Abstract
In cyanobacteria and chloroplasts, thylakoids are the complex internal membrane system where the light reactions of oxygenic photosynthesis occur. In plant chloroplasts, thylakoids are differentiated into a highly interconnected system of stacked grana and unstacked stroma membranes. In contrast, in cyanobacteria, the evolutionary progenitors of chloroplasts, thylakoids do not routinely form stacked and unstacked regions, and the architecture of the thylakoid membrane systems is only now being described in detail in these organisms. We used electron tomography to examine the thylakoid membrane systems in one cyanobacterium, Cyanothece sp. ATCC 51142. Our data showed that thylakoids form a complicated branched network with a rudimentary quasi-helical architecture in this organism. A well accepted helical model of grana-stroma architecture of plant thylakoids describes an organization in which stroma thylakoids wind around stacked granum in right-handed spirals. Here we present data showing that the simplified helical architecture in Cyanothece 51142 is lefthanded in nature. We propose a model comparing the thylakoid membranes in plants and this cyanobacterium in which the system in Cyanothece 51142 is composed of non-stacked membranes linked by fret-like connections to other membrane components of the system in a limited left-handed arrangement.
Key words: cyanobacteria, Cyanothece 51142, thylakoid membrane, electron tomography, chloroplast
Thylakoid Organization in Plants and Cyanobacteria
In cyanobacteria, algae and plants, the light reactions of photosynthesis are performed by large protein complexes embedded in the thylakoid membranes. In eukaryotic plants and algae, thylakoid membranes reside in chloroplasts, semi-autonomous organelles of prokaryotic origin. An early cyanobacterium, or cyanobacterial ancestor, was almost certainly the evolutionary precursor of chloroplasts via an endosymbiotic event involving the engulfment of a photosynthetic cell by a mitochondria-containing eukaryote.1 Despite this common ancestry, over evolutionary time the photosynthetic membrane systems in plants and cyanobacteria have evolved into architecturally dissimilar forms. Understanding in detail the morphology and the evolutionary history of these extraordinary membrane systems, upon which oxygen-requiring life depends, is a topic of outstanding interest in cell biology.
In higher plant chloroplasts, thylakoid membranes have a distinct architecture, forming an intricate network of stacks of flattened or appressed lamellae called grana that are connected by unstacked stroma thylakoids that traverse the chloroplast stroma matrix. The details of the three-dimensional arrangement of grana and stroma thylakoids have been debated for years, and different models have been proposed (reviewed in refs. 2 and 3). The foremost of these, the helical model, was originally based on serial-section electron microscopy data collected over decades and describes stroma thylakoids as joining grana at numerous frets or junctional slits or connections, forming a stair-step arrangement in which multiple right-handed helixes of stroma thylakoids wind around a granum. Together, the grana and stroma thylakoids enclose a single lumenal space.2,4,5 Recent studies and discussions have focused on the precise details of the architecture of this system. Importantly, some recent studies have taken advantage of significant technical advances in 3D data acquisition and analysis, employing electron tomography followed by computer-aided model generation. In particular, Shimoni et al.6 analyzed electron tomographic data of chloroplast thylakoid membranes in lettuce leaves and proposed a model significantly at odds with the helical model: grana membranes are formed from bifurcations of stromal sheets, so that entry and exit of the stroma membranes from the grana occur in the same plane, with no helical component. This study was followed by a refinement of the helical model,7 and subsequent discussions of the validity of the two models.8,9 Most recently a study by Austin and Staehelin10 examining tomographic data of multiple complete grana stacks and surrounding stroma thylakoids has resolved some of this controversy by confirming the basic tenants of the helical model. From these data, it is apparent that stroma thylakoids are analogous to threads on a screw that coil in right-handed helices around the central column, the granum.
While the organization of thylakoid membranes into grana and stroma regions is universally observed in mature land plant chloroplasts, cyanobacterial thylakoid membranes have not been found to form grana stacks connected by stroma thylakoids, and appression has been noted in only a few strains. In fact, only within the past few years has work using tomographic data shown that thylakoid membranes are connected by channels or bridging membranes in some cyanobacterial strains.11,12 Our recent work13 examining the thylakoid membrane system in Cyanothece 51142 depicted, for the first time, a membrane system in a cyanobacterium with strong similarities to that in plants: a highly interconnected network composed by branching membranes enclosing a single lumenal space. Furthermore, by examining the arrangement of branch points within the cell, we discovered a rudimentary helical architecture in the thylakoid membranes of Cyanothece 51142. Here we describe our additional findings regarding this helical architecture, and discuss its implications in cyanobacteria.
Handedness of the Thylakoid Arrangement in Cyanothece 51142
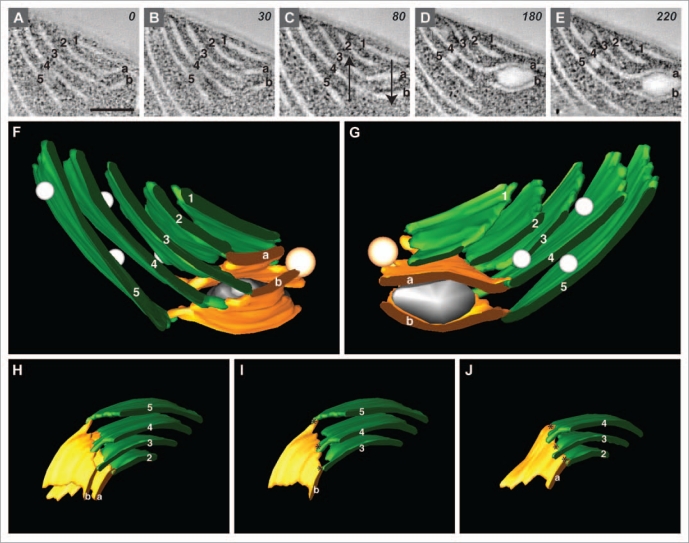
To examine the thylakoid membranes of Cyanothece 51142 cells, we prepared samples for electron microscopy by high-pressure freezing followed by freeze substitution, and used serial electron tomography and montaging to examine and model the components of cyanobacterial cells. A region of the thylakoid membrane system from a cell harvested at the D10 timepoint (after 10 h of growth in dark) of the diurnal cycle and prepared in this manner is shown in Figure 1. We found that the majority of membranes were separated from each other by approximately 50 nm, and did not form stacks or appressed areas, but could be closely associated with inclusion bodies. This architecture is similar to what we reported in our examination of cells from the L10 time point.13 In tomographic slice images, the thylakoid membranes appeared sheet-like and the areas where the membranes branched could be clearly observed (Fig. 1A–E). In order to show the helical arrangement in the tomographic slices, membrane regions were labeled with numbers and letters; in Figure 1A–E, the regions on the left are numbered and the regions on the right are lettered. By following the rearrangements to the letter-number pairs while moving through the tomogram, it was apparent that the membrane regions to the left (numbered) advanced upward relative to the regions on the right (lettered) (see arrows in Fig. 1C).
Figure 1.
Handedness of thylakoid membranes in Cyanothece 51142. (A–E) Tomographic slice images (composed from 3 superimposed serial 2 nm slices) taken through the tomogram at z = 0, 30, 80, 180 and 220 slice intervals. Membrane regions are labeled (1–5) and (a and b). Arrows in (C) show the direction of the membrane rearrangements through the tomogram. Bar = 200 nm. (F and G) Model generated from the tomographic data shown in (A–E); (G) is rotated 180 degrees relative to (F). (H) Model derived from the model shown in (F and G); the model has been rotated and inclusion bodies have been removed for clarity. (I and J) Dissected images of the model shown in (H); (I) shows only the numbered membrane regions connected to membrane “b;” (J) shows only the numbered membrane regions connected to membrane “a.” Asterisks designate the junctional connections.
We used the IMOD software to model the membranes and inclusion bodies in this area to more clearly illustrate this organization (Fig. 1F and G). We rendered the numbered membranes in green and the lettered membranes in orange; however, spatial overlapping obscured some of the details of how the green and orange regions were arranged in the complete model. We therefore pared down the model to only the relevant membranes (Fig. 1H), and next showed only the numbered membranes connected to membrane “b” (Fig. 1I) and the numbered membranes connected to membrane “a” (Fig. 1J). In the case of both membranes “a” and “b,” the green numbered membranes are connected by thin flattened membrane tubules (designated by the asterisks). These tubules appear similar to the junctional connections between stroma thylakoids and granum thylakoids, also being highly variable in size as recently found to be the case in plants.10 It was apparent that these tubules join the green numbered membranes in a stair-step arrangement that ascends to the left, as in a limited left-handed spiral (Fig. 1I and J). Our analysis of eight such regions in Cyanothece 51142 showed that all of them had the same left-handedness. These examples encompassed multiple cells from different time points and from independently grown cultures, suggesting that this architecture is widespread in Cyanothece 51142.
We then compared these data to previous studies examining the helical arrangement of chloroplast membranes. It has been shown that the association of stroma thylakoids with grana forms only right-handed helices,4 so that stroma thylakoids always ascend to the right when spiraling around grana stacks. In such data, the membrane regions to the right always advanced upward when moving through subsequent slice images.5,10 Hence, Cyanothece 51142 membranes have the opposite helical arrangement compared to chloroplast membranes.
We have created schematic models to compare the morphology of chloroplast and Cyanothece 51142 thylakoid membranes (Fig. 2). In chloroplasts, stacked thylakoids of the granum are connected to stroma thylakoids that spiral around the granum, ascending to the right (Fig. 2A). In Cyanothece 51142, thylakoid membranes are not stacked, but are connected by membrane tubules, analogous to junctional slits, arranged like stair steps ascending to the left (Fig. 2B).
Figure 2.
Schematic model of membrane architecture in chloroplasts and cyanobacteria. (A) Stroma thylakoids (orange, ST) associate with grana thylakoids (green, GT) with a right-handed orientation. (B) Thylakoid membranes (TM) in Cyanothece 51142 have limited left-handedness. Junctional connections are in dark green.
An important difference between these two systems is the extent of the helical architecture. In chloroplasts, grana typically contain 10–20 circular membrane layers that are tightly appressed to within ∼2.5 nm of each other, and are surrounded by multiple spiraling stroma thylakoids.14 In Cyanothece 51142, the helical architecture is much more limited. Membranes are not differentiated into flattened discs and sheets, but rather all membranes are sheet-like and are uniformly separated by ∼50 nm. Moreover, much fewer membrane sheets are involved, typically only 2–3 membranes forming the grana-like region and 1–2 connecting membranes (see the green and yellow membrane regions, respectively, in Fig. 1I and J). The tubular membrane connections, however, do seem to be similar in shape and size in the two systems. To date, Cyanothece 51142 is the only cyanobacterium in which any helical architecture has been reported, compared to the universality of grana-stroma architecture in higher plants.
Helical membrane organization might have evolved as a means to efficiently package a large amount of membranes into a limited cellular space. We have shown that the membrane system in Cyanothece 51142 is very dense, with a larger number of membrane layers compared to what has been observed in other cyanobacteria.13 It is possible that helical organization originated as a primarily left-handed architecture, followed by the development of grana and the attendant right-handedness of the grana-stroma architecture at a later time. It is tempting to speculate, particularly in light of the recent analysis of stroma thylakoids,10 that the chloroplast stroma thylakoid arrangement and the thylakoid architecture in Cyanothece 51142 can be compared directly, with the addition of grana in chloroplasts. Tomographic data showed that stroma thylakoids were not uniformly sheet-like in nature, nor were they thin strips of membrane that extended from the granum and met in the stroma, but that these membranes have a complex arrangement that has not previously been described.10 In models based on tomographic data, stroma membranes are sheets of varying widths that often display disruption in their continuity and subsequent fusion to other stroma membranes. Furthermore, stroma thylakoids have a complicated architecture depending on their proximity to neighboring grana stacks.
Collectively, tomographic studies of plant and cyanobacterial thylakoid membrane architecture have shown these membrane systems at a level of detail previously unknown. Further examination of the thylakoid membranes in both plants and cyanobacteria will doubtlessly reveal additional details in the architecture of these complex membrane systems, and shed light on the complicated evolutionary history of these organisms.
Acknowledgments
We thank members of the Pakrasi lab for collegial discussions. This work is part of a Membrane Biology EMSL Scientific Grand Challenge project at the W.R. Wiley Environmental Molecular Sciences Laboratory, a national scientific user facility sponsored by the US Department of Energy's Office of Biological and Environmental Research (BER) program located at Pacific Northwest National Laboratory. PNNL is operated for the Department of Energy by Battelle. This work was also partially supported as part of the Photosynthetic Antenna Research Center (PARC), an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under Award Number DE-SC 0001035.
References
- 1.Gray MW. Evolution of organellar genomes. Curr Opin Genet Dev. 1999;9:678–687. doi: 10.1016/s0959-437x(99)00030-1. [DOI] [PubMed] [Google Scholar]
- 2.Mustardy L, Garab G. Granum revisited. A three-dimensional model—where things fall into place. Trends Plant Sci. 2003;8:117–122. doi: 10.1016/S1360-1385(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 3.Nevo R, Chuartzman SG, Tsabari O, Reich Z, Charuvi D, Shimoni E. Architecture of thylakoid membrane networks. In: Wada H, Murata N, editors. Lipids in Photosynthesis: Essential and regulatory functions. Springer Science and Business Media; 2009. pp. 295–328. [Google Scholar]
- 4.Paolillo DJ., Jr The three-dimensional arrangement of intergranal lamellae in chloroplasts. J Cell Sci. 1970;6:243–255. doi: 10.1242/jcs.6.1.243. [DOI] [PubMed] [Google Scholar]
- 5.Brangeon J, Mustardy L. The ontogenetic assembly of intra-chloroplastic lamellae viewed in 3-dimension. Biol Cellul. 1979;36:71–780. [Google Scholar]
- 6.Shimoni E, Rav-Hon O, Ohad I, Brumfeld V, Reich Z. Three-dimensional organization of higher-plant chloroplast thylakoid membranes revealed by electron tomography. Plant Cell. 2005;17:2580–2586. doi: 10.1105/tpc.105.035030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mustardy L, Buttle K, Steinbach G, Garab G. The three-dimensional network of the thylakoid membranes in plants: quasihelical model of the granumstroma assembly. Plant Cell. 2008;20:2552–2557. doi: 10.1105/tpc.108.059147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brumfeld V, Charuvi D, Nevo R, Chuartzman S, Tsabari O, Ohad I, et al. A note on three-dimensional models of higher-plant thylakoid networks. Plant Cell. 2008;20:2546–2549. doi: 10.1105/tpc.108.062299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garab G, Mannella CA. Reply: On three-dimensional models of higher-plant thylakoid networks: Elements of consensus, controversies and future experiments. Plant Cell. 2008;20:2549–2551. doi: 10.1105/tpc.108.062299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Austin JR, Staehelin LA. Three-dimensional architecture of grana and stroma thylakoids of higher plants as determined by electron tomography. Plant Physiol. 2011;4:1601–1611. doi: 10.1104/pp.110.170647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nevo R, Charuvi D, Shimoni E, Schwarz R, Kaplan A, Ohad I, et al. Thylakoid membrane perforations and connectivity enable intracellular traffic in cyanobacteria. EMBO J. 2007;26:1467–1473. doi: 10.1038/sj.emboj.7601594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ting CS, Hsieh C, Sundararaman S, Mannella C, Marko M. Cryo-electron tomography reveals the comparative three-dimensional architecture of Prochlorococcus a globally important marine cyanobacterium. J Bacteriol. 2007;189:4485–4493. doi: 10.1128/JB.01948-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liberton M, Austin JR, Berg RH, Pakrasi HB. Unique thylakoid membrane architecture of a unicellular N2-fixing cyanobacterium revealed by electron tomography. Plant Physiol. 2010;4:1656–1666. doi: 10.1104/pp.110.165332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mullineaux CW. Function and evolution of grana. Trends Plant Sci. 2005;10:521–525. doi: 10.1016/j.tplants.2005.09.001. [DOI] [PubMed] [Google Scholar]