Abstract
Essential features of pollen tube growth are polarization of extracellular ion fluxes, intracellular ion gradients and oscillating dynamics. These features in pollen tube growth are regulated by a wide range of spatiotemporally organized functions such as exocytosis and endocytosis, actin cytoskeleton reorganization, cell wall deposition and assembly, intracellular signaling, fertilization and self-incompatibility. Recently, by developing a compartmental model, we have demonstrated that the tip and shank in a pollen tube combine in an integrative and self-regulatory system of ion and growth dynamics. Recent developments in modelling and the wealth of experimental data can be used to develop a systems model that provides an integrative view of the the interactions between the different functions that affect pollen tube growth.
Key words: pollen tube, tip growth, systems biology, spatiotemporal model, ion gradients, oscillatory dynamics
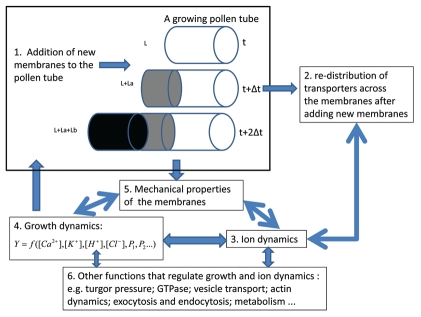
Pollen tube growth involves polarized ion fluxes, intracellular ion gradients and oscillations in ion concentrations, fluxes and cell growth.1,2 These features in pollen tube growth are regulated by a wide range of spatiotemporally organized functions such as exocytosis and endocytosis, actin cytoskeleton reorganization, cell wall deposition and assembly, phospholipid and inositol polyphosphate signaling, fertilization and self-incompatibility.3 With the accumulation of experimental data, the theoretical development of models of pollen tube growth, we have reached a stage where we can rise to the challenge of integrating experimental data and biological knowledge into a mathematical model at a systemic level. In Figure 1, we propose a framework for developing a Systems Tip Growth Model of the pollen tube.
Figure 1.
A proposed framework for developing a systems tip growth model for a pollen tube. (1) New membrane is formed at the tip when growth occurs. Due to an oscillatory growth rate, La and Lb may not be equal for the same time interval, Δt. (2) Addition of new membranes causes the redistribution of ion transporters across the membrane. The process is dynamical and it corresponds to the process for the addition of new membranes in pollen tube growth; (3–6) Pollen tube growth, ion dynamics, mechanical properties of the membranes and other factors that regulate growth and ion dynamics all form entangled dynamical systems and they regulate each other. Development of a systems tip growth model for a pollen tube requires integration of all the processes highlighted in Figure 1 into a dynamical system.
Previously we have shown, by developing a compartmental model, that the tip and shank in a pollen tube combine in an integrative and self-regulatory dynamical system of ions and growth.4 To our knowledge, our model is the first attempt to model (a) the dynamics of all four major ions (Ca2+, K+, H+ and Cl−) that arise from the interactions between transporters at the tip and the shank, and (b) the interactions between ion dynamics of all four major ions (Ca2+, K+, H+ and Cl−) and pollen tube growth. As described in Figure 1, when growth occurs, new membrane is formed at the tip. In our model, a parameter, which is the surface occupied by transporters to volume ratio, is used to describe the relationship between the surface that is occupied by transporters and the volume at both tip, RAVt and shank, RAVs.4 Our model analysis reveals the following results. If the volume or the growth rate at the tip affects neither RAVt nor the kinetics of the transporters, the oscillatory dynamics do not markedly change with respect to an increase of the tip volume or the growth rate. This implies that, if the newly added membranes increase the volume and surface area simultaneously and if the old membranes do not change the kinetic properties, the tip volume of a pollen tube will continuously increase as growth occurs. However, in pollen tube growth, the tip volume remains about the same. If we consider that a pollen tube has a regular geometrical shape, then we can assume that the surface occupied by transporters to the volume ratio will not change as growth occurs. Therefore, the membranes of a pollen tube must change the kinetic properties as the growth occurs. One factor that can affect the kinetic properties of transporters is the abundance of the transporters. Experimentally, it has been shown that the abundance of H+ ATPase at the tip is much lower than that at the shank and reduces towards the apex of the tip.5 The systemic model does require that we now determine the distribution of all relevant transporters in a growing pollen tube.
In the experiments that examine Nicotiana tabacum pollen tube growth, hypotonic treatment is shown to induce growth rate and the apical volume oscillation frequencies do change. These data provide strong evidence that hydrodynamic oscillations are closely correlated with, or form the basis of, the pollen tube oscillator that drives growth rate oscillations and the oscillations in ion fluxes and concentrations.6,7 A recent study develops a model that includes a turgor pressure, a viscous cell wall that yields under pressure, stretch-activated calcium channels that transport calcium ions into the cytoplasm and an exocytosis rate dependent on the cytosolic calcium concentration in the apex of the pollen tube.8 The model shows that an oscillating growth rate in pollen tubes requires Ca2+-dependent vesicle recycling.8 This modelling result supports the idea that turgor pressure may drive an oscillatory growth.
However, another recent study which concentrates on vesicle trafficking in Arabidopsis pollen tubes links growth rate with vesicle trafficking that moves phospholipid and pectin to the tube tip.9 In this study, the tube length is defined by the amount of pectin and cargo phospholipids transported to the tube tip. The model results adequately fit the pollen tube growth of both previously reported wild-type and the Rab GTPase, raba4d, knockout lines. Furthermore, the difference in pollen tube growth in the SNARE syp124/syp125 single and double mutations is quantitatively predicted based on the model analysis. This modelling result indicates that vesicle trafficking in Arabidopsis pollen tubes is directly related to pollen tube growth. In addition to turgor pressure and vesicle trafficking, model analysis also shows that calcium participates in feedback regulation of the oscillating ROP1 Rho GTPase that regulates pollen tube growth.10 In the compartmental model developed in our previous work,4 the growth rate of a pollen tube is correlated with the concentrations of ions and emphasizes the effects of ions on growth rate.
It is clear that different models, which concentrate on the roles of different factors that regulate pollen tube growth, may lead to different conclusions on how pollen tube growth is being regulated. A systems tip growth model is required to address the following questions: (a) as pollen tube growth can be regulated by different functions such as turgor pressure, vesicle trafficking and ion dynamics, how is it controlled in an integrative manner? (b) how does pollen tube growth itself regulate these functions? Although metabolic pathways provide fluxes of materials for biomass and other outputs to sustain life and these fluxes provide a dynamic description of the metabolic phenotype of the plant,11 modelling pollen tube growth has largely neglected the roles of metabolism. In plant cells, biomass accumulation in metabolism can be modeled in terms of metabolic fluxes using flux balance analysis.12,13 The regulation of metabolic fluxes on pollen tube growth should also be integrated into the systems tip growth model.
Another important factor that regulates pollen tube growth is mechanical properties of membranes.14 A recent study using a finite-element method divides a pollen tube into 3-dimensional discrete areas.15 The model is used to identify the requirements for spatial distribution of mechanical properties in the cell wall. It is shown that there is an agreement between the modeled gradient in mechanical properties and the experimental distribution of de-esterified pectin. As shown in Figure 1, development of a Systems Tip Growth Model for the pollen tube requires an integration of the mechanical properties of pollen tube membranes with other functions in 3-dimensional space.
In summary, understanding pollen tube growth requires a Systems Tip Growth Model. Our compartmental model4 has established the mechanism for (a) the dynamics of all four major ions (Ca2+, K+, H+ and Cl−) arising from the interactions between transporters at the tip and shank, and (b) the interactions between ion dynamics of all the four major ions (Ca2+, K+, H+ and Cl−) and pollen tube growth. We propose that the development of a Systems Tip Growth Model should integrate all the functions in pollen tube growth as illustrated in Figure 1.
Acknowledgments
We gratefully acknowledge Research Councils UK and the Biotechnology and Biological Sciences Research Council for funding in support of this work.
References
- 1.Michard E, Alves F, Feijó JA. The role of ion fluxes in polarized cell growth and morphogenesis: The pollen tube as an experimental paradigm. Int J Dev Biol. 2009;53:1609–1622. doi: 10.1387/ijdb.072296em. [DOI] [PubMed] [Google Scholar]
- 2.Holdaway-Clarke TL, Hepler PK. Control of pollen tube growth: Role of ion gradients and fluxes. New Phytol. 2003;159:539–563. doi: 10.1046/j.1469-8137.2003.00847.x. [DOI] [PubMed] [Google Scholar]
- 3.Zonia L. Spatial and temporal integration of signalling networks regulating pollen tube growth. J Exp Bot. 2010;61:1939–1957. doi: 10.1093/jxb/erq073. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Piette BMAG, Deeks MJ, Franklin-Tong VE, Hussey PJ. A compartmental model analysis of integrative and self-regulatory ion dynamics in pollen tube growth. PLoS ONE. 2010;5:13157. doi: 10.1371/journal.pone.0013157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Certal AC, Almeida RB, Carvalho LM, Wong E, Moreno N, Michard E, et al. Exclusion of a proton ATPase from the apical membrane is associated with cell polarity and tip growth in Nicotiana tabacum pollen tubes. Plant Cell. 2008;20:614–634. doi: 10.1105/tpc.106.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zonia L, Cordeiro S, Feijo JA. Ion dynamics and the control of hydrodynamics in the regulation of pollen tube growth. J Sex Plant Reprod. 2001;14:111–116. [Google Scholar]
- 7.Zonia L, Muller M, Munnik T. Hydrodynamics and cell volume oscillations in the pollen tube apical region are integral components of the biomechanics of Nicotiana tabacum pollen tube growth. Cell Biochem Biophys. 2006;46:209–232. doi: 10.1385/CBB:46:3:209. [DOI] [PubMed] [Google Scholar]
- 8.Kroeger JH, Geitmann A, Grant M. Model for calcium dependent oscillatory growth in pollen tubes. J Theor Biol. 2008;253:363–374. doi: 10.1016/j.jtbi.2008.02.042. [DOI] [PubMed] [Google Scholar]
- 9.Kato N, He HY, Steger AP. A systems model of vesicle trafficking in Arabidopsis pollen tubes. Plant Physiol. 2010;152:590–601. doi: 10.1104/pp.109.148700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan A, Xu G, Yang ZB. Calcium participates in feedback regulation of the oscillating ROP1 Rho GTPase in pollen tubes. Proc Natl Acad Sci USA. 2009;106:22002–22007. doi: 10.1073/pnas.0910811106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kruger NJ, Ratcliffe RG. Insights into plant metabolic networks from steady-state meabolic flux analysis. Biochimie. 2009;91:697–702. doi: 10.1016/j.biochi.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Poolman MG, Miguet L, Sweetlove LJ, Fell DA. A genome-scale metabolic model of Arabidopsis and some of its properties. Plant Physiol. 2009;151:1570–1581. doi: 10.1104/pp.109.141267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grafahrend-Belau E, Schreiber F, Koschutzki D, Junker BH. Flux balance analysis of barley seeds: a computational approach to study systemic properties of central metabolism. Plant Physiol. 2009;149:585–598. doi: 10.1104/pp.108.129635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chebli Y, Geitmann A. Mechanical principles governing pollen tube growth. Funct Plant Sci Biotechnol. 2007;1:232–245. [Google Scholar]
- 15.Fayant P, Girlanda O, Chebli Y, Aubin C, Villemure I, Geitmannb A. Finite element model of polar growth in pollen tubes. Plant Cell. 2010;22:2579–2593. doi: 10.1105/tpc.110.075754. [DOI] [PMC free article] [PubMed] [Google Scholar]