Abstract
Mycorrhizal fungi form a mutualistic relationship with the roots of most plant species. This association provides the arbuscular mycorrhizal (AM) fungus with sugars while the fungus improves the uptake of water and mineral nutrients in the host plant. Moreover, the induction of defense gene expression in mycorrhizal roots has been described. While salicylic acid (SA)-regulated Pathogenesis-Related (PR) proteins accumulate in rice roots colonized by the AM fungus G. intraradices , the SA content is not significantly altered in the mycorrhizal roots. Sugars, in addition to being a source of carbon for the fungus, might act as signals for the control of defense gene expression. We hypothesize that increased demands for sugars by the fungus might be responsible for the activation of the host defense responses which will then contribute to the stabilization of root colonization by the AM fungus. An excessive root colonization might change a mutualistic association into a parasitic association.
Key words: Glomus intraradices, glucose, fructose, Oryza sativa, pathogenesis-related (PR), salicylic acid (SA), sucrose, sugars
The arbuscular mycorrhizal (AM) fungi are obligate biotrophs that establish mutualistic associations with the roots of over 90% of all plant species. AM fungi improve the uptake of water and mineral nutrients in the host plant, mainly phosphorus and nitrogen, in exchange for sugars generated from photosynthesis. The benefits of the AM symbiosis on plant fitness are largely known, including increased ability to cope with biotic and abiotic stresses.1,2 In fact, the amount of carbon allocated to mycorrhizal roots might be up 20% of the total photosynthate income.3 During root colonization, the AM fungus penetrates into the root through the epidermal cells and colonizes the cortex. In the root cortical cells, the fungus forms highly branched structures, called arbuscules, which are the site of the major nutrient exchange between the two symbionts.4,5 The legumes Medicago truncatula and Lotus japonicus have been widely adopted as the reference species for studies of the AM symbiosis. Cereal crops and rice in particular are also able to establish symbiotic associations with AM fungi.6,7 Arabidopsis thaliana, the model system for functional genomics in plants, has no mycorrhization ability.
It is also well known that plants have evolved inducible defense systems to protect themselves from pathogen invasion. Challenge with a pathogen activates a complex variety of defense reactions that includes the rapid generation of reactive oxygen species (ROS), changes in ion fluxes across the plasma membrane, cell wall reinforcement and production of antimicrobial compounds (e.g., phytoalexins).8 One of the most frequently observed biochemical events following pathogen infection is the accumulation of pathogenesis-related (PR) proteins.9 For some PR proteins antimicrobial activities have been described (e.g., chitinases, β-1,3-glucanases, thionins or defensins). The plant responses to pathogen attack are activated both locally and systemically. The phytohormones salicyclic acid (SA), jasmonic acid (JA), ethylene (ET) and abscisic acid (ABA) act as defense signaling molecules for the activation of defense responses.10 Whereas SA-dependent signaling often provides resistance to biotrophic pathogens, JA/ET-dependent signaling is effective against necrotrophic pathogens.11 During plant-pathogen interactions, cross-talk between SA and JA/ET signaling pathways provides the plant with the opportunity to prioritize one pathway over another to efficiently fine-tune its defense response to the invading pathogen. Contrary to biotrophic pathogens which exhibit a high degree of host specificity, the AM fungi manage to colonize a broad range of plant species.
Evidence also exists on the existence of common mechanisms and signaling pathways governing responses to AM and pathogenic fungi.2,12,13 Alterations in the content of hormones acting as defense signals also appear to occur during the AM symbiosis. As an example, JA and its derivatives (jasmonates) are believed to play an important role during the AM symbiosis in M. truncatula or tomato plants.14,15 However, controversial data exists in the literature concerning the involvement of the various defense-related hormones during AM functioning. In particular, our current understanding of SA signaling during AM symbiosis is not clear.
We recently documented the symbiotic proteome of the rice roots during their interaction with the AM fungus Glomus intraradices.6 A majority of the proteins identified in the rice symbiotic proteome are proteins with a function in defense responses or sugar metabolism. Among the proteins that accumulated at high levels in mycorrhizal rice roots compared to non mycorrhizal roots were PR proteins belonging to different PR families, such as PR1, chitinases (PR3), PR5 and several PR10 proteins. The PR1 and PBZ1 (a member of the PR10 family of PR proteins) genes are considered markers of the activation of defense responses in rice plants.16,17 Of interest, the expression of many of the AM-regulated PR genes was previously reported to be induced by SA.16,18–20 Proteins acting as oxidative stress protectors, such as ascorbate peroxidases, peroxidases and glutathione-S-transferases, also accumulated in mycorrhizal rice roots. Together, these observations support that the plant's immune system is activated in the mycorrhizal rice root.
To gain further insights into the molecular mechanisms governing PR gene expression in mycorrhizal roots, the SA and sugar contents of mycorrhizal roots were determined. Towards this end, rice (Oryza sativa ssp. japonica cv. Senia) plants were inoculated with the AM fungus G. intraradices.6 At 42 days post-inoculation (dpi), the overall colonization of the rice roots ranged from 25 to 30% as judged by microscopical observations of trypan blue-stained roots (results not shown; similar results were reported previously in reference 6). By this time, all the events related to fungal development, namely intraradical hyphae, arbuscules at different morphological stages of formation and vesicles, were present in G. intraradices-inoculated roots, thus confirming the establishment of the symbiotic association in the rice roots.
Knowing that many AM-regulated proteins are also regulated by SA in rice roots, it was of interest to determine whether the level of endogenous SA increases in mycorrhizal roots compared to non mycorrhizal roots. In plants, intracellular SA is found predominantly as free SA and its sugar conjugate SA-glucoside (SAG). Root samples were analyzed for SA content, by measuring the level of both free SA and SAG as previously described in reference 21. This analysis revealed no significant differences, neither in free nor in SAG, between mycorrhizal and non mycorrhizal roots (Fig. 1). Then, it appears that although the expression of PR genes (functioning in a SA-dependent manner) is activated during the AM symbiosis, the fungus G. intraradices do not exploit the SA-mediated signaling pathway for induction of PR genes.
Figure 1.

SA content, free SA and SA-glucoside (SAG) conjugate, in roots of mock-inoculated (−Gi) and G. intraradices-inoculated (+Gi) rice plants. SA determination was carried out at 42 days post-inoculation with G. intraradices. Three independent biological samples and three replicates per biological sample were used for quantification of SA. Two out of the three samples were the same ones used for the characterization of the symbiotic proteome in which the accumulation of SA-regulated PR genes was observed in reference 6. FW, fresh weight. Bars represent the means ± standard error.
On the other hand, a direct link between sugar metabolism and the plant defense response has been established, including the phenomenon of high sugarmediated resistance and the finding that various key PR genes are induced by sugars. Transgenic approaches that lead to alterations in photoassimilate partitioning, either sucrose or hexoses, also alter PR gene expression.22,23 In other studies, a SA-independent induction of PR genes by soluble sugars, sucrose, glucose and fructose, was reported in reference 24.
Sucrose, the main form of assimilated carbon during photosynthesis, is transported to the root tissues via the phloem where it becomes available to the root cells. As previously mentioned, characterization of the rice symbiotic proteome revealed alterations in the accumulation of proteins involved in sugar metabolism, such as enzymes involved in glucolysis/gluconeogenesis (e.g., fructose-1,6-bisphophate aldolase, enolase) or in pentose interconversions (e.g., UDP-glucose dehydrogenase).6 Because the plant provides sugars to the fungus, it is not surprising to find alterations in enzymes involved in sugar metabolism in the mycorrhizal roots. Evidence also supports that AM fungi acquire hexoses from the host cell and transform it into trehalose and glycogen, the typical sugars in the fungus.25 Utilization of sucrose then requires hydrolysis in the plant cell which can be performed by sucrose synthase, producing UDP-glucose and fructose or invertases, producing glucose and fructose. Along with this, increased activities of invertases and sucrose synthases or increased expression of their corresponding genes, have been described during AM symbiotic interactions.26,27 Very recently, the MtSucS1 sucrose synthase gene was reported to be essential for the establishment and maintenance of the AM symbiosis in Medicago truncatula.28 In this context, we decided to explore whether colonization by G. intraradices has an effect on the accumulation of soluble sugars in rice roots.
Sucrose, glucose and fructose content were measured enzymatically23 in the rice roots at 42 days post-inoculation with G. intraradices . A tendency to a higher sucrose level was observed in mycorrhizal roots compared to non-mycorrhizal roots (Fig. 2). Concerning the hexose content, the mycorrhizal roots had a significantly lower hexose, both glucose and fructose levels, compared to non-mycorrhizal roots (p ≤ 0.05, Fig. 2). This finding is in agreement with results reported by other authors indicating that the fungal symbiont takes up and uses hexoses within the root.29,30 The observation that the sucrose content is not significantly affected by mycorrhiza functioning, indicates that the host cell is able to sense sucrose concentration in order to maintain it at sufficient but constant levels to satisfy the demand for sugars by the fungal symbiont.
Figure 2.
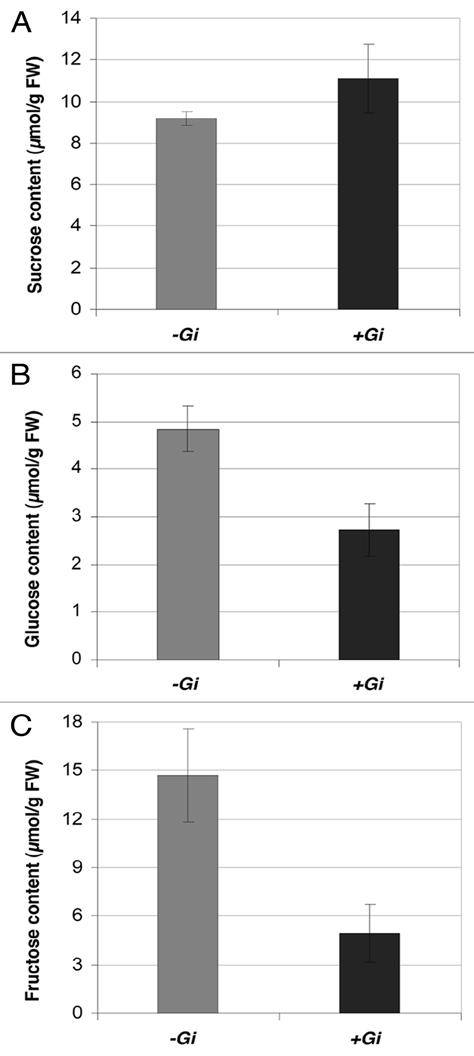
Sugar content in roots of rice plants inoculated with G. intraradices (+Gi) or mock-inoculated (−Gi). (A) Sucrose content. (B) Glucose content. (C) Fructose content. Measurements were made at 42 days post-inoculation with G. intraradices. Bars represent the means ± standard error.
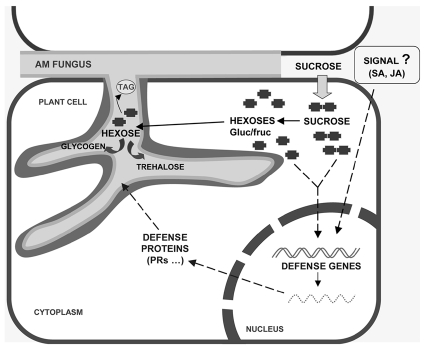
Clearly, the outcome of the AM symbiosis is an overall improvement of the fitness of both partners: the plant supplies the fungus with photosynthates whereas the fungus delivers nutrients from the soil to the host plant. Variations in the extent of colonization of the AM fungi will impose different carbon demands on the plants. However, a high demand of photosynthates by the mycorrhizal root might result in increased mycorrhization which, in turn, might be detrimental for the host plant. The rate of colonization and the amount of fungal biomass must then be tightly controlled by the host plant. We postulate that an increased sink strength by AM colonization might result in transient and/or localized increases in sugar concentrations in the root cell which might be the signal for the activation of defense gene expression. A schematic representation of plant responses associated with increased demands for sugars and deployment of defense responses is shown in Figure 3. According to this model, sugars might play a dual role during the AM symbiosis: (1) sugars are transferred from the plant to the fungus in exchange of mineral nutrients and (2) sugars alter host gene expression, leading to the activation of defense-related genes. This will allow the host plant to avoid an excessive root colonization by the AM fungus that might cause negative effects on the plant's fitness. A complex exchange and interplay of signals between plant roots and AM fungi must then operate during functioning of the AM symbiosis for coordination of joint nutrient resource explotation strategies and control of the plant's immune system. During evolution, co-adaptation between the two symbionts, the AM fungi and the host plant, must have occurred for stabilization of mycorrhizal cooperation and optimal functioning of mycorrhizal associations along the mutualism-parasitism continuum.
Figure 3.
Proposed model for a sugar mediated-activation of defense-related genes in mycorrhizal roots. In the arbuscular mycorrhizal symbiosis, the fungal symbiont colonizes root cortical cells, where it establishes differentiated hyphae called arbuscules. Arbuscules are the site of mineral nutrient transfer to the plant and the site of carbon acquisition by the fungus. Although arbuscules form within the root cortical cells, they remain separated from the plant cell cytoplasm by a plant-derived membrane, the periarbuscular membrane. In this way, an interface is created between the plant and fungal cells which appears to be optimal for nutrient transfer. Sucrose is transported through the phloem into the root. In the root cell, sucrose is hydrolyzed by host invertase and sucrose synthase activities before uptake by the AM fungus. Hexose uptake at the plant-fungus interfase might be passive with a concentration gradient maintained by rapid conversion of hexoses taken up by the fungus to trehalose and glycogen. Active mechanisms might also operate for hexose transport processes between the host cell and the symbiont. Under conditions of a high demand for sugars by the AM fungus, transient increases in sugar content will occur in the root cells which would be the signal for the activation of the host defense responses. The host-produced defense compounds would stabilize the level of root colonization by the AM fungus. An excessive root colonization might change the mutualistic association into a parasitic one.
Acknowledgments
This work was supported by grant BIO2009-08719 from the Ministerio de Ciencia e Innovación (MICINN), as well as by the Center CONSOLIDER on Agrigenomics. We thank K. Kakar for critical reading. We also thank the “Departament d'Innovació, Universitats i Empresa” from the Generalitat de Catalunya (Xarxa de Referencia en Biotecnología and SGR 09626) for substantial support.
References
- 1.Parniske M. Molecular genetics of the arbuscular mycorrhizal symbiosis. Curr Opin Plant Biol. 2004;7:414–421. doi: 10.1016/j.pbi.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Pozo MJ, Azcón-Aguilar C. Unraveling mycorrhiza-induced resistance. Curr Opin Plant Biol. 2007;10:393–398. doi: 10.1016/j.pbi.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Jakobsen I, Rosendahl L. Carbon flow into soil and external hyphae from roots of mycorrhizal cucumber plants. New Phytol. 1990;115:7–83. [Google Scholar]
- 4.Bonfante P, Genre A. Mechanisms underlying beneficial plant-fungus interactions in mycorrhizal symbiosis. Nat Commun. 2010;1:48. doi: 10.1038/ncomms1046. [DOI] [PubMed] [Google Scholar]
- 5.Harrison MJ. Signaling in the arbuscular mycorrhizal symbiosis. Ann Rev Microbiol. 2005;59:19–42. doi: 10.1146/annurev.micro.58.030603.123749. [DOI] [PubMed] [Google Scholar]
- 6.Campos-Soriano L, García-Garrido JM, San Segundo B. Activation of basal defense mechanisms of rice plants by Glomus intraradices does not affect the arbuscular mycorrhizal symbiosis. New Phytol. 2010;188:597–614. doi: 10.1111/j.1469-8137.2010.03386.x. [DOI] [PubMed] [Google Scholar]
- 7.Sawers RJH, Gutjahr C, Paszkowski U. Cereal mycorrhiza: An ancient symbiosis in modern agriculture. Trends Plant Sci. 2008;13:93–97. doi: 10.1016/j.tplants.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Zipfel C. Early molecular events in PAMP-triggered immunity. Curr Opin Plant Biol. 2009;12:414–420. doi: 10.1016/j.pbi.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 9.van Loon LC, Rep M, Pieterse CMJ. Significance of inducible defense-related proteins in infected plants. Ann Rev Phytopathol. 2006;44:135–162. doi: 10.1146/annurev.phyto.44.070505.143425. [DOI] [PubMed] [Google Scholar]
- 10.Spoel SH, Dong X. Making sense of hormone crosstalk during plant immune responses. Cell Host Microbe. 2008;3:348–351. doi: 10.1016/j.chom.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Ann Rev Phytopathol. 2005;43:205–227. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- 12.Güimil S, Chang HS, Zhu T, Sesma A, Osbourn A, Roux C, et al. Comparative transcriptomics of rice reveals an ancient pattern of response to microbial colonization. Proc Natl Acad Sci USA. 2005;102:8066–8070. doi: 10.1073/pnas.0502999102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Wees SC, Van der Ent S, Pieterse CM. Plant immune responses triggered by beneficial microbes. Curr Opin Plant Biol. 2008;11:443–448. doi: 10.1016/j.pbi.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Hause B, Mrosk C, Isayenkov S, Strack D. Jasmonates in arbuscular mycorrhizal interactions. Phytochemistry. 2007;68:101–110. doi: 10.1016/j.phytochem.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 15.López-Ráez JA, Verhage A, Fernández I, García JM, Azcón-Aguilar C, Flors V, et al. Hormonal and transcriptional profiles highlight common and differential host responses to arbuscular mycorrhizal fungi and the regulation of the oxylipin pathway. J Exp Bot. 2010;61:2589–2601. doi: 10.1093/jxb/erq089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agrawal GK, Rakwal R, Jwa NS, Agrawal VP. Signalling molecules and blast pathogen attack activates rice OsPR1a and OsPR1b genes: A model illustrating components participating during defense/stress response. Plant Physiol Biochem. 2001;39:1095–1103. [Google Scholar]
- 17.Midoh N, Iwata M. Cloning and characterization of a probenazole-inducible gene for an intracellular pathogenesis-related protein in rice. Plant Cell Physiol. 1996;37:9–18. doi: 10.1093/oxfordjournals.pcp.a028918. [DOI] [PubMed] [Google Scholar]
- 18.Ganesan V, Thomas G. Salicylic acid response in rice: influence of salicylic acid on H2O2 accumulation and oxidative stress. Plant Sci. 2001;160:1095–1106. doi: 10.1016/s0168-9452(01)00327-2. [DOI] [PubMed] [Google Scholar]
- 19.McGee JD, Hamer JE, Hodges TK. Characterization of a PR-10 pathogenesis-related gene family induced in rice during infection with Magnaporthe grisea. Mol Plant-Microbe Interact. 2001;14:877–886. doi: 10.1094/MPMI.2001.14.7.877. [DOI] [PubMed] [Google Scholar]
- 20.Muthukrishnan S, Liang G, Trick H, Gill B. Pathogenesis-related proteins and their genes in cereals. Plant Cell Tissue Organ Culture. 2001;64:93–114. [Google Scholar]
- 21.Malamy J, Hennig J, Klessig DF. Temperature-dependent Induction of salicylic acid and its conjugates during the resistance response to tobacco mosaic virus infection. Plant Cell. 1992;4:359–366. doi: 10.1105/tpc.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herbers K, Meuwly P, Frommer WB, Metraux JP, Sonnewald U. Systemic acquired resistance mediated by the ectopic expression of invertase: possible hexose sensing in the secretory pathway. Plant Cell. 1996;8:793–803. doi: 10.1105/tpc.8.5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murillo I, Roca R, Bortolotti C, Segundo BS. Engineering photoassimilate partitioning in tobacco plants improves growth and productivity and provides pathogen resistance. Plant J. 2003;36:330–341. doi: 10.1046/j.1365-313x.2003.01880.x. [DOI] [PubMed] [Google Scholar]
- 24.Herbers K, Meuwly P, Métraux JP, Sonnewald U. Salicylic acid-independent induction of pathogenesis-related protein transcripts by sugars is dependent on leaf developmental stage. FEBS Lett. 1996;397:239–244. doi: 10.1016/s0014-5793(96)01183-0. [DOI] [PubMed] [Google Scholar]
- 25.Bago B, Pfeffer PE, Abubaker J, Jun J, Allen JW, Brouillette J, et al. Carbon export from arbuscular mycorrhizal roots involves the translocation of carbohydrate as well as lipid. Plant Physiol. 2003;131:1496–1507. doi: 10.1104/pp.102.007765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franken P. In: Arbuscular Mycorrhizas: Physiology and Function. Koltai H, Kapulnik Y, editors. Springer Verlag; 2010. pp. 93–116. [Google Scholar]
- 27.Harrison MJ. A sugar transporter from Medicago truncatula: Altered expression pattern in roots during Vesicular-Arbuscular (VA) mycorrhizal associations. Plant J. 1996;9:491–503. doi: 10.1046/j.1365-313x.1996.09040491.x. [DOI] [PubMed] [Google Scholar]
- 28.Baier MC, Keck M, Gödde V, Niehaus K, Küster H, Hohnjec N. Knockdown of the symbiotic sucrose synthase MtSucS1 affects arbuscule maturation and maintenance in mycorrhizal roots of Medicago truncatula. Plant Physiol. 2010;152:1000–1014. doi: 10.1104/pp.109.149898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bago B, Pfeffer PE, Shachar-Hill Y. Carbon metabolism and transport in arbuscular mycorrhizas. Plant Physiol. 2000;124:949–958. doi: 10.1104/pp.124.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solaimanand MDZ, Saito MASA. Use of sugars by intraradical hyphae of arbuscular mycorrhizal fungi revealed by radiorespirometry. New Phytol. 1997;136:533–538. doi: 10.1046/j.1469-8137.1997.00757.x. [DOI] [PubMed] [Google Scholar]