Abstract
Potassium (K+) is an important nutrient for plants. It serves as a cofactor of various enzymes and as the major inorganic solute maintaining plant cell turgor. In a recent study, an as yet unknown role of K+ in plant homeostasis was shown. It was demonstrated that K+ gradients in vascular tissues can serve as an energy source for phloem (re)loading processes and that the voltage-gated K+ channels of the AKT2-type play a unique role in this process. The AKT2 channel can be converted by phosphorylation of specific serine residues (S210 and S329) into a non-rectifying channel that allows a rapid efflux of K+ from the sieve element/companion cells (SE/CC) complex. The energy of this flux is used by other transporters for phloem (re)loading processes. Nonetheless, the results do indicate that post-translational modifications at S210 and S329 alone cannot explain AKT2 regulation. Here, we discuss the existence of multiple post-translational modification steps that work in concert to convert AKT2 from an inward-rectifying into a non-rectifying K+ channel.
Key words: potassium, channel, potassium channel, AKT2, phloem (re)loading, post-translational modifications, potassium battery
Potassium (K+) is the most abundant mineral element in plants, and together with nitrogen and phosphorous, is limiting for plant production in many natural and agricultural habitats. Voltage-gated K+ channels are key players in the acquisition of K+ ions from the soil and in its redistribution within the plant.1 Structurally, these channels result from the assembly of four so-called α-subunits. The subunits are encoded by nine genes in Arabidopsis and both homo- and hetero-tetramers are expressed.2,3 The K+ channel α-subunits can be categorized into four different subfamilies, based on the voltage-gating characteristics of the exogenous K+ conductance when expressed in an appropriate heterologous expression system. Kin α-subunits form hyperpolarization-activated channels that mediate K+ uptake.4–7 Kout α-subunits form depolarization-activated channels that mediate K+ release from cells.8–10 Ksilent subunits appear unable to yield functional homomeric channels, but can combine with Kin subunits and fine-tune the K+-uptake properties of the resulting heteromeric channels.11–14 Finally, Kweak α-subunits form channels with complex voltage-gating; they allow both K+ uptake and release.15–19 In Arabidopsis, a single member is found in this subfamily, AKT2, and this channel can assemble in heteromeric channels with the Kin subunit KAT2.20
To date, only scarce and speculative information has been obtained for the function of Kweak channels. When expressed in heterologous expression systems, two different subpopulations of AKT2 channels differing in their sensitivity to voltage were found.21 Channels of the first type showed gating properties and currents analogous to that of Kin channels, while the other sort enabled a non-rectified (leak-like) current; they were open over the entire physiological voltage range.
A given channel can be converted from one type to the other by post-translational modifications.21 A voltage-dependent phosphorylation was found to be an essential step for this switch,22,23 although the kinase responsible for this conversion still needs to be uncovered.24 In biophysical studies, mutant versions of the Arabidopsis Kweak channel subunit AKT2 have been created that showed impaired gating mode settings.22,23 Recently, Gajdanowicz et al. generated transgenic Arabidopsis thaliana plants that express these mutant AKT2 channels in the background of the akt2-1 null-allele plant.25 The major conclusion from analyses of these mutants is that the status switching of AKT2 from an inward-rectifying to a non-rectifying channel is crucial for plants to overcome energy-limiting conditions. This function of AKT2 could be correlated to its expression in phloem tissues. Selective expression of AKT2 under the control of the phloem companion cell-specific AtSUC2 promoter rescued the akt2-1 line, but conversely, selective expression of AKT2 under the control of the guard cell-specific GC1 promoter,26 resulted in further impairment of plant growth (Fig. 1). By combining diverse experimental approaches with mathematical simulation methods, an existing model for phloem (re)loading18,27 was fundamentally improved. This allowed the uncovering of a novel and interesting role of K+ in phloem physiology: K+ gradients present between the sieve element/companion cell (SE/CC) complex and the apoplast can serve as an energy source in phloem (re)loading processes. This “potassium battery” can be tapped by means of AKT2 regulation. This clarifies the observation of Deeken et al.28 that in AKT2 loss-of-function mutant plants, assimilates leaking away from the sieve tube were not efficiently reloaded into the main phloem stream.
Figure 1.
AKT2 expressed only in guard cells delays plant development. (A–C) Representative wild-type, akt2-1 and akt2-1+pGC1:AKT2 complementation plants grown for 7 weeks (A), 9 weeks (B) and 12 weeks (C) under 12-h day/12-h night conditions at normal light intensity (150 µmol m−2 s−1). (D) akt2-1+pGC1:AKT2 developed a similar number of leaves as the akt2-1 knock out plants, but bolting-time was delayed. (B and E) After 9 weeks, wild-type plants were at an advanced bolting stage, akt2-1 plants had started bolting, but only initial signs of bolting were visible in akt2-1+pGC1:AKT2 plants. (C and F) At 12 weeks, akt2-1 plants had caught up with the wild-type and akt2-1+pGC1:AKT2 was just starting to bolt, although rosette-leaves were showing clear signs of senescence. For the generation of akt2-1+pGC1:AKT2, the AKT2 cDNA was fused to the guard cell-specific GC1 promoter26 kindly provided by J.I. Schroeder, San Diego. The pGC1:AKT2 construct was cloned into pGreen0229-35S by replacing the 35S promoter and then transformed into the akt2-1 knockout plant. All seeds were cold-treated for 24 h at 4°C. Plants were grown on artificial substrate (type GS-90, Einheitserde). After 2 weeks, seedlings were transferred to single pots. Plants were grown in 60% relative humidity at 21°C during the day and 18°C at night. Phenotypical analyses were done in the middle of the day. Data are shown as means ± SD of n ≥ 9 plants. Statistical analyses using Student's t test: (D, WT/akt2-1: p < 2e-08; D, WT/pGC-AKT2: p < 2e-08; D, akt2-1/pGC-AKT2: p < 5e-03; E, WT/akt2-1: p < 4e-06; E, WT/pGC-AKT2: p < 1e-10; E, akt2-1/pGC-AKT2: p < 5e-04; F, WT/akt2-1: p = 0.51; F, WT/pGC-AKT2: p < 1e-10; F, akt2-1/pGC-AKT2: p < 1e-10).
AKT2 expression is especially abundant in phloem tissues and the root stele, both of which are characterized by a poor availability of oxygen.29,30 This local internal hypoxia impairs respiratory activity of the vascular tissue and concomitantly, respiratory ATP production is reduced.31 As a consequence, phloem transport is very susceptible to decreasing oxygen supply to the plant.29,32 It is therefore comprehensible that the above mentioned support by the K+ driving force for sucrose retrieval is especially relevant in the phloem. Indeed Gajdanowicz et al.25 showed that transgenic plants lacking the AKT2 K+ channel were severely impaired in growth when exposed to mild hypoxia (10% v:v), whereas growth of wild-type plants was unaffected by this treatment. These observations illustrate the importance of biochemical flexibility in plant cells to cope with the energetic consequences of the steep oxygen concentration gradients that generally occur in plant stems and roots.
In fact, the role of K+ gradients in driving sugar, amino acid and organic acid transport across plant cell membranes was first suggested several decades ago.33,34 Experimental evidence for this concept was provided by various tests in which pieces of plant tissue were incubated in solutions with different K+ concentrations and pH levels.33,34 Unfortunately, at that time the lack of genetic information to support this hypothesis (e.g., identifying transporter proteins that could provide a molecular mechanism to explain the working mechanism of substrate transport driven by a K+-motive force) resulted in this idea falling into oblivion. Indeed, the unequivocal experimental observation of this new role of K+ gradients in phloem reloading is extremely challenging. Under normal experimental conditions, K+ fluxes and sucrose fluxes are coupled during phloem loading in source tissues and unloading in sink tissues. Nonetheless, computational simulations predict that under certain conditions, a local K+/Suc antiport is also thermodynamically possible. In this antiport system, the energy from the K+ gradient is used to transport Suc into the phloem. This process is only transient; flooding the apoplast with K+ will decrease the K+ gradient. However, the gradient can be maintained for longer if surrounding cells take up the apoplastic K+ for their own use. A K+/Suc antiport will not occur in obvious sink or source tissues since the energy balances in such cells are fundamentally different. Consequently, in these tissues only the coupled symport of K+ and Suc can be observed. However, the computational predictions allowed the identification of the experimental conditions under which the effect of the K+/Suc antiport system is empirically observable at the whole plant level.
An essential role in the regulation of AKT2 is played by (de)phosphorylation events of serine residues at positions S210 and S329. The replacement of both serines by asparagine (AKT2-S210N-S329N) resulted in a K+-selective leak that is locked in a continuously open mode when the channels are expressed in Xenopus oocytes. Under certain conditions, plants expressing the AKT2-S210N-S329N mutation showed growth benefits over wild-type plants; akt2-1+AKT2-S210N-S329N plants reach the generative state faster, possess an increased number of leaves and increased fresh weight (Fig. 2). Intuitively, one would expect a continuously open channel to cause severe problems for the plant, not a benefit as was observed here. We therefore have to postulate that phosphorylation at residues AKT2-S210 and AKT2-S329 is insufficient for converting AKT2 from an inward-rectifying into a non-rectifying channel; other, as yet unknown mechanisms, must contribute to the switch in the AKT2 gating mode. Such a concept would correspond to results that would otherwise be hard to explain. For instance, when both serine residues were replaced by glutamate, the mutant AKT2-S210E-S329E still showed wild-type characteristics.22 The S to E substitution is expected to mimic the phosphorylated state better than the S to N replacement. Furthermore, position AKT2-K197 has a fundamental influence on the AKT2 gating mode.23 AKT2 mutants with that particular lysine substituted with a serine are far less sensitive towards (de)phosphorylation; they display the characteristics of a pure inward-rectifying K+ channel,23 and transgenic Arabidopsis plants expressing AKT2 channels with this substitution showed the characteristics of akt2-1 knock-out plants.25 Initially, it was proposed that the positive charge is important for sensitizing AKT2 to phosphorylation. However, the charge-conserving mutant AKT2-K197R is similar to the charge inverting mutant AKT2-K197D,23 a purely inward-rectifying channel (Fig. 3). We therefore need to take into account that in plants, K197 may also be a target of post-translational modification.35 At present, we can explain the beneficial effect of the AKT2-S210N-S329N mutant on plant growth only by a multiple step regulation of AKT2 (Fig. 4). The double-N mutation would then bypass the phosphorylation step, but AKT2-S210N-S329N could still be deregulated into an inward-rectifying channel. Thus, AKT2 can be considered as a highly specialized Kin channel that can be converted into a leak-like channel by a cascade of post-translational modification steps.
Figure 2.
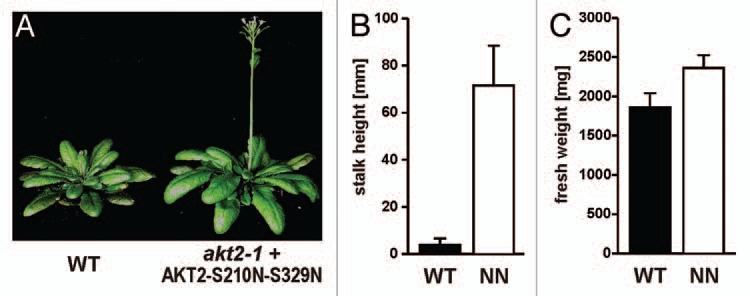
Plants expressing the AKT2-S210N-S329N mutant reach the generative state faster than wild-type plants. The mutant channel AKT2-S210N-S329N was expressed under the control of the native AKT2 promoter in the akt2-1 knock-out background. (A) Photos of representative Arabidopsis thaliana plants grown 7 weeks under short day conditions (12-h day/12-h night, light intensity = 150 µE m−2s−1). Seven weeks after sowing, plants expressing only AKT2-S210N-S329N mutant channels (n = 22) differed significantly (Student's t test, p < 4e-05) from wild-type plants (n = 20) in the height of the main inflorescent stalk (B) and fresh weight (C). At later time points, these differences decrease.25
Figure 3.
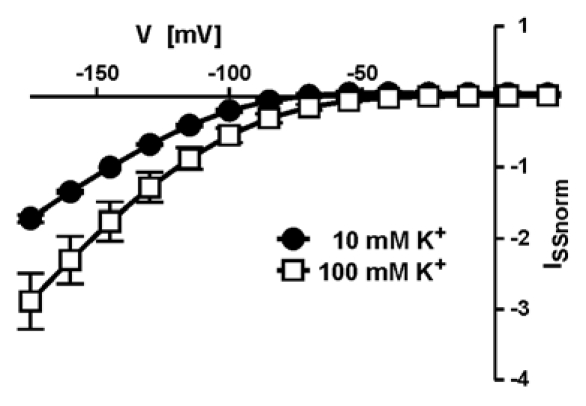
The mutant AKT2-K197R channel is inward-rectifying. Steady-state current-voltage characteristics measured at the end of activation voltage steps. Currents were normalized to the current values measured at −145 mV in 10 mM K+ and are shown as means ± SD (n = 6).
Figure 4.
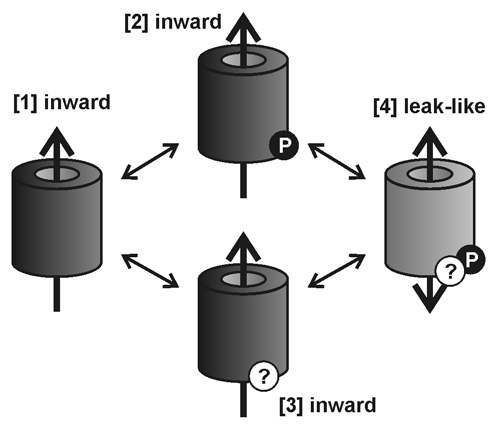
Minimal model for AKT2 gating-mode regulation. To switch AKT2 from an inward-rectifying into a non-rectifying channel, at least two post-translational steps are postulated. (1) Phosphorylation at residues AKT2-S210 and AKT2-S329 (transitions [1]→[2] and [3]→[4]) and (2) a yet unknown modification that most likely involves the residue AKT2-K197 (transitions [1]→[3] and [2]→[4]). Only after both modifications will AKT2 allow the efflux of K+ (state [4]).
Acknowledgments
This work was supported by the Abate Juan Ignacio Molina Excellence Award of the Comisión Nacional de Investigación Científica y Tecnológica de Chile (CONICYT) and the Alexander von Humboldt Foundation (to I.D.), by Grants DR430/5-1, DR430/5-2, DR430/8-1, and by a Heisenberg fellowship of the German Science Foundation (Deutsche Forschungsgemeinschaft) (to I.D.) and by the AleChile project “NiaPoc” (I.D. and W.G.) of the German Academic Exchange Service (DAAD) and CONICYT. E.M. and J.B.T. were supported by the Agropolis Fondation (Réseau Thématique de Recherche Avancée Montpellier, Grant 0803-022). K.S. is a member of the International Max-Planck Research School ‘Primary Metabolism and Plant Growth’ at the University of Potsdam and the Max-Planck Institute of Molecular Plant Physiology. T.A.C. is an EU FP7's Marie-Curie IRG fellow.
Note
The work on AKT2 is dedicated to the memory of Prof. Claude Grignon (1942–2010) who headed for some 30 years the “Biochimie et Physiologie Moléculaire des Plantes” Laboratory in Montpellier, France. There, the biophysical study of the AKT2 channel was initiated by I.D., E.M. and J.B.T. in the late '90s.
References
- 1.Dreyer I, Blatt MR. What makes a gate? The ins and outs of Kv-like K+ channels in plants. Trends Plant Sci. 2009;14:383–390. doi: 10.1016/j.tplants.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Lebaudy A, Hosy E, Simonneau T, Sentenac H, Thibaud JB, Dreyer I. Heteromeric K+ channels in plants. Plant J. 2008;54:1076–1082. doi: 10.1111/j.1365-313X.2008.03479.x. [DOI] [PubMed] [Google Scholar]
- 3.Jeanguenin L, Lebaudy A, Xicluna J, Alcon C, Hosy E, Duby G, et al. Heteromerization of Arabidopsis Kv channel alphα-subunits: Data and prospects. Plant Signal Behav. 2008;3:622–625. doi: 10.4161/psb.3.9.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lebaudy A, Vavasseur A, Hosy E, Dreyer I, Leonhardt N, Thibaud JB, et al. Plant adaptation to fluctuating environment and biomass production are strongly dependent on guard cell potassium channels. Proc Natl Acad Sci USA. 2008;105:5271–5276. doi: 10.1073/pnas.0709732105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirsch RE, Lewis BD, Spalding EP, Sussman MR. A role for the AKT1 potassium channel in plant nutrition. Science. 1998;280:918–921. doi: 10.1126/science.280.5365.918. [DOI] [PubMed] [Google Scholar]
- 6.Mouline K, Véry AA, Gaymard F, Boucherez J, Pilot G, Devic M, et al. Pollen tube development and competitive ability are impaired by disruption of a Shaker K+ channel in Arabidopsis. Genes Dev. 2002;16:339–350. doi: 10.1101/gad.213902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szyroki A, Ivashikina N, Dietrich P, Roelfsema MR, Ache P, Reintanz B, et al. KAT1 is not essential for stomatal opening. Proc Natl Acad Sci USA. 2001;98:2917–2921. doi: 10.1073/pnas.051616698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaymard F, Pilot G, Lacombe B, Bouchez D, Bruneau D, Boucherez J, et al. Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell. 1998;94:647–655. doi: 10.1016/s0092-8674(00)81606-2. [DOI] [PubMed] [Google Scholar]
- 9.Hosy E, Vavasseur A, Mouline K, Dreyer I, Gaymard F, Porée F, et al. The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proc Natl Acad Sci USA. 2003;100:5549–5554. doi: 10.1073/pnas.0733970100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ache P, Becker D, Ivashikina N, Dietrich P, Roelfsema MR, Hedrich R. GORK, a delayed outward rectifier expressed in guard cells of Arabidopsis thaliana, is a K+-selective, K+-sensing ion channel. FEBS Lett. 2000;486:93–98. doi: 10.1016/s0014-5793(00)02248-1. [DOI] [PubMed] [Google Scholar]
- 11.Geiger D, Becker D, Vosloh D, Gambale F, Palme K, Rehers M, et al. Heteromeric AtKC1/AKT1 channels in Arabidopsis roots facilitate growth under K+-limiting conditions. J Biol Chem. 2009;284:21288–21295. doi: 10.1074/jbc.M109.017574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duby G, Hosy E, Fizames C, Alcon C, Costa A, Sentenac H, et al. AtKC1, a conditionally targeted Shaker-type subunit, regulates the activity of plant K+ channels. Plant J. 2008;53:115–123. doi: 10.1111/j.1365-313X.2007.03324.x. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, He L, Li HD, Xu J, Wu WH. Potassium channel alpha-subunit AtKC1 negatively regulates AKT1-mediated K+ uptake in Arabidopsis roots under low-K(+) stress. Cell Res. 2010;20:826–837. doi: 10.1038/cr.2010.74. [DOI] [PubMed] [Google Scholar]
- 14.Reintanz B, Szyroki A, Ivashikina N, Ache P, Godde M, Becker D, et al. AtKC1, a silent Arabidopsis potassium channel alpha-subunit modulates root hair K+ influx. Proc Natl Acad Sci USA. 2002;99:4079–4084. doi: 10.1073/pnas.052677799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marten I, Hoth S, Deeken R, Ache P, Ketchum KA, Hoshi T, et al. AKT3, a phloem-localized K+ channel, is blocked by protons. Proc Natl Acad Sci USA. 1999;96:7581–7586. doi: 10.1073/pnas.96.13.7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lacombe B, Pilot G, Michard E, Gaymard F, Sentenac H, Thibaud JB. A Shaker-like K+ channel with weak rectification is expressed in both source and sink phloem tissues of Arabidopsis. Plant Cell. 2000;12:837–851. doi: 10.1105/tpc.12.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Philippar K, Fuchs I, Luthen H, Hoth S, Bauer CS, Haga K, et al. Auxin-induced K+ channel expression represents an essential step in coleoptile growth and gravitropism. Proc Natl Acad Sci USA. 1999;96:12186–12191. doi: 10.1073/pnas.96.21.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ache P, Becker D, Deeken R, Dreyer I, Weber H, Fromm J, et al. VFK1, a Vicia faba K+ channel involved in phloem unloading. Plant J. 2001;27:571–580. [PubMed] [Google Scholar]
- 19.Langer K, Ache P, Geiger D, Stinzing A, Arend M, Wind C, et al. Poplar potassium transporters capable of controlling K+ homeostasis and K+-dependent xylogenesis. Plant J. 2002;32:997–1009. doi: 10.1046/j.1365-313x.2002.01487.x. [DOI] [PubMed] [Google Scholar]
- 20.Xicluna J, Lacombe B, Dreyer I, Alcon C, Jeanguenin L, Sentenac H, et al. Increased functional diversity of plant K+ channels by preferential heteromerization of the shaker-like subunits AKT2 and KAT2. J Biol Chem. 2007;282:486–494. doi: 10.1074/jbc.M607607200. [DOI] [PubMed] [Google Scholar]
- 21.Dreyer I, Michard E, Lacombe B, Thibaud JB. A plant Shaker-like K+ channel switches between two distinct gating modes resulting in either inward-rectifying or “leak” current. FEBS Lett. 2001;505:233–239. doi: 10.1016/s0014-5793(01)02832-0. [DOI] [PubMed] [Google Scholar]
- 22.Michard E, Dreyer I, Lacombe B, Sentenac H, Thibaud JB. Inward rectification of the AKT2 channel abolished by voltage-dependent phosphorylation. Plant J. 2005;44:783–797. doi: 10.1111/j.1365-313X.2005.02566.x. [DOI] [PubMed] [Google Scholar]
- 23.Michard E, Lacombe B, Porée F, Mueller-Roeber B, Sentenac H, Thibaud JB, Dreyer I. A unique voltage sensor sensitizes the potassium channel AKT2 to phosphoregulation. J Gen Physiol. 2005;126:605–617. doi: 10.1085/jgp.200509413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Held K, Pascaud F, Eckert C, Gajdanowicz P, Hashimoto K, Corratgé-Faillie C, Offenborn JN, et al. Calcium-dependent modulation and plasma membrane targeting of the AKT2 potassium channel by the CBL4/CIPK6 calcium sensor/protein kinase complex. Cell Res. 2011 doi: 10.1038/cr.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gajdanowicz P, Michard E, Sandmann M, Rocha M, Correa LG, Ramirez-Aguilar SJ, et al. Potassium (K+) gradients serve as a mobile energy source in plant vascular tissues. Proc Natl Acad Sci USA. 2011;108:864–869. doi: 10.1073/pnas.1009777108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y, Costa A, Leonhardt N, Siegel RS, Schroeder JI. Isolation of a strong Arabidopsis guard cell promoter and its potential as a research tool. Plant Methods. 2008;4:6. doi: 10.1186/1746-4811-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carpaneto A, Geiger D, Bamberg E, Sauer N, Fromm J, Hedrich R. Phloem-localized, proton-coupled sucrose carrier ZmSUT1 mediates sucrose efflux under the control of the sucrose gradient and the proton motive force. J Biol Chem. 2005;280:21437–21443. doi: 10.1074/jbc.M501785200. [DOI] [PubMed] [Google Scholar]
- 28.Deeken R, Geiger D, Fromm J, Koroleva O, Ache P, Langenfeld-Heyser R, et al. Loss of the AKT2/3 potassium channel affects sugar loading into the phloem of Arabidopsis. Planta. 2002;216:334–344. doi: 10.1007/s00425-002-0895-1. [DOI] [PubMed] [Google Scholar]
- 29.van Dongen JT, Schurr U, Pfister M, Geigenberger P. Phloem metabolism and function have to cope with low internal oxygen. Plant Physiol. 2003;131:1529–1543. doi: 10.1104/pp.102.017202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armstrong W, Webb T, Darwent M, Beckett PM. Measuring and interpreting respiratory critical oxygen pressures in roots. Ann Bot. 2009;103:281–293. doi: 10.1093/aob/mcn177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta KJ, Zabalza A, van Dongen JT. Regulation of respiration when the oxygen availability changes. Physiol Plant. 2009;137:383–391. doi: 10.1111/j.1399-3054.2009.01253.x. [DOI] [PubMed] [Google Scholar]
- 32.van Dongen JT, Roeb GW, Dautzenberg M, Froehlich A, Vigeolas H, Minchin PE, et al. Phloem import and storage metabolism are highly coordinated by the low oxygen concentrations within developing wheat seeds. Plant Physiol. 2004;135:1809–1821. doi: 10.1104/pp.104.040980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Bel AJ, van Erven AJ. A model for proton and potassium co-transport during the uptake of glutamine and sucrose by tomato internode disks. Planta. 1979;145:77–82. doi: 10.1007/BF00379930. [DOI] [PubMed] [Google Scholar]
- 34.van Bel AJ, van Erven AJ. Potassium co-transport and antiport during the uptake of sucrose and glutamic acid from the xylem vessels. Plant Science Letters. 1979;15:285–291. [Google Scholar]
- 35.Walsh CT, Garneau-Tsodikova S, Gatto GJ., Jr Protein posttranslational modifications: the chemistry of proteome diversifications. Angew Chem Int Ed Engl. 2005;44:7342–7372. doi: 10.1002/anie.200501023. [DOI] [PubMed] [Google Scholar]



