrifugation cells were resuspended in incubation buffer for 30 min on ice and centrifuged at 15,000 rpm for 10 min at room temperature. The resulting supernatant was collected and stored at −20°C prior to analysis. DNA fragmentation, a hallmark of apoptosis, was measured using a Cell Death ELISA assay (Roche Diagnostics, Cat. #11 544 675 001).
Protein gel blot analysis.
C4-2, PC-3 and LNCaP cells were plated at a density of 500,000 cells per 10 cm culture dish. Cells were then treated with vehicle (100% ethanol or DMSO) or the PPARγ ligands ciglitazone, pioglitazone, rosiglitazone or troglitazone (0–45 µM) for the indicated times. In co-treatment experiments involving the inhibitors MG132 (0–20 µM), GW9662 (0–10 µM), LY294002 (20–40 µM), SP600125 (10–20 µM) or SB216763 (20 µM), drugs were added immediately before vehicle or PPARγ ligand treatment. Following treatment, cells were lysed in RIPA lysis buffer (0.1% SDS, 0.5% sodium deoxycholate, 1% NP-40, 150 mM NaCl, 50 mM Tris pH 8.0) containing the phosphatase inhibitor sodium vanadate (NaV, 1 mM) and the protease inhibitor phenylmethylsulphonyl fluoride (PMSF, 100 µg/ml). Protein concentrations were calculated using the Bradford protein assay (BioRad, Cat. #500-0001). Equal amounts of protein were separated on SDS-PAGE gels and transferred to a nitrocellulose membrane. Membrane blots were then blocked in TBST (1x TBS, 0.1% Tween 20) containing 1% non-fat powdered milk (p21 and PPARγ blots), 5% non-fat powdered milk (c-Myc, CDK4, E2F1, phospho-JNK, JNK), 5% BSA (phospho-Rb) or 1% BSA (phospho-c-Myc, actin). The membrane was then incubated with primary antibody diluted in the blocking solution noted above for 1 h at room temperature (actin) or overnight at 4°C (other antibodies). The primary antibodies used were as follows: a c-Myc mouse monoclonal antibody (Santa Cruz, Cat. #sc-41; 1:500), a phospho-c-Myc rabbit polyclonal antibody (Cell Signaling, Cat. #9401; 1:1,000), a p21 mouse monoclonal antibody (Calbiochem, Cat. #OP64; 1:200), a PPARγ rabbit polyclonal antibody (Santa Cruz Biotechnology, Cat. #sc-271392; 1:200), a CDK4 mouse monoclonal antibody (BD Biosciences, Cat. #610147; 1:1,000), a phospho-Rb polyclonal rabbit antibody (Cell Signaling, Cat. #9308; 1:1,000), a total Rb monoclonal mouse antibody (Cell Signaling, Cat. #9309; 1:1,000), an E2F1 polyclonal rabbit antibody (Santa Cruz, Cat. #sc-193; 1:500), a phospho-JNK polyclonal rabbit antibody (Cell Signaling, Cat. #4668; 1:1,000), a total JNK polyclonal rabbit antibody (Cell Signaling, Cat. #9258; 1:1,000) and a mouse monoclonal primary actin antibody (Chemicon International, Cat. #MAB1501R; 1:15,000). For the p21 protein gels, the blots were washed and next incubated with a rabbit anti-mouse secondary antibody (1:10,000). The blots were then incubated with a horseradish peroxidase-conjugated donkey anti-rabbit antibody (1:10,000). For PPARγ, c-Myc, phospho-c-Myc, phospho-Rb, phospho-JNK, JNK, E2F1 and CDK4 protein gels, following the primary antibody the blots were incubated in a horseradish peroxidase-conjugated donkey anti-rabbit secondary antibody (1:10,000). For protein gels to detect total Rb and actin protein, the blots were washed and exposed to horseradish peroxidase-conjugated sheep anti-mouse antibody (1:10,000). All membranes were next incubated in ECL reagent according to the manufacturer's instructions (GE Healthcare, Cat. #RPN2132) and exposed to BioMax Light autoradiography film (Kodak, Cat. #IB-1788207). Data from protein gel blots were quantified using the UN-SCAN-IT program (Silk Scientific).
c-Myc T58A mutant studies.
The QuickChange II Site-Directed Mutagenesis Kit (Stratagene, Cat. #200524) was used to introduce the T58A single point mutation into the pcDNA3.1-c-Myc plasmid construct (Addgene). The resulting plasmid was then sequenced by the Molecular Biology Core at Meharry Medical College to confirm the presence of the mutation. Electroporation was then used to transfect 4.5 µg of the pcDNA-c-Myc T58A or pCDNA3.1 parent vector into 10 million C4-2 cells. Following transfection, the cells were plated in 10 cm dishes at a density of 750,000 cells per 10-cm dish and allowed to attach for 48 h. The cells were next treated with DMSO vehicle or troglitazone 45 µM for 48 h. Following treatment, cells were harvested by scraping. Protein gel blot analysis was used to detect levels of c-Myc and actin protein as described above.
qRT-PCR analysis.
C4-2 cells were plated at a density of 500,000 cells per 10 cm culture dish. Cells were then treated with DMSO vehicle or troglitazone (45 µM) for the indicated times. Cells were harvested by scraping and total RNA was isolated using TRIZOL reagent (Invitrogen, Cat. #15596-026) according to the manufacturer's protocol. For each sample the iScript cDNA Synthesis Kit (BioRad, Cat. #170-8891) was used to synthesize cDNA from 1 µg of total RNA. The cDNA was then amplified by quantitative PCR using a reaction involving iQ SYBR Green Supermix reagent (BioRad, Cat. #170-8880). This PCR reaction consisted of an initial denaturation step (3 min at 95°C) and 40 cycles of PCR (95°C for 30 s, 55°C for 30 s and 72°C for 30 s). The following primer sets were used to amplify regions of c-Myc and 18S rRNA: c-Myc Fwd 5′-CTC CTG GCA AAA GGT CAG AG-3′ c-Myc Reverse 5′-CAA GCA GAG GAG CAA AAG CT-3′, 18s Forward 5′-ATC AAC TTT CGA TGG TAG TCG-3′ 18s Reverse 5′-TCC TTG GAT GTG GTA GCG-3′. The ΔΔCt algorithm was used to calculate the relative amounts of c-Myc mRNA and 18S rRNA in each sample. The level of c-Myc mRNA was then normalized to 18S rRNA levels.
siRNA experiments.
C4-2 cells were transfected with non-specific control SMARTpool (Dharmacon, Cat. #D-001206-13-05, siRNA negative control), PPARγ SMARTpool siRNA (Dharmacon, Cat. #M-003436-02) or c-Myc SMARTpool siRNA (Dharmacon, Cat. # L-003282-00-0005) at a concentration of 10 pmol/well by electroporation. The transfected cells were then plated in six-well plates at a density of 200,000 cells/well and allowed to attach for 48 h. Next the cells were treated for two days with DMSO vehicle or troglitazone (45 µM). Following treatment the cells were harvested by scraping. Protein gel blot analysis was then performed as described above to detect c-Myc, PPARγ and actin protein in treated cells. In parallel plates, the cells were detached with trypsin-EDTA and counted as described above using a Z1 coulter counter.
Statistical analyses.
For growth assays, flow cytometry data and protein gel blot quantitation, One way analysis of variance (ANOVA) was performed using the SigmaStat program (Systat Software, Inc.) to detect differences between treatment groups. A p value < 0.05 was considered statistically significant. Each experiment was repeated at least three times. A representative experiment is shown.
Acknowledgments
We thank the laboratory of Dr. Simon Hayward, Vanderbilt University Medical Center, for providing us with the E2F1 primary antibody. This work was supported by an NCI Mentored Career Development Award (1KO1 CA114253), Ruth L. Kirchstein National Research Service Award (5 T32 HL007735-12) and the MMC/VICC U54 Cancer Partnership (5 U54 CA091408-8).
Abbreviations
- PPARγ
peroxisome proliferator activated receptor gamma
- TZD
thiazolidinedione
- CDK4
cyclin dependent kinase 4
- Rb
retinoblastoma protein
- PI3Kinase
phosphatidylinositol-3-kinase
- GSK3β
glycogen synthase kinase 3beta
- ERK
extracellular signal-regulated kinases
- JNK
jun N-terminal kinase
References
- 1.American Cancer Society; 2010. [Google Scholar]
- 2.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPARgamma) J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 3.Lyles BE, Akinyeke TO, Moss PE, Stewart LV. Thiazolidinediones regulate expression of cell cycle proteins in human prostate cancer cells via PPARgamma-dependent and PPARgamma-independent pathways. Cell Cycle. 2009;8:268–277. doi: 10.4161/cc.8.2.7584. [DOI] [PubMed] [Google Scholar]
- 4.Papageorgiou E, Pitulis N, Manoussakis M, Lembessis P, Koutsilieris M. Rosiglitazone attenuates insulin-like growth factor 1 receptor survival signaling in PC-3 cells. Mol Med. 2008;14:403–411. doi: 10.2119/2008-00021.Papageorgiou. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Y, Iyengar S, Roberts RL, Shappell SB, Peehl DM. Primary culture model of peroxisome proliferator-activated receptor gamma activity in prostate cancer cells. J Cell Physiol. 2003;196:131–143. doi: 10.1002/jcp.10281. [DOI] [PubMed] [Google Scholar]
- 6.Annicotte JS, Iankova I, Miard S, Fritz V, Sarruf D, Abella A, et al. Peroxisome proliferator-activated receptor gamma regulates E-cadherin expression and inhibits growth and invasion of prostate cancer. Mol Cell Biol. 2006;26:7561–7574. doi: 10.1128/MCB.00605-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagata D, Yoshihiro H, Nakanishi M, Naruyama H, Okada S, Ando R, et al. Peroxisome proliferator-activated receptor-gamma and growth inhibition by its ligands in prostate cancer. Cancer Detect Prev. 2008;32:259–266. doi: 10.1016/j.cdp.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Smith MR, Manola J, Kaufman DS, George D, Oh WK, Mueller E, et al. Rosiglitazone versus placebo for men with prostate carcinoma and a rising serum prostate-specific antigen level after radical prostatectomy and/or radiation therapy. Cancer. 2004;101:1569–1574. doi: 10.1002/cncr.20493. [DOI] [PubMed] [Google Scholar]
- 9.Hisatake JI, Ikezoe T, Carey M, Holden S, Tomoyasu S, Koeffler HP. Down regulation of prostate-specific antigen expression by ligands for peroxisome proliferator-activated receptor gamma in human prostate cancer. Cancer Res. 2000;60:5494–5498. [PubMed] [Google Scholar]
- 10.Mueller E, Smith M, Sarraf P, Kroll T, Aiyer A, Kaufman DS, et al. Effects of ligand activation of peroxisome proliferator-activated receptor gamma in human prostate cancer. Proc Natl Acad Sci USA. 2000;97:10990–10995. doi: 10.1073/pnas.180329197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukherjee S, Conrad SE. c-Myc suppresses p21WAF1/CIP1 expression during estrogen signaling and antiestrogen resistance in human breast cancer cells. J Biol Chem. 2005;280:17617–17625. doi: 10.1074/jbc.M502278200. [DOI] [PubMed] [Google Scholar]
- 12.Miliani de Marval PL, Macias E, Rounbehler R, Sicinski P, Kiyokawa H, Johnson DG, et al. Lack of cyclin-dependent kinase 4 inhibits c-myc tumorigenic activities in epithelial tissues. Mol Cell Biol. 2004;24:7538–7547. doi: 10.1128/MCB.24.17.7538-7547.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amendola D, De Salvo M, Marchese R, Verga Falzacappa C, Stigliano A, Carico E, et al. Myc downregulation affects cyclin D1/cdk4 activity and induces apoptosis via Smac/Diablo pathway in an astrocytoma cell line. Cell Prolif. 2009;42:94–109. doi: 10.1111/j.1365-2184.2008.00576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung S, Park S, Yang CH. Unsaturated fatty acids bind Myc-Max transcription factor and inhibit Myc-Max-DNA complex formation. Cancer Lett. 2002;188:153–162. doi: 10.1016/s0304-3835(02)00455-x. [DOI] [PubMed] [Google Scholar]
- 15.Mallette FA, Gaumont-Leclerc MF, Huot G, Ferbeyre G. Myc downregulation as a mechanism to activate the Rb pathway in STAT5A-induced senescence. J Biol Chem. 2007;282:34938–34944. doi: 10.1074/jbc.M707074200. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Ge L, Wang M, Carr BI. Phosphorylation regulates Myc expression via prolonged activation of the mitogen-activated protein kinase pathway. J Cell Physiol. 2006;208:133–140. doi: 10.1002/jcp.20649. [DOI] [PubMed] [Google Scholar]
- 17.Jeon WJ, Kim SH, Seo MS, Kim Y, Kang UG, Juhnn YS, et al. Repeated electroconvulsive seizure induces c-Myc downregulation and Bad inactivation in the rat frontal cortex. Exp Mol Med. 2008;40:435–444. doi: 10.3858/emm.2008.40.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotliarova S, Pastorino S, Kovell LC, Kotliarov Y, Song H, Zhang W, et al. Glycogen synthase kinase-3 inhibition induces glioma cell death through c-MYC, nuclear factor-kappaB and glucose regulation. Cancer Res. 2008;68:6643–6651. doi: 10.1158/0008-5472.CAN-08-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cerbone A, Toaldo C, Laurora S, Briatore F, Pizzimenti S, Dianzani MU, et al. 4-Hydroxynonenal and PPARgamma ligands affect proliferation, differentiation and apoptosis in colon cancer cells. Free Radic Biol Med. 2007;42:1661–1670. doi: 10.1016/j.freeradbiomed.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Kanunfre CC, da Silva Freitas JJ, Pompeia C, Goncalves de Almeida DC, Cury-Boaventura MF, Verlengia R, et al. Ciglitizone and 15d PGJ2 induce apoptosis in Jurkat and Raji cells. Int Immunopharmacol. 2004;4:1171–1185. doi: 10.1016/j.intimp.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Ming M, Yu JP, Meng XZ, Zhou YH, Yu HG, Luo HS. Effect of ligand troglitazone on peroxisome proliferator-activated receptor gamma expression and cellular growth in human colon cancer cells. World J Gastroenterol. 2006;12:7263–7270. doi: 10.3748/wjg.v12.i45.7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiao L, Dai Y, Gu Q, Chan KW, Ma J, Lan HY, et al. Loss of XIAP sensitizes colon cancer cells to PPARgamma independent antitumor effects of troglitazone and 15-PGJ2. Cancer Lett. 2008;268:260–271. doi: 10.1016/j.canlet.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Sharma C, Pradeep A, Pestell RG, Rana B. Peroxisome proliferator-activated receptor gamma activation modulates cyclin D1 transcription via beta-catenin-independent and cAMP-response element-binding protein-dependent pathways in mouse hepatocytes. J Biol Chem. 2004;279:16927–16938. doi: 10.1074/jbc.M309045200. [DOI] [PubMed] [Google Scholar]
- 24.Yang YC, Ho TC, Chen SL, Lai HY, Wu JY, Tsao YP. Inhibition of cell motility by troglitazone in human ovarian carcinoma cell line. BMC Cancer. 2007;7:216. doi: 10.1186/1471-2407-7-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim J, Roh M, Abdulkadir SA. Pim1 promotes human prostate cancer cell tumorigenicity and c-MYC transcriptional activity. BMC Cancer. 2010;10:248. doi: 10.1186/1471-2407-10-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Washington MN, Kim JS, Weigel NL. 1alpha,25-dihydroxyvitamin D3 inhibits C4-2 prostate cancer cell growth via a retinoblastoma protein (Rb)-independent G1 arrest. Prostate. 2011;71:98–110. doi: 10.1002/pros.21226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baena E, Ortiz M, Martinez AC, de Alboran IM. c-Myc is essential for hematopoietic stem cell differentiation and regulates Lin(−)Sca-1(+)c-Kit(−) cell generation through p21. Exp Hematol. 2007;35:1333–1343. doi: 10.1016/j.exphem.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Hwang-Verslues WW, Sladek FM. Nuclear receptor hepatocyte nuclear factor 4alpha1 competes with oncoprotein c-Myc for control of the p21/WAF1 promoter. Mol Endocrinol. 2008;22:78–90. doi: 10.1210/me.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang LG, Liu XM, Fang Y, Dai W, Chiao FB, Puccio GM, et al. De-repression of the p21 promoter in prostate cancer cells by an isothiocyanate via inhibition of HDACs and c-Myc. Int J Oncol. 2008;33:375–380. [PubMed] [Google Scholar]
- 30.Yan KH, Yao CJ, Chang HY, Lai GM, Cheng AL, Chuang SE. The synergistic anticancer effect of troglitazone combined with aspirin causes cell cycle arrest and apoptosis in human lung cancer cells. Mol Carcinog. 2010;49:235–246. doi: 10.1002/mc.20593. [DOI] [PubMed] [Google Scholar]
- 31.Yao CJ, Lai GM, Chan CF, Cheng AL, Yang YY, Chuang SE. Dramatic synergistic anticancer effect of clinically achievable doses of lovastatin and troglitazone. Int J Cancer. 2006;118:773–779. doi: 10.1002/ijc.21361. [DOI] [PubMed] [Google Scholar]
- 32.Hann SR. Role of post-translational modifications in regulating c-Myc proteolysis, transcriptional activity and biological function. Semin Cancer Biol. 2006;16:288–302. doi: 10.1016/j.semcancer.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Sears RC. The life cycle of C-myc: from synthesis to degradation. Cell Cycle. 2004;3:1133–1137. [PubMed] [Google Scholar]
- 34.Wei S, Lin LF, Yang CC, Wang YC, Chang GD, Chen H, et al. Thiazolidinediones modulate the expression of beta-catenin and other cell cycle regulatory proteins by targeting the F-box proteins of Skp1-Cul1-F-box protein E3 ubiquitin ligase independently of peroxisome proliferator-activated receptor gamma. Mol Pharmacol. 2007;72:725–733. doi: 10.1124/mol.107.035287. [DOI] [PubMed] [Google Scholar]
- 35.Yang CC, Wang YC, Wei S, Lin LF, Chen CS, Lee CC, et al. Peroxisome proliferator-activated receptor gamma-independent suppression of androgen receptor expression by troglitazone mechanism and pharmacologic exploitation. Cancer Res. 2007;67:3229–3238. doi: 10.1158/0008-5472.CAN-06-2759. [DOI] [PubMed] [Google Scholar]
- 36.Gregory MA, Qi Y, Hann SR. Phosphorylation by glycogen synthase kinase-3 controls c-myc proteolysis and subnuclear localization. J Biol Chem. 2003;278:51606–51612. doi: 10.1074/jbc.M310722200. [DOI] [PubMed] [Google Scholar]
- 37.Laidler P, Dulinska J, Mrozicki S. Does the inhibition of c-myc expression mediate the antitumor activity of PPAR's ligands in prostate cancer cell lines? Arch Biochem Biophys. 2007;462:1–12. doi: 10.1016/j.abb.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 38.Kim HG, Han EH, Jeong HG. Effect of troglitazone on CYP1A1 induction. Toxicology. 2008;246:166–171. doi: 10.1016/j.tox.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Kim KR, Choi HN, Lee HJ, Baek HA, Park HS, Jang KY, et al. A peroxisome proliferator-activated receptor gamma antagonist induces vimentin cleavage and inhibits invasion in high-grade hepatocellular carcinoma. Oncol Rep. 2007;18:825–832. [PubMed] [Google Scholar]
- 40.Wei S, Yang HC, Chuang HC, Yang J, Kulp SK, Lu PJ, et al. A novel mechanism by which thiazolidinediones facilitate the proteasomal degradation of cyclin D1 in cancer cells. J Biol Chem. 2008;283:26759–26770. doi: 10.1074/jbc.M802160200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coras R, Holsken A, Seufert S, Hauke J, Eyupoglu IY, Reichel M, et al. The peroxisome proliferator-activated receptor-gamma agonist troglitazone inhibits transforming growth factor-beta-mediated glioma cell migration and brain invasion. Mol Cancer Ther. 2007;6:1745–1754. doi: 10.1158/1535-7163.MCT-06-0763. [DOI] [PubMed] [Google Scholar]
- 42.Plosker GL, Faulds D. Troglitazone: a review of its use in the management of type 2 diabetes mellitus. Drugs. 1999;57:409–438. doi: 10.2165/00003495-199957030-00014. [DOI] [PubMed] [Google Scholar]
- 43.Campbell SE, Stone WL, Whaley SG, Qui M, Krishnan K. Gamma (gamma) tocopherol upregulates peroxisome proliferator activated receptor (PPAR) gamma (gamma) expression in SW 480 human colon cancer cell lines. BMC Cancer. 2003;3:25. doi: 10.1186/1471-2407-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stone WL, Krishnan K, Campbell SE, Qui M, Whaley SG, Yang H. Tocopherols and the treatment of colon cancer. Ann NY Acad Sci. 2004;1031:223–233. doi: 10.1196/annals.1331.022. [DOI] [PubMed] [Google Scholar]
- 45.Donapaty S, Louis S, Horvath E, Kun J, Sebti SM, Malafa MP. RRR-alpha-tocopherol succinate downregulates oncogenic Ras signaling. Mol Cancer Ther. 2006;5:309–316. doi: 10.1158/1535-7163.MCT-05-0330. [DOI] [PubMed] [Google Scholar]
- 46.Sun W, Wang Q, Chen B, Liu J, Liu H, Xu W. Gamma-tocotrienol-induced apoptosis in human gastric cancer SGC-7901 cells is associated with a suppression in mitogen-activated protein kinase signalling. Br J Nutr. 2008;99:1247–1254. doi: 10.1017/S0007114507879128. [DOI] [PubMed] [Google Scholar]
- 47.Ahn KS, Sethi G, Krishnan K, Aggarwal BB. Gamma-tocotrienol inhibits nuclear factor-kappaB signaling pathway through inhibition of receptor-interacting protein and TAK1 leading to suppression of antiapoptotic gene products and potentiation of apoptosis. J Biol Chem. 2007;282:809–820. doi: 10.1074/jbc.M610028200. [DOI] [PubMed] [Google Scholar]
- 48.Lee T, Yao G, Nevins J, You L. Sensing and integration of Erk and PI3K signals by Myc. PLoS Comput Biol. 2008;4:1000013. doi: 10.1371/journal.pcbi.1000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu J, Blenis J, Yuan J. Activation of PI3K/Akt and MAPK pathways regulates Myc-mediated transcription by phosphorylating and promoting the degradation of Mad1. Proc Natl Acad Sci USA. 2008;105:6584–6589. doi: 10.1073/pnas.0802785105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han L, Yang Y, Yue X, Huang K, Liu X, Pu P, et al. Inactivation of PI3K/AKT signaling inhibits glioma cell growth through modulation of beta-catenin-mediated transcription. Brain Res. 2010;1366:9–17. doi: 10.1016/j.brainres.2010.09.097. [DOI] [PubMed] [Google Scholar]
- 51.Ikezoe T, Nishioka C, Bandobashi K, Yang Y, Kuwayama Y, Adachi Y, et al. Longitudinal inhibition of PI3K/Akt/mTOR signaling by LY294002 and rapamycin induces growth arrest of adult T-cell leukemia cells. Leuk Res. 2007;31:673–682. doi: 10.1016/j.leukres.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 52.Jeay S, Sonenshein GE, Kelly PA, Postel-Vinay MC, Baixeras E. Growth hormone exerts antiapoptotic and proliferative effects through two different pathways involving nuclear factor-kappaB and phosphatidylinositol-3-kinase. Endocrinology. 2001;142:147–156. doi: 10.1210/endo.142.1.7892. [DOI] [PubMed] [Google Scholar]
- 53.Laurora S, Pizzimenti S, Briatore F, Fraioli A, Maggio M, Reffo P, et al. Peroxisome proliferator-activated receptor ligands affect growth-related gene expression in human leukemic cells. J Pharmacol Exp Ther. 2003;305:932–942. doi: 10.1124/jpet.103.049098. [DOI] [PubMed] [Google Scholar]
- 54.Takenokuchi M, Saigo K, Nakamachi Y, Kawano S, Hashimoto M, Fujioka T, et al. Troglitazone inhibits cell growth and induces apoptosis of B-cell acute lymphoblastic leukemia cells with t(14;18) Acta Haematol. 2006;116:30–40. doi: 10.1159/000092345. [DOI] [PubMed] [Google Scholar]
- 55.Yamakawa-Karakida N, Sugita K, Inukai T, Goi K, Nakamura M, Uno K, et al. Ligand activation of peroxisome proliferator-activated receptor gamma induces apoptosis of leukemia cells by downregulating the c-myc gene expression via blockade of the Tcf-4 activity. Cell Death Differ. 2002;9:513–526. doi: 10.1038/sj.cdd.4401000. [DOI] [PubMed] [Google Scholar]
- 56.Rohan JN, Weigel NL. 1Alpha,25-dihydroxyvitamin D3 reduces c-Myc expression, inhibiting proliferation and causing G1 accumulation in C4-2 prostate cancer cells. Endocrinology. 2009;150:2046–2054. doi: 10.1210/en.2008-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bernard D, Pourtier-Manzanedo A, Gil J, Beach DH. Myc confers androgen-independent prostate cancer cell growth. J Clin Invest. 2003;112:1724–1731. doi: 10.1172/JCI19035. [DOI] [PMC free article] [PubMed] [Google Scholar]