Abstract
The indirect effect of ants on plants through their mutualism with honeydew-producing insects has been extensively investigated. Honeydew-producing insects that are tended by ants impose a cost on plant fitness and health by reducing seed production and/or plant growth. This cost is associated with sap intake and virus transmissions but may be overcompesated by tending ants if they deter or prey on hebivorous insects. The balance between cost and benefits depends on the tending ant species. In this study we report other indirect effects on plants of the mutualism between aphids and ants. We have found that two Lasius ant species, one native and the other invasive, may change the composition of volatile organic compounds (VOCs) of the holm oak (Quercus ilex) blend when they tend the aphid Lachnus roboris. The aphid regulation of its feeding and honeydew production according to the ant demands was proposed as a plausible mechanism that triggers changes in VOCs. Additionally, we now report here that aphid feeding, which is located most of the time on acorns cap or petiole, significantly increased the relative content of linolenic acid in acorns from holm oak colonized by the invasive ant. This acid is involved in the response of plants to insect herbivory as a precursor or jasmonic acid. No effect was found on acorn production, germination or seedlings quality. These results suggest that tending-ants may trigger the physiological response of holm oaks involved in plant resistance toward aphid herbivory and this response is ant species-dependent.
Key words: tended aphid, invasive ants, linolenic acid, jasmonic acid, monoterpene emissions
To achieve an indirect effect it is necessary to have a minimun of three species, two focal species that interact directly and an associate species whose presence promotes an indirect effect on one or both focal species. In general, indirect effects of a third species are defined by how and to what degree a pairwise species interaction is influenced by the presence and density of this third species.1 There are several examples of interactions presenting indirect effects: apparent competition,1 facilitation,2 tri-trophic level interactions,3 cascading effects4 and exploitative competition. 5 But, indirect effects have been studied most extensively in the context of trophic cascades when top predators are removed6 or added7 and in the context of mutualisms.8–10 Usually, indirect effects are investigated as changes in abundance of the focal species occur. However, indirect effects may result in biologically significant changes in a species that are not reflected only to its abundance.11 There are many examples of changes in physiology, behavior, morphology and/or genotypic composition of the focal species.11,12 These changes on density and/or morphological, physiological and behavioral traits of the focal species are not mutually exclusive, and all can act at the same time.13 The magnitude and direction of both direct and indirect effects should influence the relative resilience of communities to perturbation, which in turn will affect species coexistence and community evolution.14 In this regard, indirect effects had been postulated as one of the main forces structuring communities2 and shaping the evolution of communities.14
In terrestrial communities ants interact with plants both directly and indirectly. They can disperse or consume seeds, feed from specialized plant structures such as food bodies and extrafloral nectaries, act as or deter pollinitators, prey on herbivorous insects and/or develop mutualisms with honeydew-producing insects indirectly modifying plant fitness.15–17 Additionally, through their nesting activities in soil, ants increase soil nutrient content available to plants, may change water infiltration and soil holding-capacity and modify biodiversity and abundance of soil organisms related to the decomposition process.18,19 As a consequence of their activities, ants may thus change behavior, density, physiology or fitness of other species.12,22,23 In the case of ants that tend honeydew-producing insects, evidence shows that their attention may change some traits of insect life history, 22 their abundance or physiology.18 For the plant, the net outcome of the mutualism between ants and honeydew-producing insects will depend on the balance between the costs for plant fitness via consumption of plant sap and transmission of plant pathogens and the benefit of ants deterring herbivorous insects.18,23 As a consequence, plant seed production, pod production or even plant growth may decrease when the cost of honeydew-producing insects exceed the benefit provided by tending ants.18,23
Recently, we have described the changes that two tending ant species may exert indirectly on monoterpene emissions of holm oak (Quercus ilex) saplings through its mutualism with Lachnus roboris aphids.24 One of these tending ant species was Lasius neglectus, an invasive ant species that displaces the local ant Lasius grandis. We found that aphids feeding on holm oak increased the emission of total volatile organic carbon (VOCs) by 31%. In particular, aphids feeding elicited the emission of a new monoterpene, Δ3-carene, and increased the emission of myrcene (mean ± SE; sapling alone: 0.105 ± 0.011 µg g−1 h−1; sapling plus not tended aphid: 0.443 ± 0.057 µg g−1 h−1) and γ-terpinene (sapling alone: 0.0013 ± 0.0001; sapling plus not tended aphid: 0.0122 ± 0.0022 µg g−1 h−1) (Mann-Whitney, sapling alone vs. sapling plus not tended aphids, U4,4 = 0, p < 0.05 for both compounds). Changes of VOC emission in response to aphid infestation were noticed also in boreal trees.24 When the aphids became tended by the invasive ant, L. neglectus, VOCs emissions increased only 19% because myrcene, the main compound of the blend, decreased significantly (Table 1). On the contrary, the native ant, L. grandis promoted a 400% increase of total VOCs compared to the situation of not tended aphids. The astonishing increment of VOCs was due to the increase of α- and β-pinene, sabinene, camphene and Δ3-carene (Table 1). We suggested that the aphids' regulation of their feeding and honeydew production according to ant attention could explain these results.25 When our data was recalculated on leaf area basis (nmol m−2 s−1), the general pattern was the same independently of the units, but the differences among treatments were not statistically significant (Table 1). The change of statistical differences depending on which unit basis data VOC emissions are calculated has been reported in other studies also in holm oaks.26 These slight differences in the statitiscal significance of the differences of VOC emissions depending on the reference unit may be due to differences in leaf morphology, i.e., changes of leaf area and mass. However, in our study, all holm oaks showed a similar leaf morphology among treatments (Kruskal-Wallis, leaf mass: H3,20 = 2.16, p = 0.53; leaf area: H3,20 = 2.64, p = 0.45) (Table 1). Leaf area and mass may be also modified by aphid feeding. But the few studies that reported the effect of aphids feeding on leaf area and mass showed either decreases or no changes with aphid feeding.24,27 This lack of consistence of aphid effect on leaf area and mass limits the development of a clear pattern linking aphids feeding, leaf area or mass and VOC emissions. On the other hand, to achieve statistical significance of emitted VOCs among treatments, values should differ strongly given the high variability of VOC emission within treatments.26 Under this scenario, we recommend giving the values of leaf morphology and to give VOC emissions on both unit bases to facilite comparisons among different studies.
Table 1.
Means and standard error of the emission rates of the main compounds emitted by Quercus ilex saplings (n = 4 for T1 and T2 and n = 8 for T3) infested with untended aphids (T1) or infested with aphids tended by the native ant Lasius grandis (T2) or by the invasive ant Lasius neglectus (T3)
| Emission rates: µg g−1 h−1 above and nmol m−2 s−1 below | |||
| Compound | T1 | T2 | T3 |
| Non tended | Tended by native ant | Tended by invasive ant | |
| α-Thujene | 0.007 ± 0.004a | 0.015 ± 0.005a | 0.005 ± 0.001a |
| 0.006 ± 0.004a | 0.006 ± 0.003a | 0.009 ± 0.008a | |
| α-Pinene | 0.391 ± 0.182a | 2.072 ± 0.033b | 0.551 ± 0.105a |
| 0.244 ± 0.139a | 0.532 ± 0.082a | 0.244 ± 0.127a | |
| Camphene | 0.007 ± 0.003a | 0.047 ± 0.014b | 0.012 ± 0.004ab |
| 0.005 ± 0.003a | 0.014 ± 0.004a | 0.007 ± 0.004a | |
| Sabinene | 0.084 ± 0.042a | 0.387 ± 0.045b | 0.075 ± 0.017a |
| 0.100 ± 0.076a | 0.210 ± 0.097a | 0.128 ± 0.107a | |
| β-Pinene | 0.227 ± 0.105a | 1.454 ± 0.269b | 0.306 ± 0.075a |
| 0.159 ± 0.097a | 0.322 ± 0.134a | 0.179 ± 0.097a | |
| Myrcene | 0.443 ± 0.057a | 0.482 ± 0.044a | 0.093 ± 0.020b |
| 0.101 ± 0.034a | 0.119 ± 0.026a | 0.060 ± 0.034a | |
| Δ3-Carene | 0.003 ± 0.002a | 0.018 ± 0.001b | 0.010 ± 0.003ab |
| 0.001 ± 0.001a | 0.004 ± 0.001a | 0.002 ± 0.001a | |
| α-Terpine | 0.004 ± 0.001a | 0.003 ± 0.001a | 0.001 ± 0.000a |
| 0.001 ± 0.000a | 0.004 ± 0.003a | 0.001 ± 0.001a | |
| γ-Terpinene | 0.012 ± 0.002a | 0.011 ± 0.004a | 0.013 ± 0.005a |
| 0.003 ± 0.001a | 0.013 ± 0.010a | 0.006 ± 0.003a | |
| Terpinolene | 0.001 ± 0.000a | 0.002 ± 0.001a | 0.005 ± 0.002a |
| 0.001 ± 0.000a | 0.002 ± 0.001a | 0.001 ± 0.001a | |
| Leaf mass (g) | 0.001 ± 0.000a | 0.002 ± 0.001a | 0.005 ± 0.002a |
| Leaf area (m2) | 0.104 ± 0.005a | 0.146 ± 0.026a | 0.113 ± 0.006a |
The emission rate were compared first by Kruskal-Wallis test. Values given above were calculated as µg g−1 h−1, while values below were calculated as nmols m−2 s−1. At the last row, leaf morphology is shown for each treatment. Different letters indicate statistical differences of multiple non parametrical post hoc comparisons (Dunn's test, p < 0.05).
The tended aphid, Lachnus roboris, feed most of the time on the petiole or on the cap of acorns of holm oaks.28 Therefore, acorn quantity and quality (lipid content) and seedlings quality could be affected by tending ants through their mutualism with aphids. We analyzed lipid content as an estimator of acorn quality. Lipids and starches are synthetized in acorns from carbohydrates translocated from leaves.29 However, before being used for metabolic functions, lipid content of acorns must be transformed into glucids and then can be used as respiratory substrate during germination.29 As a consequence, when aphids suck sap from acorns they may act as a sink of translocated carbohydrates, thus decreasing the amount that reaches the seeds.30
During two consecutive years, we counted all acorns from one branch (8–11 cm diameter) for each one of 6 holm oaks colonized by L. neglectus and 6 holm oaks colonized by L. grandis that we studied. We followed them at different stages of their development (July, September and December). Among holm oaks, the loss of acorn production varied between 87.9–96.8%. Acorn production (acorns that started to develop and reached maturity) did not differ between the tree colonized by one or another ant species (mean number of acorns per branch ± SE, 2003: L. neglectus trees: 2.67 ± 1.38, L. grandis trees: 2.67 ± 2.01; Mann Whitney, U = 15, p = 0.69; 2004: L. neglectus trees: 35.83 ± 19.23, L. grandis trees: 49.80 ± 27.99; Mann Whitney, U = 12, p = 0.66). The only work in which researchers evaluated the effect of ants on acorn production was conducted by Ito and Higashi.31 These authors showed that the acorn production of Quercus dentata in the presence of the tending ant Formica yessensis did not differ either. However, there was a significantly lower proportion of infested acorns with weevil larvae when Formica yessensis were tending aphids.31 So, ants may indirectly increase the probability that acorns reach the maturity in healthy conditions, improving in this way one component of the fitness of the oak. In the case of the larvae of weevils, wasps and moth species that infest holm oak acorns32 during their development, they do not move to other acorn as in the case reported by Ito and Higashi.31 This behavior prevents ant predation during the move from one acorn to another.
Lipid content of acorn cotyledons was analyzed by gas cromatography-flame ionization detector (FID) after performing the derivatization of lipid acids to methyl esters with BF3 in methanol.33 Acorn quality only differed in the content of linolenic acid, which was significantly higher in acorns from oaks colonized by the invasive ant Lasius neglectus (Fig. 1). Linolenic acid acts as a precursor for the synthesis of jasmonic acid,34 a signaling molecule involved in responses associated with insect herbivory.35 The increase of linolenic acid suggests that a local response to aphid feeding was triggered during acorn development. In boreal trees, aphid feeding increased up to 50% the emission of methyl salicylate, a defence compound of plants, that acts as aphid repellent and an attractor of foraging predators and parasitoids.24
Figure 1.
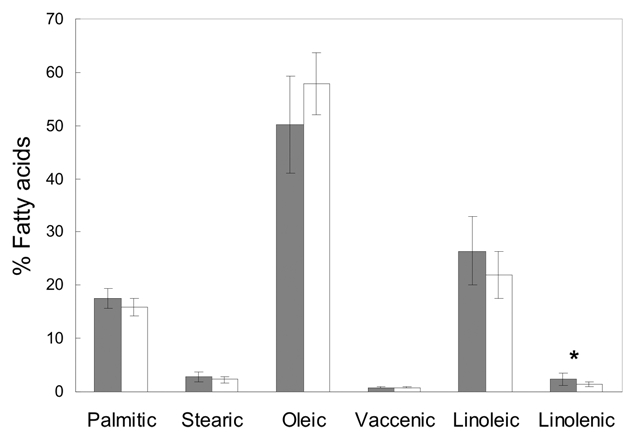
Mean (±SE) of the percentage of each fatty acid relative to the total amount of fatty acids of acorns from holm oaks colonized by invasive ants L. neglectus (in grey) or by native ants L. grandis (in white). Asterisk shows significant differences of linolenic content (Mann Whitney, U = 7.5, p = 0.026).
We then performed a germination test at the second year when enough acorns reached maturity. We picked mature acorns from trees colonized by the invasive or by the native ant. Those acorns with visual evidence of being infested by insect larvae were discarded as non-viable. From the group of healthy acorns, we chose randomly between 6 to 18 acorns per tree comprising in total 94 or 97 acorns for holm oaks colonized by L. neglectus or L. grandis, respectively. We performed a laboratory germination test at 20–25°C under natural light conditions. Acorns were planted in nursery flats of 300 cc filled with commercial compost (70% organic matter, pH = 6.5), watered twice a week and inspected daily from January to April until emergency. After 90 days, acorn viability (germination + seedling emergence) was 89% and 87% for acorns from holm oaks colonized by the invasive or by native ant, respectevily. Puerta-Piñeiro et al. obtained a 90% acorn viability when acorns where sown in sterilized river sand. On the other hand, Leiva and Fernαndez-Alés37 sowed 20 acorns per 7l pots filled with peat and obtained 59% of acorn viability. In our test, we sowed acorns in separate flats under a less competitive environment. The mean time of seedling emergence was 47.8 ± 13.1 days for acorns from holm oaks colonized by L. neglectus and 47.3 ± 14.1 days for acorns from holm oaks colonized by L. grandis. We randomly chose 10 one-month-old seedlings to calculate their quality using the Dickson index.38 This index indicates the potentiality of a seedling to survive and to grow by combining the ratio between root biomass and total biomass with the height and the diameter of the sapling. Seedlings with a higher quality have a higher index. Seedlings showed a very low and similar Dickson index (Mann-Whitnney, L. neglectus: 0.072 ± 0.015; L. grandis: 0.075 ± 0.015, U = 44, p = 0.68, n = 10 seedlings). The low values of Dickson index of the two treatments suggest that from the chosen acorns, emerged seedlings had, per se, a low quality. Only a long term experiment, i.e., at least 10 years to achieve at least two masting years with reproductive holm oaks that never had been infested with aphids, and another group that was infested, could reveal if the effect of aphid feeding on acorns really affect holm oak fitness.
We conclude that ants, through their mutualism with tended aphids, may promote considerable changes of holm oaks VOCs emission and acorn quality. However, there was no effect on seedling quality in spite of the decrease of linolenic acid content of acorns from holm oaks where aphids were tended by the invasive ant. These results indicate that the physiological response of acorns to aphid feeding tended by invasive or local ants does not necessary imply a low quality of seedlings as we previously expected. Under natural conditions, the emission of mature holm oak doubled those of saplings from a plantation.39 So considering that we performed our experiment using 4-year-old saplings, it is probable that the indirect effect of ants on VOCs emissions and acorn quality could be magnified when aphid outbreaks occur in mature holm oak forest. Taking into account the contribution of monoterpenes and isoprene emitted by mediterranean and boreal forests to atmospheric VOC pools40 and the species richness of aphids in the north hemisphere,41 we suggest, in agreement with Blande et al., that aphid infestations should be considered in future models of biogenic VOC emissions from forests.
Acknowledgments
Carolina Paris would like to thank the Government of Catalonia for the grant for young researchers (2003FI 00342) and to Helena Barril and Angela Ribas for taking care of the germination experiment. Funding was provided by Spanish Government grants CGL 2004-05240-CO2/01, CGL2006-04025/BOS, CGL2006-01293/BOS, CGL2007-64080-C02-01, CGL2010-17172/BOS and Consolider-Ingenio Montes CSD2008-00040, and by the Catalan Government grants SGR 2009-458 and SGR 2009-1511.
References
- 1.Miller TE, Kerfoot WC. Redefining indirect effects. In: Kerfoot WC, Sih A, editors. Predation: Direct and indirect impacts on aquatic communities. Hanover: University Press of New England; 1987. pp. 33–37. [Google Scholar]
- 2.Arkema KK, Reed DC, Schroeter SC. Direct and indirect effects of giant kelp determine benthic community structure and dynamics. Ecology. 2009;90:3126–3137. doi: 10.1890/08-1213.1. [DOI] [PubMed] [Google Scholar]
- 3.Heil M. Indirect defence via tritrophic interactions. New Phytol. 2008;178:41–61. doi: 10.1111/j.1469-8137.2007.02330.x. [DOI] [PubMed] [Google Scholar]
- 4.Wardle DA, Williamson WM, Yeates GW, Bonner KI. Trickle-down effects of aboveground trophic cascades on the soil food web. Oikos. 2005;111:348–358. [Google Scholar]
- 5.Reitz SR, Thrumble JT. Competitive displacement among insects and arachnids. Annu Rev Entomol. 2002;47:435–465. doi: 10.1146/annurev.ento.47.091201.145227. [DOI] [PubMed] [Google Scholar]
- 6.Larson AJ, Paine RT. Ungulate herbivory: Indirect effects cascade into the treetops. Proc Natl Acad Sci USA. 2007;104:5–6. doi: 10.1073/pnas.0610198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Croll DA, Maron JL, Estes JA, Danner EM, Byrd GV. Introduced predators transform subarctic islands from grassland to tundra. Science. 2005;307:1959–1961. doi: 10.1126/science.1108485. [DOI] [PubMed] [Google Scholar]
- 8.Boucher D, James S, Keeler K. The ecology of mutualism. Ann Rev System. 1984;13:315–347. [Google Scholar]
- 9.Ness JH. A mutualism's indirect costs: the most aggressive plant bodyguards also deter pollinators. Oikos. 2006;113:506–514. [Google Scholar]
- 10.Wolf B, Husband B, Klironomonos J. Effects of the bellowground mutualism on an aboveground mutualim. Ecol Lett. 2005;8:218–223. [Google Scholar]
- 11.Strauss SY. Indirect effects in community ecology: Their definition, study and importance. Trends Ecol Evol. 1991;6:206–210. doi: 10.1016/0169-5347(91)90023-Q. [DOI] [PubMed] [Google Scholar]
- 12.Gastreich KR. Trait-mediated indirect effects of a Theridiid spider on ant-plant mutualism. Ecology. 1999;80:1066–1070. [Google Scholar]
- 13.Schimidtz OJ, Beckerman AP, O'Brien KM. Behaviorally mediated trophic cascades: effects of predation risk on food web interactions. Ecology. 1997;78:1388–1399. [Google Scholar]
- 14.Miller TE, Travis J. The evolutionary role of indirect effects in communities. Ecology. 1996;77:1329–1335. [Google Scholar]
- 15.Rico-Gray V, Oliveira P. In: The ecology and evolution of ant-plant interactions. Thompson J, editor. The University of Chicago Press; 2007. pp. 1–332. [Google Scholar]
- 16.de Vega C. The importance of floral signals in the establishment of plant-ant mutualisms. Plant Signal Behav. 2009;4:517–518. doi: 10.4161/psb.4.6.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Styrsky JD, Eubanks MD. Ecological consequences of interactions between ants and honeydew-producing insects. Proc Roy Soc London Ser-B. 2007;274:151–164. doi: 10.1098/rspb.2006.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boulton A, Amberman K. How ant nests increase soil biota richness and abundance: A field experiment. Biodivers Conserv. 2006;15:69–82. [Google Scholar]
- 19.Frouz J, Jilkova V. The effects of ants on soil properties and effects (Hymenoptera: Formicidae) Mirmecol News. 2008;11:191–199. [Google Scholar]
- 20.Fagundes M, Neves FS, Fernandes GW. Direct and indirect interactions involving ants, insect herbivores, parasitoids and the host plant Baccharis dracunculifolia (Asteraceae) Ecol Entomol. 2005;30:28–35. [Google Scholar]
- 21.Stadler B, Dixon AFG. Ant attendance in aphids: Why different degrees of myrmecophily? Ecol Entomol. 1999;24:363–369. [Google Scholar]
- 22.Flatt T, Weisser W. The effects of mutualistic ants on aphid life history traits. Ecology. 2000;81:3522–3529. [Google Scholar]
- 23.Renault CK, Buffa LM, Delfino MA. An aphid-ant interaction: Effects on different trophic levels. Ecol Res. 2005;20:71–74. [Google Scholar]
- 24.Blande J, Korjus M, Holopainen J. Foliar methyl salicylate emissions indicate prolonged aphid infestation on silver birch and black alder. Tree Physiol. 2010;30:404–416. doi: 10.1093/treephys/tpp124. [DOI] [PubMed] [Google Scholar]
- 25.Paris C, Llusia J, Peñuelas J. Changes in monoterpene emission rates of Quercus ilex infested by aphids tended by native or invasive Lasius ant species. J Chem Ecol. 2010;36:689–698. doi: 10.1007/s10886-010-9815-1. [DOI] [PubMed] [Google Scholar]
- 26.Staudt M, Joffre R, Rambal S. How growth conditions affect the capacity of Quercus ilex leaves to emit monoterpenes. New Phytol. 2003;158:61–73. [Google Scholar]
- 27.Meyer G, Whitlow T. Effects of leaf and sap feeding insects on photosynthetic rates of goldenrod. Oecologia. 1992;92:480–489. doi: 10.1007/BF00317839. [DOI] [PubMed] [Google Scholar]
- 28.Paris C, Espadaler X. Honeydew collection by the invasive garden ant Lasius neglectus versus the native ant L. grandis. Arthropod-Plant Interact. 2009;3:75–85. [Google Scholar]
- 29.Kramer P, Kozlowski T. Physiology of trees. Mc Graw-Hill Book Company; 1960. pp. 148–153. [Google Scholar]
- 30.Dixon AFG. Aphids and translocation. In: Zimmermann MH, Milburn JA, editors. Transport in Plants I Phloem transport. Verlag Berlin Heidelberg New York: Springer; 1975. pp. L156–L170. [Google Scholar]
- 31.Ito F, Higashi S. An indirect mutualism between oaks and wood ants via aphids. J Anim Ecol. 1991;60:463–470. [Google Scholar]
- 32.Siscart D, Diego V, Lloret F. Acorn ecology. In: Roda F, Retana J, Gracia CA, Bellot J, editors. Ecology of Mediterranean evergreen oak forest. First edition. Berlin Heidelberg: Springer-Verlag; 1999. pp. 75–87. [Google Scholar]
- 33.Metcalfe LD, Schmitz AA, Wang CN. The gas-chromatography of commercial fatty-acid derivatives. J Amer Oil Chem Soc. 1982;59:268. [Google Scholar]
- 34.Goggin F. Plant-aphid interactions: Molecular and ecological perspectives. Curr Opin Plant Biol. 2007;10:399–408. doi: 10.1016/j.pbi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Gatehouse JA. Plant resistance towards insect herbivores: A dynamic interaction. New Phytol. 2002;156:145–169. doi: 10.1046/j.1469-8137.2002.00519.x. [DOI] [PubMed] [Google Scholar]
- 36.Puerta-Piñeiro C, Gómez JM, Zamora R. Species-specific effects on topsoil development affect Quercus ilex seedling performance. Acta Oecol. 2006;29:65–71. [Google Scholar]
- 37.Leiva MJ, Fernández-Alés R. Holm oak (Quercus ilex subsp. ballota) acorns infestation by insects in Mediterranean dehesas and shrublands. Its effect on acorn germination and seedlings emergence. Forest Ecol Manag. 2005;212:221–229. [Google Scholar]
- 38.Dickson A, Leaf AL, Hosner JF. Quality appraisal of white spruce and white pine seedling stock in nurseries. Forestry Chron. 1960;36:10–13. [Google Scholar]
- 39.Street RA, Owen S, Duckham SC, Boissard C, Hewitt CN. Effect of habitat and age on variations in volatile organic compound (VOC) emissions form Quercus ilex and Pinus pinea. Atmos Environ. 1997;31:89–100. [Google Scholar]
- 40.Karl M, Guenther A, Köble R, Leip A, Seufert G. A new European plant-specific emission inventory of biogenic volatile organic compounds for use in atmospheric transport models. Biogeosciences. 2009;6:1059–1087. [Google Scholar]
- 41.Dixon AFG, Kindlmann P, Lepš J, Holman J. Why there are so fewspecies of aphids, especially in the tropics. Am Nat. 1987;129:580–592. [Google Scholar]


