Abstract
Cis-jasmone is a volatile organic compound emitted constitutively by flowers or leaves of several plant species where it acts as an attractant for pollinators and as a chemical cue for host localization (or avoidance) for insects.1–3 It is also released by some plant species after feeding damage inflicted by herbivorous insects and in this case might serve as a chemical cue for parasitoids to guide them to their prey (so called “indirect defense”).4,5 Moreover, we have recently shown that plants can perceive cis-jasmone and that it acts as a signaling molecule in A. thaliana, inducing a discrete and distinctive suite of genes, of which a large subset is putatively involved in metabolism and defense responses.6 Cytochrome P450s feature prominently in these functional subsets and of these the highest fold change upon cis-jasmone treatment occurred with the cytochrome CYP81D11 (At3g28740).6 Hence this gene was chosen for a more thorough analysis of the potential biological relevance of the cis-jasmone induced defense response. Although the precise function of CYP81D11 remains to be determined, we could previously demonstrate its involvement in the indirect defense response in Arabidopsis, as plants exposed to cis-jasmone ceased to be attractive to the aphid parasitoid Aphidius ervi when this P450 was inactivated by T-DNA insertion mutagenesis.6 Here we report additional experiments which give further support to a role of CYP81D11 in the direct or indirect defense response of A. thaliana.
Key words: cis-Jasmone, Cytochrome P450, indirect defense, tritrophic interactions, volatile signaling
CYP81D11 is Induced by Herbivorous Insects and Bacteria
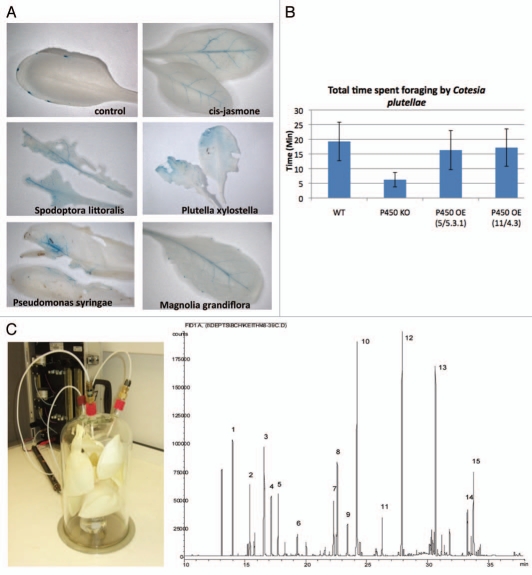
We recently described a transgenic A. thaliana line containing the GUS reporter under the control of the cis-jasmone inducible promoter of CYP81D11 (At3g28740).6 In order to confirm the functionality of the construct, this line was exposed to cis-jasmone and then assayed for GUS expression. As shown in Figure 1A, in the untreated control GUS staining was detected only in very discrete leaf cell types (the hydathodes), whereas in the cis-jasmone-treated leaves GUS staining was mainly detected in the vascular tissue. We then challenged this promoter: GUS line with larvae of either the diamond-back moth Plutella xylostella, a specialist Brassicaceae feeder, or the generalist-herbivore Spodoptora littoralis. In both cases, herbivore induced damage led to the activation of CYP81D11, as can be seen in Figure 1A. In the case of the P. xylostella induced damage, GUS staining was mainly detected around the feeding site and slightly in the vascular tissue, whereas feeding by S. littoralis led to induction mainly in the vasculature, similar to the induction by cis-jasmone. Infection of A. thaliana with the bacterial pathogen Pseudomonas syringae (DC3000) resulted in GUS staining coinciding with bacteria induced lesions and in the vasculature. These data clearly suggest that CYP81D11 is responsive to various biological stresses.
Figure 1.
(A) CYP81D11 expression is induced by biological stresses. Larvae of P. xylostella and S. littoralis were allowed to feed for 48 h on 8-week-old plants of A. thaliana plants containing the CYP81D11 promoter::GUS reporter line and leaves were then stained for GUS. Alternatively, the reporter lines were sprayed with a P. syringae (DC 3000) bacterial solution containing 106 cfu/ml in 10 mM MgCl2 and after 5 days leaves showing lesions were harvested and tested for GUS expression. In a separate experiment the reporter lines were kept for 48 h in an air tight chamber with one Magnolia grandiflora flower and then leaves were isolated and stained. (B) CYP81D11 expression is required for the arrestment of foraging C. plutellae: walking parasitoids (n = 8) were observed individually after being released directly onto the center of individual Arabidopsis plants of different genotype. Mean time spent on plants before flying off was significantly shorter on CYP81D11 knock-out plants (p < 0.05). (C) The most abundant component of the volatiles emitted from Magnolia grandiflora flowers is cis-jasmone. GC-traces, on an HP-1 column, of volatiles sampled over a period of 12 h from flowers. Peaks were tentatively identified by GC-mass spectrometry as 1 α-pinene, 2 β-pinene, 3 benzyl alcohol, 4 limonene, 5 (E)-ocimene, 6 linalool, 7 α-terpineol, 8 verbenone, 9 citronellol, 10 geraniol (15%), 11 methyl decanoate, 12 cis-jasmone (17%), 13 methyl dodecanoate (8%), 14 unknown, 15 methyl tetradecanoate. The identities of cis-jasmone and geraniol were confirmed by peak enhancement by co-injection on two columns of different polarities.
CYP81D11 Plays a Role in Tritrophic Interactions
Barker et al. described the suitability of A. thaliana as a model for investigating host plant-P. xylostella-Cotesia plutellae interactions.7 As P. xylostella larval feeding leads to the activation of CYP81D11, we investigated whether this cytochrome could be involved in interactions with the diamondback moth parasitoid C. plutellae. A. thaliana WT, CYP81D11 knock-out and overexpressing lines were tested in a foraging behavior bioassay with C. plutellae and as shown in Figure 1B, foraging time of the parasitoid was significantly decreased when the cytochrome P450 was not functional. As CYP81D11 is consitutively expressed in the hydathodes (Fig. 1A), we would infer that this is the site of synthesis of the arrestant semiochemical for C. plutellae in the un-induced state. That hydathodes can function as organs where semiochemical synthesis occurs has been demonstrated by Ro et al. who could localise terpene synthases responsible for γ-bisabolene synthesis to these organs. However, the CYP81D11 overexpressing lines were not more attractive to C. plutellae than the wild-type which could be explained by a lack of substrate for CYP81D11 in the tissues where it is not normally expressed.
CYP81D11 is Induced by cis-jasmone Emitted by Another Plant
Conveyance of information between plants via release of volatile compounds is a potentially controversial subject. Several cases have been described where chemical information emitted from wounded plants has an effect on undamaged plants.10–12 cis-jasmone is known to be a prominent component in the floral bouquet emitted constitutively from flowers of Jasminum officinale or Magnolia grandiflora.13 Volatiles were collected from one freshly picked M. grandiflora flower obtained from trees growing on the Rothamsted estate and analyzed using GC and GC-MS. As shown in Figure 1C, we could identify cis-jasmone as the major component. We put one cut M. grandiflora flower together with 8-week-old Arabidopsis CYP81D11 promoter::GUS reporter plants in an airtight glass tank for 48 h, after which leaves from the Arabidopsis lines were stained for GUS activity. As can be seen in Figure 1A, GUS staining replicated the results obtained by exposure to cis-jasmone alone. This result clearly demonstrates that A. thaliana can respond to cis-jasmone emitted by a neighboring plant and there remains the possibility that floral signaling to the vegetative tissue, even of other plants, occurs naturally. Although we could show that cis-jasmone emitted by M. grandiflora flowers is inducing CYP81D11 expression in a neighboring A. thaliana plant, it remains to be determined if cis-jasmone released by wounded plants is capable of inducing this P450 as well.
Conclusions
cis-jasmone has recently been identified as a “plant activator” capable of switching on plant defense prior to attack and its potential application has already been demonstrated in field experiments.14–16 Using the model plant A. thaliana, we provided further evidence for the defense-inducing properties of this volatile compound by showing that genes that are activated by cis-jasmone are also responsive to naturally occurring biotic stresses and are involved in an enhanced performance of foraging natural enemies of the damage-inducing herbivore.
Acknowledgments
Rothamsted Research receives grant-aided support from BBSRC (UK).
References
- 1.El-Sayed AM, Mitchell VJ, McLaren GF, Manning LM, Bunn B, Suckling DM. Attraction of New Zealand Flower Thrips, Thrips obscuratus, to cis-Jasmone, a volatile identified from Japanese Honeysuckle flowers. J Chem Ecol. 2009;35:656–663. doi: 10.1007/s10886-009-9619-3. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka K, Uda Y, Ono Y, Nakagawa T, Suwa M, Yamaoka R, et al. Highly selective tuning of a silkworm olfactory receptor to a key mulberry leaf volatile. Curr Biol. 2009;9:881–890. doi: 10.1016/j.cub.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 3.Schlotzhauer WS, Pair SD, Horvart RJ. Volatile constituents from flowers of Japanese Honeysuckle (Lonicera japonica) J Agri Food Chem. 1996;44:206–209. [Google Scholar]
- 4.Loughrin JH, Manukian A, Heath RR, Tumlinson JH. Volatiles emitted by different cotton varieties damaged by feeding Beet Armyworm larvae. J Chem Ecol. 1995;21:1217–1227. doi: 10.1007/BF02228321. [DOI] [PubMed] [Google Scholar]
- 5.Degen T, Dillmann C, Marion-Poll F, Turlings TCJ. High genetic variability of herbivore-induced volatile emission within a broad range of maize inbred lines. Plant Physiol. 2004;135:1928–1938. doi: 10.1104/pp.104.039891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matthes MC, Bruce TJA, Ton J, Verrier PJ, Pickett JA, Napier JA. The transcriptome of cis-jasmone-induced resistance in Arabidopsis thaliana and its role in indirect defense. Planta. 2010;232:1163–1180. doi: 10.1007/s00425-010-1244-4. [DOI] [PubMed] [Google Scholar]
- 7.Barker JE, Poppy GM, Payne CC. Suitability of Arabidopsis thaliana as a model for host plant—Plutella xylostella-Cotesia plutellae interactions. Ent Exp Appl. 2007;122:17–26. [Google Scholar]
- 8.Ro DK, Ehlting J, Keeling CI, Lin R, Mattheus N, Bohlman J. Microarray expression profiling and functional characterisation of AtTPS genes: Duplicated Arabidopsis thaliana sesquiterpene synthase genes At4g13280 and At4g1330 encode root-specific and wound-inducible (Z)-γ-bisabolene synthases. Archives Biochem Biophys. 2006;448:104–116. doi: 10.1016/j.abb.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Dicke M, Bruin J. Chemical information transfer between plants: back to the future. Biochem Syst Ecol. 2001;29:981–994. [Google Scholar]
- 10.Dicke M, Agrawal AA, Bruin J. Plants talk, but are they deaf? Trends Plant Sci. 2003;9:403–405. doi: 10.1016/S1360-1385(03)00183-3. [DOI] [PubMed] [Google Scholar]
- 11.Baldwin IT, Halitschke R, Paschold A, von Dahl CC, Preston CA. Volatile signaling in plant-plant interactions: “Talking trees” in the genomics era. Science. 2006;311:812–815. doi: 10.1126/science.1118446. [DOI] [PubMed] [Google Scholar]
- 12.Dicke M, Baldwin IT. The evolutionary context for herbivore-induced plant volatiles: Beyond the ‘cry for help’. Trends Plant Sci. 2010;15:167–175. doi: 10.1016/j.tplants.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Raguso RA. Why are some floral nectars scented? Ecology. 2004;85:1486–1494. [Google Scholar]
- 14.Pickett JA, Bruce TJA, Chamberlain K, Hassanali A, Khan ZR, Matthes MC, et al. Plant volatiles yielding new ways to exploit plant defense. Chemical Ecology: From Gene to Ecosystem. 2006:161–173. [Google Scholar]
- 15.Pickett JA, Birkett MA, Bruce TJA, Chamberlain K, Gordon-Weekes R, Matthes MC, et al. Developments in aspects of ecological phytochemistry: The role of cis-jasmone in inducible defense systems in plants. Phytochemistry. 2007;68:2937–2945. doi: 10.1016/j.phytochem.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 16.Bruce TJA, Martin JL, Pickett JA, Pye BJ, Smart LE, Wadhams LJ. Cis-Jasmone treatment induces resistance in wheat plants against grain aphid, Sitobion avenae (Fabricius) (Homoptera: Aphididae) Pest Management Sci. 2003;59:1031–1036. doi: 10.1002/ps.730. [DOI] [PubMed] [Google Scholar]