Abstract
Land plants (embryophytes) evolved in the presence of prokaryotic microbes. As a result, numerous mutually beneficial associations (symbioses) developed that can be analyzed using a variety of methods. Here we describe the isolation and characterization of a new pink-pigmented facultatively methylotrophic symbiotic bacterium of the genus Methylobacterium (laboratory strain F3.2) that was isolated from the gametophytic phylloids of the common cord moss Funaria hygrometrica Hedw. Plantlets were collected in the field and analyzed in the laboratory. Colonies of methylobacteria were obtained by the agar-impression-method. Based on its unique phenotype (the bacterial cells are characterized by fimbriae-like appendages), a comparative 16S rRNA gene (DNA) sequence analysis and an average DNA-DNA hybridization value of 8.4%, compared with its most closely related sister taxon, this isolate is described as a new species, Methylobacterium funariae sp. nov. (type strain F3.2). This new epiphytic bacterium inhabits the leaf surface of “primitive” land plants such as mosses and interacts with its host organism via the secretion of phytohormones (cytokinines, auxins). These external signals are perceived by the plant cells that divide and grow more rapidly than in the absence of their prokaryotic phytosymbionts. We suggest that M. funariae sp. nov. uses methanol emitted from the stomatal pores as principal carbon source for cell metabolism. However, our novel data indicate that, in this unique symbiotic plant-microbe interaction, the uptake of amino acids leached from the surface of the epidermal cells of the green host organism may be of importance as microbial carbon- and nitrogen-source.
Key words: growth-promoting bacteria, methylobacteria, mosses, plant-microbe-interactions, symbiosis
Introduction
In 1897, the German plant pathologist and doyen of slime mold research (myxomycetology), Anton de Bary (1831–1888) introduced the term “symbiosis” to refer to “the living together of differently named organisms”. This classical definition encompasses both parasitic and mutualistic interactions.1 Today, the word “symbiosis” denotes a mutually beneficial association between two (or more) genetically unrelated organisms, inclusive of endosymbiotic relationships of organelles and cytoplasmatic components within eukaryotic cells.2,3
Over the past decades, the nitrogen-fixing interactions between root bacteria of the genus Rhizobium and legumes such as soybean, and the mycorrhizal association between fungi and the majority of land plants (embryophytes), respectively, were the two most commonly analyzed symbioses with respect to the kingdom Plantae.3 However, the above-ground parts of all green, photosynthetic organisms, notably the leaf surfaces (phylloplane), have also become a focus of microbiological research. In the phyllosphere of “lower living fossil plants,” such as liverworts and mosses, pink-pigmented facultatively methylotrophic microbes, prokaryotes that are ubiquitous in nature and hence are found in a variety of habitats (inclusive of the leaf surfaces of seed plants), were discovered.4,5 These growth-promoting methylobacteria form symbiotic interactions with their host organism that were characterized in detail.6,7 One isolate obtained from the free-living thallus of the liverwort Marchantia polymorpha was recently described as a novel bacterial species, Methylobacterium marchantiae Schauer et al.,8 a microbe that is characterized by a unique phenotype.
In this report, we describe a related strain isolated from the phylloids of the common cord moss Funaria hygrometrica Hedw. Based on morphological and molecular data, this symbiotic, growth-stimulating, plant-associated bacterium is named here Methylobacterium funariae and described as a new species. In addition, we further characterize the mutualistic interaction between this novel methylobacterium and its eukaryotic, photoautotrophic host organism.
Results and Discussion
Nine years ago, we isolated several Methylobacterium strains from the upper side of the phylloids of the leafy gametophytes of the common cord moss Funaria hygrometrica. This bryophyte, which is one of the most widely distributed mosses in the world, forms dense colonies in the moist areas in the Botanical Garden of the University of Kassel. Figure 1 shows groups of upright leafy haploid gametophytes of F. hygrometrica, with diploid sporophytes (stems or setae) attached. At the tips of the setae mature (closed) and older (open) spore capsules are visible.
Figure 1.
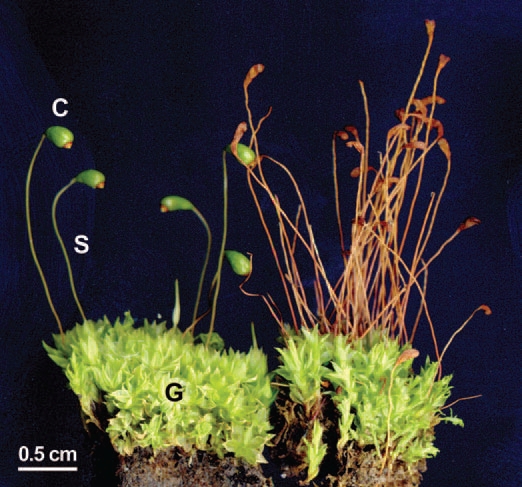
Photograph of leafy gametophytes (G), with attached stalk-like sporophytes S, (inclusive of the closed, turgid spore capsules, C) of the common cord moss Funaria hygrometrica (left half of the picture). In the older group of plantlets depicted on the right side, the spore capsules are empty and desiccated. Note the twisted stalks, which is a characteristic morphological feature of this moss species.
In order to isolate epiphytic bacteria from this “lower land plant,” single leaflets (phylloids, size ca. 1 × 3 mm) were removed from the gametophytes with sterile forceps and pressed onto the surface of germ-free agar plates.9 After removal of the samples and incubation of the petri dishes, colonies of methylobacteria developed. One laboratory strain (F3.2), isolated in November 2001, was analyzed in more detail in reference 9.
In a “moss protonema-bioassay”, these microbes (Methylobacterium sp. F3.2) were found to stimulate cell- and organ growth via the secretion of cytokinins.9 Later, we discovered that M. sp. F3.2 produces and releases a second phytohormone, indole-3-acetic acid (auxin).10 Base on these and other findings, we classified these methylobacteria, microbes that can grow on the one-carbon compound methanol released via the stomatal pores as a by-product of cell-wall metabolism, as phytosymbionts.6
The unique phenotype of M. sp. F3.2 is shown in Figure 2. All methylobacteria obtained by sequential cultivation of this isolate (“founder population”) were Gram-negative, aerobic rods (size: 1.0–1.4 × 1.7–3.4 µm) which, when maintained in liquid culture, occur singly or, at high density, in large clusters/aggregates of up to 2,000 cells. In liquid culture, the microbes live in a so-called “planctonic form.” These methylobacteria are motile by a single polar flagellum, whereas in microbes that have settled down and formed aggregates (or biofilms) no such corkscrew-like appendages were detected. On the outer surface of the cells, numerous fimbriae-like structures occur, with densities of 100 to 150 appendages per µm2. These “mini-pili” (diameter: 25–40 nm; length up to ca. 50 nm) are similar to those observed in the related species M. marchantiae,8 but on average they are shorter. Additional phenotypic features to distinguish M. sp. F3.2 from related methylobacteria are listed in Table 1. In contrast to the closely related type strain of M. marchantiae,8 M. sp. F3.2 forms aggregates (or rosettes) in liquid culture and is motile when the cells are cultivated on semi-solid agar plates.
Figure 2.
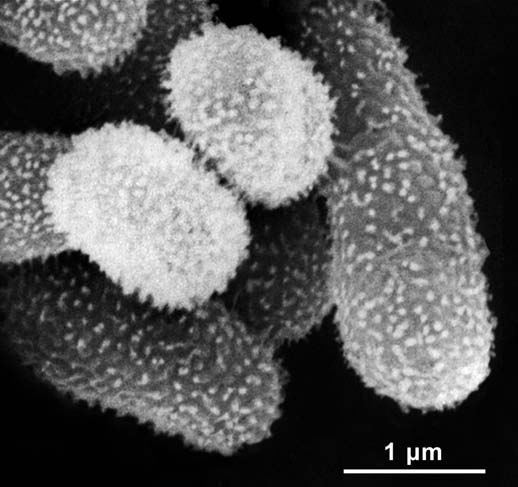
Scanning electron micrograph of a cluster of cells of Methylobacterium funariae F3.2T. The microbes were isolated from the upper side of a moss phylloid as shown in Figure 1. The bacteria depicted here grew on a protonema cell of the moss F. hygrometrica before the sample was fixed and prepared for microscopic examination.
Table 1.
Differential phenotypic characteristics of Methylobacterium funariae sp. nov. (1) and other closely related species of the genus Methylobacterium
| 1 | 2 | 3 | 4 | 5 | |
| Cells occur | |||||
| singly | + | + | + | + | + |
| in pairs | rarely | rarely | rarely | rarely | rarely |
| in aggregates/rosettes | + | − | + | + | + |
| Cell length (µm) | 1.7–3.4 | 1.9–3.9 | 1.3–3.2 | 1.6–4.0 | 1.8–3.5 |
| Cell width (µm) | 1.0–1.4 | 1.0–1.3 | 1.0–1.4 | 1.1–1.5 | 0.8–1.2 |
| Fimbriae | + | + | − | − | − |
| Motility on semi-solid agar | + | − | + | + | − |
Species/strains: 1, F3.2T; 2, M. marchantiae JT1T; 3, M. adhaesivum DSM 17169T; 4, M. ‘goesingense’ iEII3T; 5, M. jeotgali S2R03-9T. +, positive; −, negative.
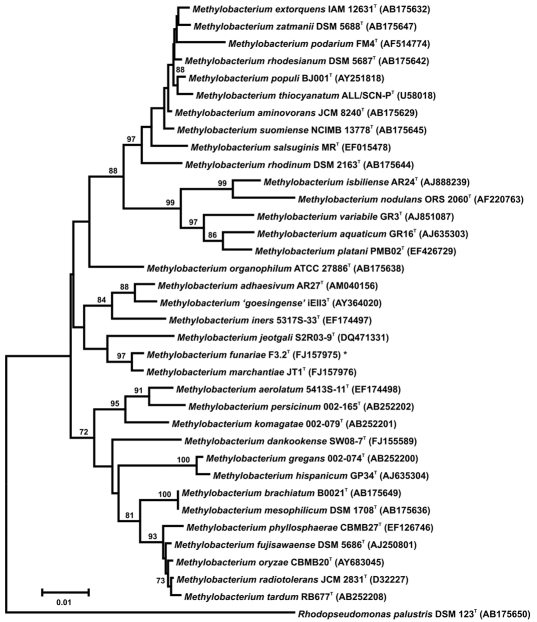
A phylogenetic tree of all known species of the genus Methylobacterium, based on 16S rRNA gene (DNA) sequences (length: 1,332–1,480 nt) is shown in Figure 3. These data document that M. marchantiae and M. sp. F3.2 are closely related sister taxa. A comparison with other validly described species of the genus Methylobacterium shows that the sister taxa juxtaposed here are to the same degree phylogenetically related as the pairs M. mesophilicum/M. brachiatum, M. hispanicum/M. gregans and M. thiocyanatum/M. populi. However, these data alone are insufficient to establish our isolate M. sp. F3.2 as a nova species.
Figure 3.
Phylogenetic tree reconstruction, based on a comparative analysis of 36 individual 16S rRNA gene (DNA) sequences (1,332–1,480 nt), documenting the evolutionary relationships between strain F3.2T (GenBank accession number FJ157975), named Methylobacterium funariae sp. nov. (*) and related methylobacteria. The evolutionary history was inferred using the Neighbor-Joining method and Jukes-Cantor evolutionary distance matrix. All positions containing gaps were eliminated from the dataset. Bootstrap values (expressed as percentage of 10,000 replications) higher than 70% are shown at the nodes. The sequence of Rhodopseudomonas palustris DSM 123T was included as an outgroup. Bar = 1% sequence dis-similarity.
According to the currently accepted prokaryotic species definition (which is not a species concept), microbes are described on the basis of both phenotypic and genotypic properties. The most important genotypic feature is the degree of deoxyribonucleic acid (DNA)-DNA hybridization (in %) between the isolate (i.e., our strain) under investigation and a reference taxon. If an un-identifiable isolate shows 70% or larger hybridization to a designated “type strain,” deposited in an accessible culture collection, then these bacteria are considered to be of the same species. However, if the hybridization level is lower than 70%, the isolate can be named as a new species.11,12
In two independent DNA-DNA hybridization (i.e., re-association) experiments, strain M. sp. F3.2 displayed values of 9.2 and 7.6%, respectively, (average 8.4%), compared with its sister taxon M. marchantiae (type strain JT1T). In addition, we compared the DNA-DNA similarities of strain M. sp. F3.2 with those of the described species M. adhaesivum DSM 17169T and M. ‘goesingense’ iEII3T. In two sets of hybridization experiments, values of 25.0/20.5% and 11.5/13.5%, respectively, were obtained. These quantitative data document the significant phylogenetic distance between M. sp. F3.2 and its sister taxa M. marchantiae,8 M. adhaesivum, and M. ‘goesingense’.
Based on our phenotypic characterization (Fig. 2 and Table 1), the molecular phylogeny (Fig. 3), and the hybridization values of less than 70% we conclude that the isolate M. sp. F3.2 represents an un-described taxon.
Species description.
Methylobacterium funariae sp. nov. (fu.na.ri.ae. N.L. gen. n. funariae, of Funaria), isolated from the phylloid of a free-living gametophyte of the cord moss Funaria hygrometrica Hedw. (Fig. 1).
The bacterial cells (Fig. 2) are rod-shaped (size: 1.0–1.4 × 1.7–3.4 µm) and stain Gram-negative. After 7 days of incubation on R2A agar at 28°C in darkness, colonies of Methylobacterium funariae sp. nov. show a pink to red color, are smooth, shiny, circular and convex with entire margins, and have diameters of 1.5–2.0 mm (Fig. 4A; concerning the colony morphologies on two alternative media, see Fig. 4B and C). A series of cultivation experiments have documented that the bacterial cells grow on R2A medium, plate count agar, nutrient agar, glycerol-peptone agar (Fig. 4C) or mineral salts media23 (Fig. 4B) supplemented with the C1-compound methanol (CH3OH, 0.1–1.0%, v/v).
Figure 4.
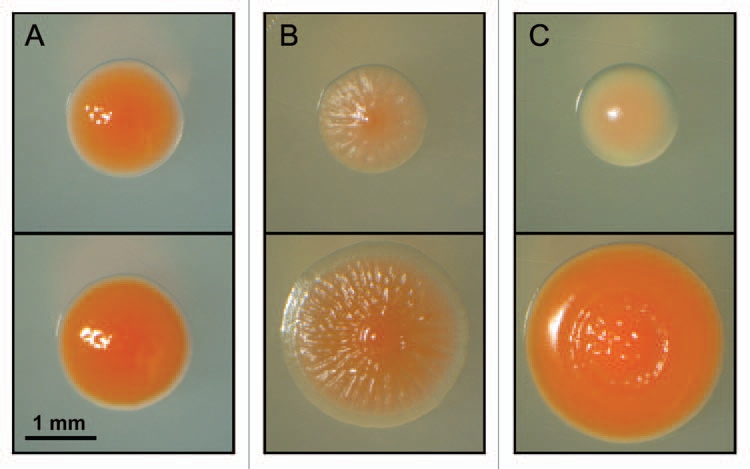
Representative colonies of Methylobacterium funariae sp. nov. F3.2 that were grown for 7 and 14 days at 28°C in darkness (upper and lower photographs, respectively). Media: R2A-agar (A), Choi-agar supplemented with 1.0% (v/v) methanol (B) and GP-agar (C). Note the pink color of the bacterial colonies, which is due to the biosynthesis of carotenoids (reviewed in ref. 4).
Methylobacterium funariae sp. nov. can utilize the alcohols methanol and ethanol, the amino acids L-aspartate and L-glutamate, and formate, citrate, glycerol and succinate as carbon source. The cells are incapable of utilizing D-xylose, tartrate and betaine. In contrast to its sister taxon M. marchantiae,8 M. funariae sp. nov. (Fig. 2) can not utilize tartrate as sole carbon source.
Potassium nitrate, ammonium sulphate, ammonium chloride and the amino acids L-aspartate and L-glutamate are utilized as sole nitrogen sources. Cultivated cells of M. funariae sp. nov. are capable of reducing nitrate to nitrite. When maintained in liquid culture, populations of M. funariae sp. nov. grow at initial pH-values of 6.0–10.0 and salt (NaCl) concentrations of up to 2% (w/v). On agar plates and in liquid cultures, bacterial cells grow at 32°C, but not at 35°C (optimum temperature: 25–30°C). The cells produce the enzymes oxidase, urease and katalase and are incapable to hydrolyse starch, CM-cellulose and gelatine. To further characterize the biochemical capacities of the cells, we also performed two enzyme tests, β-glucosidase and β-galactosidaseassays, which yielded negative results.
The type strain of M. funariae is F3.2T, isolated from the phylloid of the common cord moss Funaria hygrometrica Hedw., which was harvested in 2002 in the Botanical Garden of the University of Kassel, Germany (Central Europe, 51° 47′ N 9° 75′ E).
Summary and conclusions.
The results presented here document that our isolate M. sp. strain F3.2, microbes characterized before as phytosymbionts on the leaf surface of the common moss Funaria hygrometrica,6,9,10 represents a new bacterial species.
Our phenotypic characterization and phylogenetic analysis revealed that Methylobacterium funariae sp. nov. is a sister taxon of M. marchantiae that inhabits a similar ecological niche as its closest relative. However, more work is required to further characterize the carbon-containing nutrients on the thallus and leaf surfaces from which M. marchantiae and M. funariae sp. nov. were isolated. In our model describing the symbiotic relationship between these methylobacteria and the gametophytes of “lower living fossil plants,” methanol is assumed to be the major carbon source on which the phytohormone-producing epiphytes subsist.6 Since simple sugars such as glucose, fructose, sucrose and a variety of amino acids leach from the interior of the leaves of higher plants,13 the symbiotic methylobacteria-Marchantia/Funaria-relationship6,9,10 may be more complicated and diverse than proposed by us and others.5,6,13–15 Our data show that M. funariae sp. nov. can use at least two amino acids as carbon and nitrogen sources. Hence, we suggest that these organic substances may be important nutrients for the prokaryotic epiphytes that inhabit the outer surface of the cord moss and other “lower plants”. However, more work is required to further corroborate this tentative conclusion.
Finally, we want to emphasize that our physiological characterization of the mutualistic association between the methylobacteria M. marchantiae8 and M. funariae sp. nov. (Fig. 2) and their host organisms is restricted to the haploid (gametophytic) phase of the life cycle of bryophytes.6,9,15 Currently, we do not know whether or not the diploid sporophytes of Marchantia polymorpha (reviewed in ref. 15) and Funaria hygrometrica (Fig. 1), respectively, respond with a promotion of cell elongation in the presence of added methylobacteria, as the haploid gametophytes do.6 This lack of knowledge is due to the fact that our attempts to analyze the entire life cycle of these “lower land plants” in laboratory cultures have not yet yielded positive results. Circumstantial evidence suggests that the earliest, ca. 410- to 415-million-year-old land plants (Zosterophyllum, Cooksonia) had a dimorphic diplobiontic life cycle in which the haploid (gametophytic) phase dominated.16–18 If this hypothesis, which rests on several lines of independent evidence,18–20 proves to be correct, our deduced bryophyte (gametophyte)/methylobacteria-interaction may shed light on an ancestral host/symbiont-relationship of considerable evolutionary significance.18
Materials and Methods
In November 2001, samples of the common cord moss Funaria hygrometrica Hedw. were collected from the moist soil in the Botanical Garden of the University of Kassel, Germany. In order to isolate epiphytic, pink-pigmented, facultative methylotrophic bacteria and to analyze their growth-promoting effect on microbe-free fragments of “lower plants” such as bryophytes, the agar-impression-method and other techniques were used (reviewed in refs. 9 and 10).
Scanning electron-micrographs of methylobacteria were prepared as described by Hornschuh et al.9 Cellular morphology and motility were determined microscopically (Photomikroskop III, Carl Zeiss, Oberkochen, Germany) with exponentially growing R2A-broth cultures (see ref. 25). Flagellation of bacterial cells maintained in liquid culture (i.e., planctonic forms) was analyzed by the method of Heimbrook et al.21 Motility was also tested on one-tenth-strength R2A-medium supplemented with 0.2% agar.22
Gram staining was performed with a commercial kit (Sigma Diagnostics, Deisenhofen, Germany). Production of the enzymes catalase and oxidase was assayed with ‘ID Color Catalase’ and ‘Oxidase Reagent’ respectively (bioMérieux, Nürtingen, Germany), according to the manufacturer's instructions. Hydrolysis of gelatine as well as the production of the enzymes urease, β-galactosidase and β-glucosidase were determined using API 20 NE test strips (bioMérieux, Nürtingen, Germany). Due to the slow growth of Methylobacterium strains, the results were read after 7 days of incubation. Hydrolysis of CM-cellulose and starch was assayed on R2A agar medium using standard protocols. Physiological tests were performed as described in reference 8.
Genomic DNA required for sequencing the 16S ribosomal DNA (rDNA) was extracted using a commercially available kit (DNeasy Blood & Tissue Kit, Qiagen, Hilden, Germany) according to the manufacturer's instruction for Gram-positive bacteria. Nearly full-length 16S rDNA (1,421 nt) was amplified with the universal eubacterial 16S rDNA primers FGPS6 and FGPS1509.24 Polymerase chain reaction (PCR) amplification, sequencing and phylogenetic tree reconstruction was performed as detailed in reference 8.
DNA-DNA hybridization studies were performed at the laboratories of the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ, Braunschweig, Germany), which encompassed genomic DNA isolation using a French pressure cell (Thermo Spectronic) and purification by chromatography on hydroxyapatite as described by Cashion et al.26 DNA-DNA hybridization was carried out as described by De Ley et al.27 taking into account the modifications described by Huss et al.28 using a model Cary 100 Bio UV/VIS-spectrophotometer equipped with a Peltier-thermostatted 6 × 6 multicell changer and a temperature controller with in situ temperature probe (Varian).
Acknowledgments
We thank C. Spröer (Deutsche Sammlung von Mikroorganismen und Zellkulturen, DSMZ, Braunschweig, Germany) for the provision of the DNA-DNA hybridization data and H. Rühling (Abt. Zellbiologie, University of Kassel, Germany) for help with the scanning electron microscopy.
References
- 1.Hoppe T, Kutschera U. In the shadow of Darwin: Anton de Bary's origin of myxomycetology and a molecular phylogeny of the plasmodial slime molds. Theory Biosci. 2010;129:15–23. doi: 10.1007/s12064-009-0079-7. [DOI] [PubMed] [Google Scholar]
- 2.Kutschera U, Niklas KJ. Endosymbiosis, cell evolution and speciation. Theory Biosci. 2005;124:1–24. doi: 10.1016/j.thbio.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Douglas AE. Symbiotic interactions. Oxford: Oxford University Press; 1994. [Google Scholar]
- 4.Green PN. Methylobacterium. In: Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E, editors. The Prokaryotes. A handbook on the biology of bacteria. 3rd edition. Vol. 5. New York, NY: Springer; 2006. pp. 257–265. [Google Scholar]
- 5.Holland MA. Methylobacterium and plants. Rec Res Devel Plant Physiol. 1997;1:207–213. [Google Scholar]
- 6.Kutschera U. Plant-associated methylobacteria as co-evolved phytosymbionts: a hypothesis. Plant Signal Behav. 2007;2:74–78. doi: 10.4161/psb.2.2.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schauer S, Kutschera U. Methylotrophic bacteria on the surfaces of field-grown sunflower plants: a biogeographic perspective. Theory Biosci. 2008;127:23–29. doi: 10.1007/s12064-007-0020-x. [DOI] [PubMed] [Google Scholar]
- 8.Schauer S, Kåmpfer P, Wellner S, Spröer C, Kutschera U. Methylobacterium marchantiae sp. nov. a pink-pigmented, facultatively methylotrophic bacterium isolated from the thallus of a liverwort. Int J Syst Evol Microbiol. 2010;61(Pt 4):870–876. doi: 10.1099/ijs.0.021915-0.. [DOI] [PubMed] [Google Scholar]
- 9.Hornschuh M, Grotha R, Kutschera U. Epiphytic bacteria associated with the bryophyte Funaria hygrometrica: effects of Methylobacterium strains on protonema development. Plant Biol. 2002;4:682–687. [Google Scholar]
- 10.Hornschuh M, Grotha R, Kutschera U. Moss-associated methylobacteria as phytosymbionts: an experimental study. Naturwissenschaften. 2006;93:480–486. doi: 10.1007/s00114-006-0137-7. [DOI] [PubMed] [Google Scholar]
- 11.Wayne LG, Brenner DJ, Colwell RR, Grimont PAD, Kandler O, Krichevsky MI, et al. Report of the ad hoc committee on reconciliation of approaches to bacterial systematics. Int J Syst Bacteriol. 1987;37:463–464. [Google Scholar]
- 12.Gevers D, Cohan FM, Lawrence JG, Spratt BG, Coenye T, Feil EJ, et al. Re-evaluating prokaryotic species. Nat Rev Microbiol. 2005;3:733–739. doi: 10.1038/nrmicro1236. [DOI] [PubMed] [Google Scholar]
- 13.Lindow SE, Brandl MT. Microbiology of the phyllosphere. Appl Environ Microbiol. 2003;69:1875–1883. doi: 10.1128/AEM.69.4.1875-1883.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivanova EG, Doronina NV, Trotsenko YA. Aerobic methylobacteria are capable of synthesizing auxins. Microbiology. 2001;70:392–397. [PubMed] [Google Scholar]
- 15.Kutschera U, Koopmann V. Growth in liverworts of the Marchantiales is promoted by epiphytic methylobacteria. Naturwissenschaften. 2005;92:347–349. doi: 10.1007/s00114-005-0640-2. [DOI] [PubMed] [Google Scholar]
- 16.Niklas KJ. The evolutionary biology of plants. Chicago: The University of Chicago Press; 1997. [Google Scholar]
- 17.Niklas KJ, Kutschera U. The evolutionary development of plant body plans. Funct Plant Biol. 2009;36:682–695. doi: 10.1071/FP09107. [DOI] [PubMed] [Google Scholar]
- 18.Niklas KJ, Kutschera U. The evolution of the land plant life cycle. New Phytol. 2010;185:27–41. doi: 10.1111/j.1469-8137.2009.03054.x. [DOI] [PubMed] [Google Scholar]
- 19.Willis KJ, McElwain JC. The evolution of plants. Oxford: Oxford University Press; 2002. [Google Scholar]
- 20.Lewis LA, McCourt RM. Green algae and the origin of land plants. Amer J Bot. 2004;91:1535–1556. doi: 10.3732/ajb.91.10.1535. [DOI] [PubMed] [Google Scholar]
- 21.Heimbrook ME, Wang WL, Campbell G. Staining bacterial flagella easily. J Clin Microbiol. 1989;27:2612–2615. doi: 10.1128/jcm.27.11.2612-2615.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weon HY, Kim BY, Joa JH, Son JA, Song MH, Kwon SW, et al. Methylobacterium iners sp. nov. and Methylobacterium aerolatum sp. nov., isolated from air samples in Korea. Int J Syst Evol Microbiol. 2008;58:93–96. doi: 10.1099/ijs.0.65047-0. [DOI] [PubMed] [Google Scholar]
- 23.Choi JH, Kim JH, Daniel M, Lebeault JM. Optimization of growth medium and poly-β-hydroxybutyric acid production from methanol in Methylobacterium organophilum. Kor J Appl Microbiol Bioeng. 1989;17:392–396. [Google Scholar]
- 24.Sy A, Giraud E, Jourand P, Garcia N, Willems A, De Lajudie P, et al. Methylotrophic Methylobacterium bacteria nodulate and fix nitrogen in symbiosis with legumes. J Bacteriol. 2001;183:214–220. doi: 10.1128/JB.183.1.214-220.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reasoner DJ, Geldreich EE. A new medium for the enumeration and subculture of bacteria from potable water. Appl Environ Microbiol. 1985;49:1–7. doi: 10.1128/aem.49.1.1-7.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cashion P, Hodler-Franklin MA, McCully J, Franklin M. A rapid method for base ratio determination of bacterial DNA. Anal Biochem. 1977;81:461–466. doi: 10.1016/0003-2697(77)90720-5. [DOI] [PubMed] [Google Scholar]
- 27.De Ley J, Cattoir H, Reynaerts A. The quantitative measurement of DNA hybridization from renaturation rates. Eur J Biochem. 1970;12:133–142. doi: 10.1111/j.1432-1033.1970.tb00830.x. [DOI] [PubMed] [Google Scholar]
- 28.Huss VAR, Festl H, Schleifer KH. Studies on the spectrophotometric determination of DNA hybridization from renaturation rates. Syst Appl Microbiol. 1983;4:184–192. doi: 10.1016/S0723-2020(83)80048-4. [DOI] [PubMed] [Google Scholar]