Abstract
Most organisms have developed an internal timing mechanism or circadian clock that is able to generate 24-hour biological rhythms in synchronization with the diurnal environmental changes. Despite our increasing understanding of the molecular machinery underlying circadian clock function, a complete picture of the components and regulatory mechanisms governing the circadian system in Arabidopsis thaliana is still lacking. In a recent study, we have characterized the role of the MYB-like transcription factor REVEILLE8/LHY-CCA1-LIKE5 (RVE8/LCL5) within the Arabidopsis circadian clock. We have generated RVE8/LCL5 mutant and overexpressing plants and showed that similar to the MYB-like transcription factor CIRCADIAN CLOCK-ASSOCIATED1 (CCA1), RVE8/LCL5 binds to the promoter of key clock component TOC1 (Timing of CAB expression 1) and regulates its circadian expression. However, the mechanisms of RVE8/LCL5 and CCA1 circadian function seem to differ: while CCA1 represses TOC1 expression by facilitating a hypo-acetylated state of Histone H3, RVE8/LCL5 contributes to TOC1 expression by favouring H3 acetylation at the TOC1 locus. Although CCA1 has a more predominant role on this regulation, our results showing the opposing function of RVE8/LCL5 open interesting questions about the complex networks of transcriptional regulators and chromatin remodelling activities that need to be integrated in synergistic and antagonistic ways to generate the circadian periodicity.
Key words: Arabidopsis thaliana, circadian clock, transcriptional regulation, single MYB, histone acetylation
The circadian clock is able to generate 24-hour biological oscillations in resonance with the diurnal environmental changes.1 The mechanisms underlying circadian clock function are complex and seem to be conserved throughout evolution. In broad sense, the 24-hour period oscillations rely at its basis on negative feedback loops at the core of the circadian oscillator.2 In addition to a very precise transcriptional/translational regulation of clock gene and protein expression, recent evidence is also pointing towards changes in chromatin structure as a key mechanism for circadian clock progression.3,4 In Arabidopsis, the transcriptional oscillation of the clock component TOC1 (Timing of CAB expression 1) is preceded by rhythmic changes in the pattern of histone H3 acetylation.5 The histone acetylation state seems to be regulated, at least in part, by the clock component CCA1 (CIRCADIAN CLOCK-ASSOCIATED1) as plants misexpressing this MYB-like transcription factor exhibit an altered pattern of histone acetylation at the TOC1 locus.5 CCA1 might thus facilitate repressive chromatin structures at the TOC1 locus to regulate TOC1 expression at dawn. In a recent study, we have characterized the implication on the Arabidopsis circadian system of RVE8/LCL5, a MYB-like transcription factor with a high degree of sequence homology to CCA1.6 By using RVE8/LCL5 overexpressing and mutant plants, we found that RVE8/LCL5 binds to the TOC1 promoter and contributes to proper circadian expression of TOC1. Our studies also show that in contrast to CCA1, RVE8/LCL5 seems to favor H3 acetylation, most likely by antagonizing CCA1 function during TOC1 raising phase. Therefore, the counteracting functions of CCA1-RVE8/LCL5 (and possibly other members of the LCL subfamily) might regulate chromatin compaction to precisely shape the waveform of TOC1 circadian expression.
The Expression of the MYB-Like Transcription Factor RVE8/LCL5 is Regulated by the Circadian Clock
Two essential regulators of the Arabidopsis circadian system, CCA1 and LHY (ELONGATED HYPOCOTYL) belong to the REVEILLE (RVE) family composed of eleven proteins. These proteins show a high amino acid sequence similarity within the MYB-like domain (SHAQKYF class).7,8 Five of the eleven proteins can also be grouped into a subfamily, previously identified as LHY/ CCA1-LIKE (LCL) proteins.9 The LCL protein subfamily shows a domain at the C-terminal end of the proteins (LCL domain) that is not present in CCA1, LHY or in the rest of the RVE protein family. Based on the sequence similarities and divergences, we explored the possible role of one of the RVE/LCL proteins, RVE8/LCL5, within the Arabidopsis circadian system. To that end, we first analyzed RVE8/LCL5 expression in wild-type (WT) plants grown for two days under constant light (LL) conditions after synchronization under light:dark cycles. Northern-blot and RT-Q-PCR analysis showed that the expression of RVE8/LCL5 was controlled by the circadian clock, with a rhythmic oscillation under constant light (LL) conditions. RVE8/ LCL5 circadian waveform showed a morning acrophase similar to that reported for CCA110,11 and almost antiphasic to the one described for TOC1.12,13
TOC1 Rhythmic Expression is Affected by Overexpression and Mutation of RVE8/LCL5
To analyze the role of RVE8/LCL5 in the Arabidopsis circadian system, we characterized two independent T-DNA insertion lines, RVE8/LCL5-E5 and RVE8/LCL5-I2. We also generated different lines overexpressing RVE8/LCL5 and examined the effects of RVE8/LCL5 mis-expression on circadian gene expression. Northern-blot and RT-Q-PCR analysis revealed that overexpression of RVE8/LCL5 advanced the phase of the evening-expressed gene TOC1, leading to higher abundance than in WT plants, particularly during the subjective day. Luminescence assays of TOC1:LUC plants transformed with the RVE8/LCL5-ox construct were consistent with the northern-blot and RT-Q-PCR assays and revealed that TOC1 promoter activity rose earlier in RVE8/LCL5-ox than in WT plants, leading to an advanced phase and a short-period phenotype. RT-Q-PCR and luminescence analysis of RVE8/LCL5 mutant plants revealed the opposite phenotypes, i.e., a delayed phase and a long-period phenotype with decreased TOC1 accumulation mostly during the subjective day. Altogether, these results suggest that directly or indirectly RVE8/LCL5 contributes to proper regulation of TOC1 circadian oscillation.
In vivo Binding of RVE8/LCL5 to the TOC1 Promoter
We next examined whether regulation of TOC1 expression by RVE8/LCL5 might be achieved by binding of RVE8/LCL5 to the TOC1 promoter. To that end, we performed chromatin immunoprecipitation (ChIP) experiments with RVE8/LCL5-ox plants. Our results showed an evident amplification of the Evening Element (EE)-containing region of the TOC1 promoter, with a peak during the subjective day, at times when TOC1 expression is clearly affected by overexpression of RVE8/LCL5. No evident amplification was observed when other clock-unrelated genes were analyzed or when samples were equally processed but in the absence of GFP antibody. Together, the results suggest that in a similar way to CCA1,5 RVE8/LCL5 is able to bind to the TOC1 promoter to regulate TOC1 expression.
RVE8/LCL5 Regulates the Pattern of H3 Acetylation at the TOC1 Promoter
Based on previous results showing that circadian rhythms of H3 acetylation at the TOC1 locus are important for TOC1 circadian expression,5 we next examined whether mis-expression of RVE8/LCL5 affected the pattern of H3 acetylation at the TOC1 promoter. Our ChIP analysis with an anti-acetylated H3 antibody showed that the pattern of H3 acetylation was significantly increased in RVE8/LCL5-ox plants compared to that of WT, particularly during the subjective day. As H3 acetylation favors transcription, these results were consistent with the early raising phase of TOC1:LUC expression observed in RVE8/LCL5-ox plants. We also performed ChIP experiments using the rve8/lcl5 T-DNA insertion lines. Our studies showed that H3 acetylation was reduced during the subjective day with a delayed raising phase. These results suggest that RVE8/LCL5 overexpression and mutation alter the normal pattern of H3 acetylation at the TOC1 promoter. This regulation might be particularly relevant during the subjective day, at times when TOC1 expression is most affected in RVE8/LCL5-ox and in rve8/lcl5 T-DNA insertion lines.
Effects on TOC1 Circadian Waveform after Pharmacological Inhibition of Histone Deacetylation
To further confirm our hypotheses, we examined TOC1:LUC expression in the absence or in the presence of trichostatin A (TSA), a potent HDAC inhibitor (Chang and Pikaard, 2005). As previously reported in reference 5, TSA treatment of WT plants delayed TOC1:LUC expression but the phase-delays were more evident when TSA was applied at the beginning or at the middle of TOC1 raising phase, which suggested that treatments closer to the time of HDAC action (just after TOC1 peak of expression) had more severe effects on TOC1:LUC waveform. In RVE8/LCL5-ox plants, TSA treatment led to a high and close to arrhythmic TOC1:LUC expression regardless the time of inhibitor administration. It is therefore possible that the HDAC activities that contribute to TOC1 declining phase5 might interfere with RVE8/LCL5 regulatory function at the TOC1 promoter. When TSA effects on TOC1:LUC expression were examined in rve8/lcl5 T-DNA insertion lines, we observed that adding the inhibitor at the mid of TOC1:LUC raising phase led to a very evident upregulation of TOC1:LUC expression suggesting that adding TSA is enough to partially restore the TOC1:LUC amplitude phenotypes in rve8/lcl5 T-DNA insertion lines. Together, our results suggest that at the beginning of TOC1 declining phase, HDAC activities might antagonize RVE8/LCL5 function while TOC1 raising phase would be regulated by antagonistic functions in which CCA1 repression would be counterbalanced by RVE8/LCL5 activating function.
Conclusions
Our results suggest that RVE8/LCL5 (and most likely other members of the RVE/LCL subfamily) might function as a molecular rheostat of the CCA1-mediated H3 deacetylation at the TOC1 promoter. We propose that CCA1 repressive function at dawn might act by antagonizing RVE/LCL and HAT activities (Fig. 1). Decreased CCA1 protein abundance throughout the day together with RVE8/LCL5 association to the TOC1 promoter may lead to transcriptional activation by the recruitment of HAT activities (Fig. 1). In turn, HDAC activities contributing to TOC1 declining phase might interfere with the RVE8/LCL5 function. Additional factors and different mechanisms of circadian gene regulation (X in Fig. 1) might account for TOC1 declining phase until a rhythmic cycle starts again with CCA1 repressive binding. We suggest that regulated patterns of H3 acetylation by RVE/LCL-CCA1 and by HAT-HDAC activities might provide a fine-tune mechanism for accurate clock-controlled regulation of TOC1 gene expression.
Figure 1.
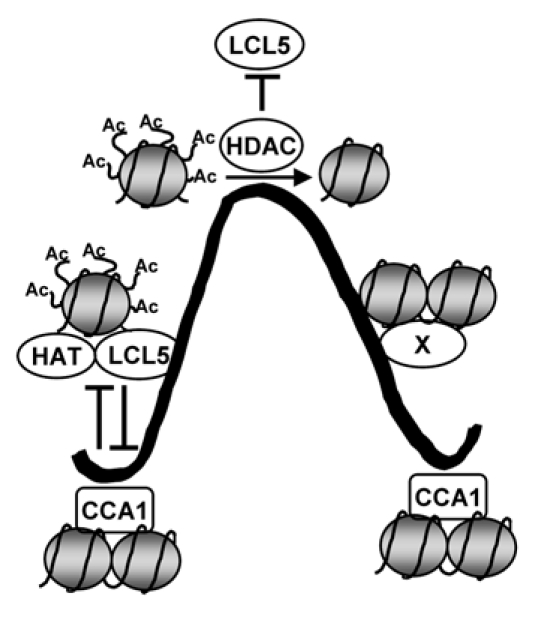
Schematic representation depicting the rhythmic regulation of TOC1 expression. CCA1 contributes to TOC1 repression at dawn most likely by antagonizing HAT activities and favoring a hypo-acetylated state of H3. Decreased CCA1 binding throughout the day together with the counteracting function of RVE8/LCL5 and HAT activities allow transcriptional activation by facilitating histone acetylation. After TOC1 peak of expression, HDAC activities interfere with HAT/LCL5 activating function, promoting the switch to repressive chromatin structures and contributing to the declining phase of TOC1 waveform. Additional unknown factors and mechanisms of circadian gene regulation (X) can account for TOC1 declining phase until a rhythmic cycle starts again with CCA1 binding. The thick black line represents TOC1 circadian waveform; nucleosomes are shown as gray circles with the H3 N-terminal tails as black, curved lines; lines ending in perpendicular dashes indicate antagonistic function.
References
- 1.Wijnen H, Young MW. Interplay of circadian clocks and metabolic rhythms. Annu Rev Genet. 2006;40:409–448. doi: 10.1146/annurev.genet.40.110405.090603. [DOI] [PubMed] [Google Scholar]
- 2.Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, et al. Circadian rhythms from multiple oscillators: Lessons from diverse organisms. Nat Rev Genet. 2005;6:544–556. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakahata Y, Grimaldi B, Sahar S, Hirayama J, Sassone-Corsi P. Signaling to the circadian clock: plasticity by chromatin remodeling. Curr Opin Cell Biol. 2007;19:230–237. doi: 10.1016/j.ceb.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Stratmann T, Mas P. Chromatin, photoperiod and the Arabidopsis circadian clock: a question of time. Semin Cell Dev Biol. 2008;19:554–559. doi: 10.1016/j.semcdb.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Perales M, Mas P. A functional link between rhythmic changes in chromatin structure and the Arabidopsis biological clock. Plant Cell. 2007;19:2111–2123. doi: 10.1105/tpc.107.050807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farinas B, Mas P. Functional implication of the MYB transcription factor RVE8/LCL5 in the circadian control of histone acetylation. Plant J. 2011;2:318–329. doi: 10.1111/j.1365-313X.2011.04484.x. [DOI] [PubMed] [Google Scholar]
- 7.Carré IA, Kim JY. MYB transcription factors in the Arabidopsis circadian clock. J Exp Bot. 2002;53:1551–1557. doi: 10.1093/jxb/erf027. [DOI] [PubMed] [Google Scholar]
- 8.Andersson CR, Harmer SL, Schultz TF, Kay SA. The Reveille (REV) family of DNA binding proteins and the circadian clock; Abstracts of the 10th international conference on Arabidopsis research; 4–8 July 1999; Melbourne. Available at: http://arabidopsis.org/abstract_australia.pdf. [Google Scholar]
- 9.Schmied KC, Merkle T. A small family of LHY-CCA1-like (LCL) MYB1R transcription factors: potential co-regulators of the circadian oscillator. EMBL/GenBank/DDBJ databases. 2005 [Google Scholar]
- 10.Wang ZY, Tobin EM. Constitutive expression of the CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) gene disrupts circadian rhythms and suppresses its own expression. Cell. 1998;93:1207–1217. doi: 10.1016/s0092-8674(00)81464-6. [DOI] [PubMed] [Google Scholar]
- 11.Schaffer R, Ramsay N, Samach A, Corden S, Putterill J, Carré IA, et al. The late elongated hypocotyl mutation of Arabidopsis disrupts circadian rhythms and the photoperiodic control of flowering. Cell. 1998;93:1219–1229. doi: 10.1016/s0092-8674(00)81465-8. [DOI] [PubMed] [Google Scholar]
- 12.Matsushika A, Makino S, Kojima M, Mizuno T. Circadian waves of expression of the APRR1/TOC1 family of pseudo-response regulators in Arabidopsis thaliana: insight into the plant circadian clock. Plant Cell Physiol. 2000;41:1002–1012. doi: 10.1093/pcp/pcd043. [DOI] [PubMed] [Google Scholar]
- 13.Strayer C. Cloning of the Arabidopsis clock gene TOC1, an autoregulatory response regulator homo-log. Science. 2000;289:768–771. doi: 10.1126/science.289.5480.768. [DOI] [PubMed] [Google Scholar]


