Abstract
The RD20 gene encodes a member of the caleosin family, which is primarily known to function in the mobilization of seed storage lipids during germination. In contrast to other caleosins, RD20 expression is early-induced by water deficit conditions and we recently provided genetic evidence for its positive role in drought tolerance in Arabidopsis. RD20 is also responsive to pathogen infection and is constitutively expressed in diverse tissues and organs during development suggesting additional roles for this caleosin. This addendum describes further exploration of phenotypic alterations in T-DNA insertional rd20 mutant and knock-out complemented transgenic plants in the context of early development and susceptibility to a phytopathogenic bacteria. We show that the RD20 gene is involved in ABA-mediated inhibition of germination and does not play a significant role in plant defense against Pseudomonas syringae.
Key words: ABA, Arabidopsis thaliana, biotic stress, caleosin, germination, RD20
RD20, a Positive Regulator of Drought Tolerance
For many years, RD20 (Responsive to Dehydration, 20) was considered as one of the genes the most highly and rapidly induced by various abiotic stresses and it is often used as a convenient water stress- or ABA-responsive marker gene.1,2 The protein encoded by RD20 belongs to the caleosin family that comprises at least 7 members in Arabidopsis (AtCLO1-7).3 As demonstrated for AtCLO1 and AtCLO2, caleosins are oil-body-associated proteins that possess a Ca2+-dependent peroxygenase activity.4 RD20, also named AtCLO3, is associated to oil-body membranes and shares all the biochemical characteristics required to act as a peroxygenase.5 Until now, the functional role of RD20 in water-stress responses was not known, but using combined whole-plant physiology and reverse genetic approaches, we demonstrated that RD20 participates in drought tolerance mechanisms through the regulation of stomatal aperture, plant growth and water use efficiency.5
RD20 is Involved in ABA-Mediated Inhibition of Germination
The importance of abscisic acid (ABA) in various physiological processes such as germination, seedling growth or regulation of evapotranspiration has been well documented.6,7 We have previously reported that exogenous ABA application is responsible for rapid and transient RD20 gene induction and that RD20 gene expression is also finely controlled during the germination process with an enhanced expression in cotyledons and hypocotyl compared to roots.5 Thus, the behavior of the available knock out rd20 and knock out complemented transgenic lines ORK5 (for Overexpression of RD20 in KO rd20) was analyzed following exogenous ABA treatment through germination and cotyledon opening and greening assays.8 Sterilized seeds were sown on half-strength MS medium containing 1% agar supplemented or not with (±)-ABA. For germination assays, about 30 seeds from WT and mutant seeds were sown in triplicate in the same Petri dish, incubated for four days at 4°C in the dark to break any residual dormancy and transferred to the growth chamber. Germination was scored as the emergence of the radical, and seeds developing fully expanded green cotyledons were counted. All experiments were repeated at least three times and the results are presented in Figure 1. As expected, increasing ABA concentrations inhibited germination and seedling growth in a dose-dependent manner in all genotypes. However, compared to WT and ORK5, rd20 mutant seeds exhibited an enhanced sensitivity to ABA-induced inhibition of germination in response to 1 and 2 µM ABA three days after transfer to the growth chamber (Fig. 1A). Time course experiments showed that all the genotypes were able to germinate fully in two days in the absence of ABA but that germination of rd20 knock-out seeds was significantly delayed on medium containing 2 µM ABA (Fig. 1B). About 50% of WT and ORK5 seeds had germinated in 4 days but rd20 seeds reached a comparable level of germination only at day six. ORK5 complemented lines were generated using RD20 cDNA under the control of the constitutive 35S promoter. Interestingly, these lines displayed a weak but a significantly higher germination rate than the WT suggesting that overexpression of RD20 decreases ABA sensitivity during germination. Scoring of green and fully developed cotyledons after six days also revealed that rd20 cotyledon opening and greening was significantly reduced in response to 0.5 µM ABA (Fig. 1C and 1D) compared to WT and ORK5 lines. This indicates that mutation in RD20 gene can alter ABA responses during early developmental stages but also stomata opening in the whole plant.5 The recent discovery of multiple ABA perception systems as well as several ABA-signaling components such as kinases, phosphatases, G-proteins and secondary messengers show that ABA acts through a complex signaling cascade to induce changes and fine-tuning of multiple physiological processes during plant development and in abiotic and biotic stress responses.9–15
Figure 1.
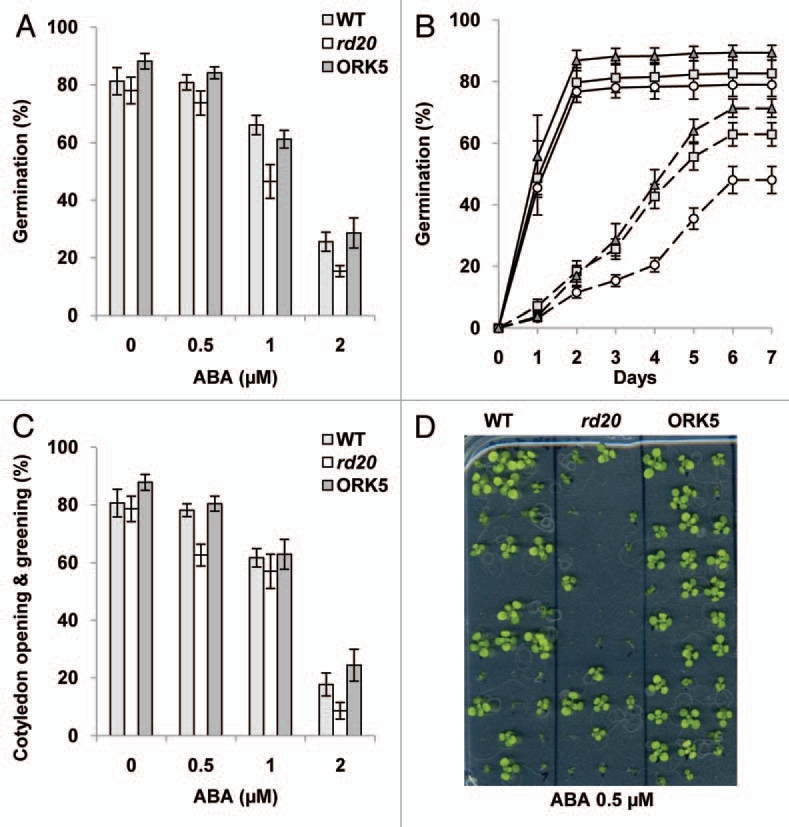
Effects of exogenous ABA on seed germination and seedling growth. (A) ABA-dose response of seed germination of WT, rd20 and ORK5 transgenic lines three days after transfer to growth chamber. (B) Time-course representation of WT (grey squares), rd20 (white circles) and ORK5 (dark grey triangles) seed germination during the 7 days following transfer to growth chamber on half-strength MS medium supplemented or not with 2 µM ABA (continuous and dashed lines respectively). (C) Seedling development of WT, rd20 and ORK5 transgenic lines. The data are means of 3 repetitions (±SEM). Each bar or point represents the mean value of at least 350 seeds. (D) Representative 10-day-old seedlings of WT, rd20 and ORK5 plants grown on MS medium supplemented with ABA (0.5 µM) are shown.
RD20 is Not Involved in Defense against Pseudomonas syringae
Thanks to microarray data,16 it is known that RD20 gene induction is not limited to abiotic stresses and exogenous ABA treatment. Several transcriptome analyses have shown that RD20 is induced by the presence of pathogens such as the bacterial phytopathogen Pseudomonas syringae or the fungus Botritys cinerea,16 and recent evidences suggests that ABA can act as a positive or negative regulator of plant responses to biotic stress by interfering with stress signaling pathways at multiple levels.14,15,17 We first validated some of these expression data by analyzing RD20 gene induction in a time-course experiment using Real-Time PCR with RNA prepared from plants challenged by virulent and avirulent P. syringae strains. Levels of RD20 mRNAs were enhanced 6 h after inoculation by both strains and this expression level was maintained for at least 24 h (not shown). To assess the physiological relevance of RD20 gene induction during biotic stresses, we evaluated its role in plant-pathogen interactions using the epiphytic bacterium P. syringae and the available knock-out rd20 and complement ORK5 lines. Although RD20 gene expression was induced during P. syringae infection, no significant differences were observed on initiation of symptoms and on disease development between the genotypes. These results were validated by in planta bacterial numeration indicating that RD20 do not contribute to plant defense against P. syringae.
Concluding Remarks
Under standard culture conditions, non-significant defects were observed on germination, growth or morphology of rd20 knock-out plants compared to WT and complemented lines. The impact of RD20 mutation was only revealed under stress conditions5 and ABA treatment indicating that RD20, like many stress responsive genes, is responsible for conditional phenotypes. Compared to other caleosins, RD20 is quite particular because of its gene induction by various abiotic factors.18 Future works aiming to decipher the molecular processes controlled by RD20 should improve our understanding of the role of this particular lipid-body peroxygenase in response to environmental stress. Increasing evidence indicates that lipids can act as mediators in many physiological processes19 and although our knowledge on the role of lipid-body associated proteins is still scarce, RD20 might contribute to lipid signaling/modifications in plant stress responses.
Acknowledgments
University Paul Sabatier (Toulouse III), the CNRS, CEA and the ANR (Grant ANR-06-BLAN-0122) supported this work. We thank M. Charpenteau, M. Pervent, D. Vile and T. Simonneau for their contribution to this work.
References
- 1.Magnan F, Ranty B, Charpenteau M, Sotta B, Galaud JP, Aldon D. Mutations in AtCML9, a calmodulin-like protein from Arabidopsis thaliana, alter plant responses to abiotic stress and abscisic acid. Plant J. 2008;56:575–589. doi: 10.1111/j.1365-313X.2008.03622.x. [DOI] [PubMed] [Google Scholar]
- 2.Alexandre C, Möller-Steinbach Y, Schönrock N, Gruissem W, Hennig L. Arabidopsis MSI1 is required for negative regulation of the response to drought stress. Mol Plant. 2009;2:675–687. doi: 10.1093/mp/ssp012. [DOI] [PubMed] [Google Scholar]
- 3.Partridge M, Murphy DJ. Role of membrane-bound caleosin and putative peroxygenase in biotic and abiotic stress responses in Arabidopsis. Plant Physiol Biochem. 2009;47:796–806. doi: 10.1016/j.plaphy.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Hanano A, Burcklen M, Flenet M, Ivancich A, Louwagie M, Garin J, et al. Plant seed peroxygenase is an original heme-oxygenase with an EF-hand calcium binding motif. J Biol Chem. 2006;281:33140–33151. doi: 10.1074/jbc.M605395200. [DOI] [PubMed] [Google Scholar]
- 5.Aubert Y, Vile D, Pervent M, Aldon D, Ranty B, Simonneau T, et al. RD20, a stress inducible caleosin, participates in stomatal control, transpiration and drought tolerance in Arabidopsis thaliana. Plant Cell Physiol. 2010;51:1975–1987. doi: 10.1093/pcp/pcq155. [DOI] [PubMed] [Google Scholar]
- 6.Finkelstein RR, Gampala SSL, Srinivas, Rock CR. Abscisic acid signalling in seeds and seedlings. Plant Cell. 2002:15–45. doi: 10.1105/tpc.010441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schroeder JI, Kwak JM, Allen GJ. Guard cell abscisic acid signalling and engeneering drought hardiness in plants. Nature. 2001;410:327–330. doi: 10.1038/35066500. [DOI] [PubMed] [Google Scholar]
- 8.Verslues PE, Agarwal M, Katiyar-Agarwal S, Zhu J, Zhu JK. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006;45:523–539. doi: 10.1111/j.1365-313X.2005.02593.x. [DOI] [PubMed] [Google Scholar]
- 9.Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol. 2010;61:651–679. doi: 10.1146/annurev-arplant-042809-112122. [DOI] [PubMed] [Google Scholar]
- 10.Hubbard KE, Nishimura N, Hitomi K, Getzoff ED, Schroeder JI. Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions. Genes Dev. 2010;24:1695–1708. doi: 10.1101/gad.1953910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raghavendra AS, Gonugunta VK, Christmann A, Grill E. ABA perception and signalling. Trends Plant Sci. 2010;15:395–401. doi: 10.1016/j.tplants.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Klinger JP, Batelli G, Zhu JK. ABA receptors: the start of a new paradigm in phytohormone signaling. J Exp Bot. 2010;12:3199–3210. doi: 10.1093/jxb/erq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kline KG, Sussman MR, Jones AM. Abscisic acid receptors. Plant Physiol. 2010;154:479–482. doi: 10.1104/pp.110.160846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robert-Seilaniantz A, Navarro L, Bari R, Jones JDG. Pathological hormone imbalances. Curr Op Plant Biol. 2007;10:372–379. doi: 10.1016/j.pbi.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Ton J, Flors V, Mauch-Mani B. The multifaceted role of ABA in disease resistance. Trends Plant Sci. 2009;14:310–317. doi: 10.1016/j.tplants.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Hruz T, Laule O, Szabo G, Wessendrop F, Bleuler S, Oertle L, et al. Genevestigator V3: A reference expression database for the meta-analysis of transcriptomes. Adv Bioinformatics. 2008:420747. doi: 10.1155/2008/420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asselbergh B, De Vleesschauwer D, Höfte M. Global switches and fine-tuning-ABA modulates plant pathogen defense. MPMI. 2008;21:709–719. doi: 10.1094/MPMI-21-6-0709. [DOI] [PubMed] [Google Scholar]
- 18.Kant P, Gordon M, Kant S, Zolla G, Daydov O, Heimer YM, et al. Functional-genomics-based identification of genes that regulate Arabidopsis response to multiple abiotic stresses. Plant Cell Env. 2008;31:697–714. doi: 10.1111/j.1365-3040.2008.01779.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang X. Lipid signaling. Curr Opin Plant Biol. 2004;7:329–336. doi: 10.1016/j.pbi.2004.03.012. [DOI] [PubMed] [Google Scholar]


