Abstract
A wide range of pharmacological properties are ascribed to natural saponins, in addition to their biological activities against herbivores, plant soil-borne pathogens and pests. As for animal cells, the cytotoxicity and the chemopreventive role of saponins are mediated by a complex network of signal transduction pathways which include reactive oxygen species (ROS) and nitric oxide (NO). The involvement of other relevant components of the saponin-related signaling routes, such as the Tumor Necrosis Factor (TNF)α, the interleukin (IL)-6 and the Nuclear Transcription FactorκB (NFκB), has been highlighted in animal cells. By contrast, information concerning the response of plant cells to saponins and the related signal transduction pathways is almost missing. To date, there are only a few common features which link plant and animal cells in their response to saponins, such as the early burst in ROS and NO production and the induction of metallothioneins (MTs), small cysteine-rich, metal-binding proteins. This aspect is discussed in the present paper in view of the recent hypothesis that MTs and NO are part of a novel signal transduction pathway participating in the cell response to oxidative stress.
Key words: plant saponins, metallothionein, nitric oxide, plant cell, reactive oxygen species, signal transduction pathway
Metallothionein and Nitric Oxide Signaling
In plants, metallothioneins (MTs) (small cysteine-rich proteins) accumulate in response to toxic levels of heavy metals.1 Besides their role in heavy metal detoxification, the antioxidant function of plant MTs has been recently highlighted in a few reports. Balestrazzi et al.2 demonstrated that the expression in white poplar (Populus alba L.) of the PsMTA1 gene, encoding a metallothionein-like protein from Pisum sativum, confers protection against oxidative injuries. In leaf tissues of transgenic white poplar plants, ROS were accumulated, but to a lower extent. In addition, plant cells showed increased tolerance to photo-oxidative stress induced by paraquat and low levels of DNA damage in leaf cell nuclei.2
The free radical nitric oxide (NO) can form stable complexes with MT, inducing conformational changes in the protein and the selective release of metals (e.g., zinc).3 S-nitrosation of the MT sulphydryl groups, the mechanism responsible for the loss of metal from the binding centers of the protein,4 is also a key event of NO signaling.5 Stitt et al. demonstrated that the metal responsive transcription factor MTF-1 required for the activation of genes, including the MT genes involved in the cell response to various stresses, is one of the cellular target of the zinc released from metallothionein and it has been hypothesized that the release of zinc caused by NO might act as a novel signal transduction pathway participating in the cell response to oxidative stress.
In plants, the link between metallothionein and nitric oxide still needs to be clarified. We recently demonstrated that white poplar cell suspension cultures challenged with heavy metals (copper, zinc and cadmium) show an early burst in NO production.7 Moreover, in the same cells, the VFMT2 gene, encoding a type 2 metallothionein from P. alba, was upregulated in response to heavy metal treatments.8
Role of Metallothionein and Nitric Oxide in the Cell Response to Saponins
Saponins represent a relevant class of plant secondary metabolites involved in the defense response against herbivores, soil-borne pathogens and pests.9 Most of them are currently investigated for their antitumor activity.10,11 Extensive studies on the relationship between saponin structure and activity, described and summarized in a recent review,10 confirm that the biological activity of saponins is influenced both by the aglycone and the sugar mojeties in the molecule. The cytotoxic action of saponins is likely to result from their ability to form complexes with the cell membrane cholesterol, leading to pore formation and cell permeabilization. In animal cells, some reports are available dealing with the possible signal transduction pathways activated by saponins12,13 while similar information in plant cells is absent.
The possible involvement of metallothioneins in the cell response to toxic saponins has been first reported by Itoh et al. who found that both glycyrrhizin and glycyrrhizinic acid induce MT expression in mice. Similarly, the triterpenoid saponin α-hederin can induce upregulation of MT genes in hepatic cells, possibly through a signaling route which involves the Tumor Necrosis Factor (TNF)α and the interleukin (IL)-6.15
As previously reported for animal cells, our recent investigations underlined the involvement of NO in the response to saponins also in plant cells.16 When different alfalfa (Medicago sativa L.) saponin extracts (obtained from plant aerial parts, seed and roots) were added to white poplar cell suspension cultures, only the root saponins were able to induce significant cell death rates associated with enhanced ROS and NO production.16 In order to assess the possible involvement of plant MTs in this process, the expression profile of the VFMT2 gene encoding a type 2 metallothionein was analyzed in poplar cell suspension cultures exposed to root alfalfa saponins, at two different concentrations (25 and 50 µg ml−1, respectively).16 The results of these experiments are shown in Figure 1. Saponins were added to 4-day-old, exponentially growing cell cultures and aliquots were collected at different times (0, 2, 4 and 6 h) following treatments. The expression profiles of the VFMT2 gene, evaluated by Quantitative RealTime Polymerase Chain Reaction (QRT-PCR) revealed an upregulation of this gene in response to treatments with alfalfa root saponins. As shown in Figure 1, 2 and 4 h following exposure to the lower dose (25 µg ml−1), the level of VFMT2 transcript significantly increased (1.5- and 2.5-fold respectively), compared with the untreated sample, while at 6 h the amount of VFMT2 mRNA, although reduced, was still high. Treatment with 50 µg ml−1 also led to significant upregulation of the VFMT2 gene (Fig. 1). However, 2 and 4 h following treatment the estimated amount of VFMT2 mRNA was 1.0- and 0.8-fold higher compared with the untreated sample. At 6 h, the level of VFMT2 mRNA was even lower (0.5-fold) than the untreated sample. The different levels of upregulation observed for the VFMT2 gene in cell cultures exposed to alfalfa root saponins at 25 and 50 µg ml−1, respectively, are consistent with our previous data on cell death rates.16 Exposure to the lower saponin dose resulted in 25% cell death while the highest dose was associated with 75% cell death (data not shown), therefore this higher citotoxicity might explaine the observed reduction in VFMT2 mRNA. To our knowledge this is the first description of saponin-induced metallothionein upregulation in plants.
Figure 1.
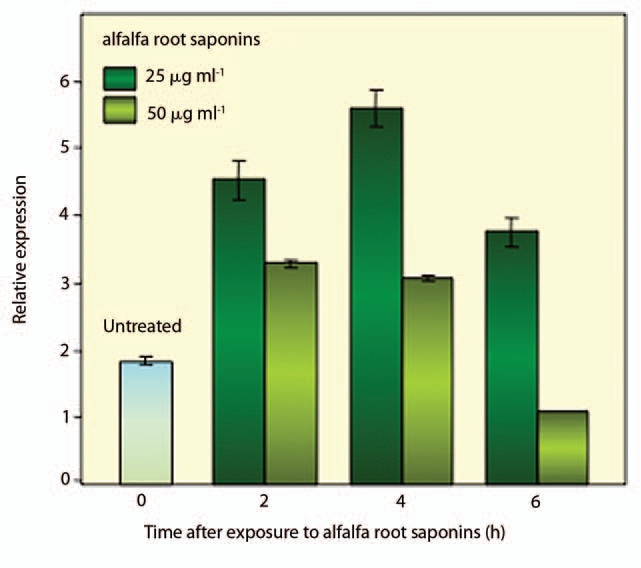
Expression profiles of the VFMT2 gene, encoding a type 2 metallothionein, in white poplar cell suspension cultures challenged with alfalfa root saponins (25 and 50 µg ml−1, respectively). The analysis was carried out at different time points (0, 2, 4 and 6 h) from the beginning of the experiment using Quantitative RealTime Polymerase Chain Reaction as previously described in reference 8. Values are expressed as means ± SD of three independent experiments.
Animal and Plant Cells Share Common Elements in the Saponin-Induced Signaling Pathway: A Temptative Model
To date, the unique common elements found in the response of animal and plant cells to saponins are restricted to the early steps of the signaling route(s) where ROS and NO act as signal molecules in both cell types16–18 (Fig. 2). The upregulation of the VFMT2 gene occurring in poplar cells exposed to root saponins, as documented in the present work, brings an additional finding and demonstrates that also in plants, as previously reported for animals,14,15 MTs play a role in the response to cytotoxic saponins.
Figure 2.
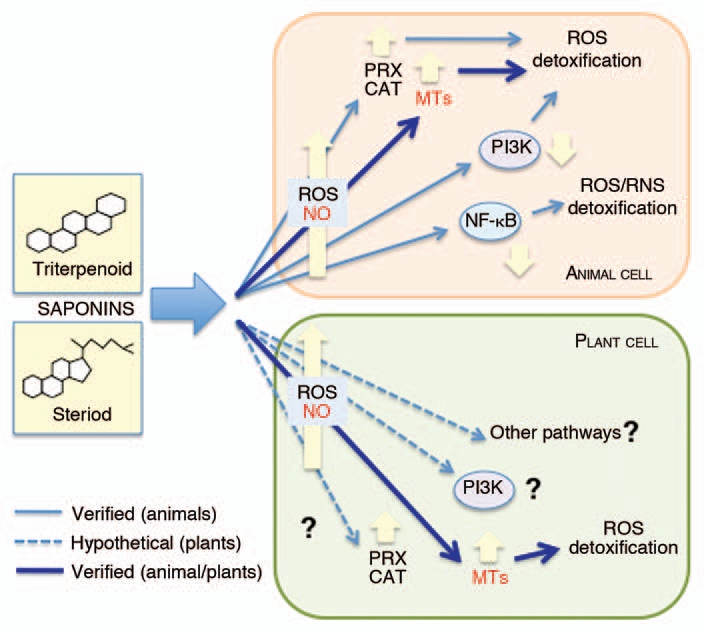
Signal transduction pathways involved in the cell response to cytotoxic saponins. Besides the known signaling pathways common to animal and plant cells, additional routes which still need to be verified are shown. CAT, catalase; MT s, metallothioneins; NFκB, nuclear transcription factorκB; NO, nitric oxide; PI3K, phosphatidylinositol-3-kinase; PRX, peroxiredoxin; ROS, Reactive Oxygen Species.
In animal cells, a novel aspect of the saponin-related signal transduction pathway(s) has been evidenced by the studies with avicins, a family of triterpenoid saponins from Acacia victoriae (Bentham).12 Avicins can block the activation of nuclear transcription factorκB (NFκB), possibly by modifying a critical sulphydryl group of this protein.12 It is worth noting that NFκB enhances the expression of the inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX-2) which contribute to cellular oxidative and nitrosative stress conditions, favouring carcinogenesis. Furthermore, it has been reported that avicins can enhance ROS detoxification by activating some scavenger functions, such as catalase and peroxiredoxins.12 The involvement of phosphatidylinositol-3-kinase (PI3K), a component of the G Protein-dependent signaling pathway,19 in the response to saponins has been also proposed12 (Fig. 2). In plant cells, PI3K participates in several processes,20 among which intracellular ROS production in salt tolerance response.21 To date, a role of PIK3 in the response of plant cells to saponins cannot be ruled out.
Additional work will be carried out in order to better define novel components of the saponin-induced signaling pathways in plant cells, possibly using purified saponin molecules and focusing on the cellular antioxidant response inside and outside the nuclear compartment.
References
- 1.Cobbet C, Goldsbrough P. Phytochelatins and metallothioneins: Roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol. 2002;53:159–182. doi: 10.1146/annurev.arplant.53.100301.135154. [DOI] [PubMed] [Google Scholar]
- 2.Balestrazzi A, Botti S, Zelasco S, Biondi S, Franchin C, Calligari P, et al. Expression of the PsMTA1 gene in white poplar engineered with the MAT system is associated to heavy metal tolerance and protection against 8-hydroxy-2′-deoxyguanosine mediated-DNA damage. Plant Cell Rep. 2009;28:1179–1192. doi: 10.1007/s00299-009-0719-x. [DOI] [PubMed] [Google Scholar]
- 3.Zangger K, Oz G, Haslinger E, Kunert O, Armitage IM. Nitric oxide selectively releases metals from the amino-terminal domain of metallothioneins: Potential roles at inflammatory sites. FASEB J. 2001;15:1303–1305. doi: 10.1096/fj.00-0641fje. [DOI] [PubMed] [Google Scholar]
- 4.Liu SX, Fabisiak JP, Tyurin V, Borisenko GG, Pitt BR, Lazo JS, et al. Nitric oxide-dependent pro-oxidant and pro-apoptotic effect of HL-60 cells challenged with cupric nitrilo-triacetate. Biochem J. 2001;345:397–406. doi: 10.1042/0264-6021:3540397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stamler JS, Lamas S, Fang FC. Nitrosylation: The prototypic redox-based signaling mechanisms. Cell. 2001;106:675–683. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- 6.Stitt MS, Wasserloos KJ, Tang X, Liu X, Pitt BR, St. Croix CM. Nitric oxide-induced nuclear translocation of the metal responsive transcription factor, MTF-1 is mediated by zinc release from metallothionein. Vasc Pharmacol. 2006;44:149–155. doi: 10.1016/j.vph.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Balestrazzi A, Macovei A, Testoni C, Raimondi E, Donà M, Carbonera D. Nitric oxide biosynthesis in white poplar (Populus alba L.) suspension cultures challenged with heavy metals. Plant Stress. 2009;1:1–6. [Google Scholar]
- 8.Macovei A, Ventura L, Donà M, Faí M, Balestrazzi A, Carbonera D. Effects of heavy metal treatments on metallothionein expression profiles in white poplar (Populus alba L.) cell suspension cultures. Analele Universitatii din Oradea-Fascicula Biologie. 2010 Available at: http://www.bioresearch.ro/revistaen.html. [Google Scholar]
- 9.Francis G, Kerem Z, Makkar HPS, Becker K. The biological action of saponins in animal systems: A review. British J Nutr. 2002;88:587–605. doi: 10.1079/BJN2002725. [DOI] [PubMed] [Google Scholar]
- 10.Tava A, Avato P. Chemical and biological activity of triterpene saponins of Medicago species. Nat Prod Commu. 2006;1:1159–1180. [Google Scholar]
- 11.Podolak I, Galanty A, Sobolewska D. Saponins as cytotoxic agents: A review. Phytochem Rev. 2010;9:425–474. doi: 10.1007/s11101-010-9183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haridas V, Arntzen CJ, Gutterman JU. Avicins, a family of triterpenoid saponins from Acacia victoriae (Bentham), inhibit activation of nuclear factorκB by inhibiting both its nuclear localization and ability to bind DNA. Proc Natl Acad Sci USA. 2001;98:11557–11562. doi: 10.1073/pnas.191363498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakaya Y, Mawatari K, Takahashi A, Harada N, Hata A, Yasui S. The phytoestrogen ginsensoside Re activates potassium channels of vascular smooth muscle cells through P13k/Akt and nitric oxide pathways. J Med Inv. 2007;54:381–384. doi: 10.2152/jmi.54.381. [DOI] [PubMed] [Google Scholar]
- 14.Itoh N, Morishita Y, Tanaka T, Muto N, Kobayashi M, Kitagawa I, et al. Metallothionein induction and hepatoprotection by echinoside A and sakurasosaponin. Phytoter Res. 1997;11:132–135. [Google Scholar]
- 15.Kim JY, Choi CY, Jeong HG. Involvement of cytokines in the hepatic metallothionein expression by alpha-hederin. Planta Med. 2005;71:743–747. doi: 10.1055/s-2005-864185. [DOI] [PubMed] [Google Scholar]
- 16.Balestrazzi A, Agoni V, Tava A, Avato P, Biazzi E, Raimondi E, et al. Cell death induction and nitric oxide biosynthesis in white poplar (Populus alba) suspension cultures exposed to alfalfa saponins. Physiol Plant. 2011;1:227–238. doi: 10.1111/j.13993054.2010.01436.x. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Che CM, Chlu JF, He QY. Dioscin (saponin)-induced generation of reactive oxygen species through mitochondria disfunction: A proteomicbased study. J Prot Res. 2007;6:4703–4710. doi: 10.1021/pr070399r. [DOI] [PubMed] [Google Scholar]
- 18.Ito SI, Ihara T, Tamura H, Tanaka S, Ikeda T, Kajihara H, et al. α-tomatine, the major saponin in tomato, induces programmed cell death mediated by reactive oxygen species in the fungal pathogen Fusarium oxysperum. FEBS Lett. 2000;581:3217–3222. doi: 10.1016/j.febslet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Castaneda CA, Cortes-Funes H, Gomez HL, Ciruelos EM. The phosphatidyl inositol 3-kinase/AKT signaling pathway in breast cancer. Cancer Metastasis Rev. 2010;29:751–759. doi: 10.1007/s10555-010-9261-0. [DOI] [PubMed] [Google Scholar]
- 20.Meijer HJG, Munnik T. Phospholipid-based signaling in plants. Annu Rev Plant Biol. 2003;54:265–306. doi: 10.1146/annurev.arplant.54.031902.134748. [DOI] [PubMed] [Google Scholar]
- 21.Leshem Y, Seri L, Levine A. Induction of phosphatidylinositol-3-kinase-mediated endocytosis by salt stress leads to intracellular production of reactive oxygen species and salt tolerance. Plant J. 2007;51:185–197. doi: 10.1111/j.1365-313X.2007.03134.x. [DOI] [PubMed] [Google Scholar]


