Abstract
Auxin is an indispensable hormone throughout the lifetime of nearly all plant species. Several aspects of plant growth and development are rigidly governed by auxin, from micro to macro hierarchies; auxin also has a close relationship with plant-pathogen interactions. Undoubtedly, precise auxin levels are vitally important to plants, which have many effective mechanisms to maintain auxin homeostasis. One mechanism is conjugating amino acid to excessive indole-3-acetic acid (IAA; main form of auxin) through some GH3 family proteins to inactivate it. Our previous study demonstrated that GH3-2 mediated broad-spectrum resistance in rice (Oryza sativa L.) by suppressing pathogen-induced IAA accumulation and downregulating auxin signaling. Here, we further investigated the expression pattern of GH3-2 and other GH3 family paralogs in the life cycle of rice and presented the possible function of GH3-2 on rice root development by histochemical analysis of GH3-2 promoter:GUS reporter transgenic plants.
Key words: auxin, GH3 gene, indole-3-acetic acid, Oryza sativa, root
The phytohormone auxin regulates tropism and organ development and influences phyllotaxis, vascular canalization and root patterning by exerting its effect on cell division, elongation and differentiation in plants.1,2 Indole-3-acetic acid (IAA) is the most widespread form of auxin in most plants. Supraoptimal or insufficient concentration of auxin will cause plants to exhibit abnormal phenotypes. 3-9 Auxin homeostasis is partly sustained by the GH3 gene family, a supervisor of the fluctuation of auxin. Most GH3 genes contain auxin-responsive cis-acting elements (AuxRE) in their promoter regions and react rapidly and transiently to auxin signaling.1 Nineteen GH3 paralogs have been discovered in Arabidopsis.10 According to the phylogenetic relationship and acyl acid substrate preference, these genes are classified into three groups (I, II and III), which catalyze the formation of jasmonates, salicylic acid, 4-substituted benzoates or IAA acyl acid amido conjugates.11,12 The rice GH3 gene family includes 13 paralogs, 4 belonging to group I (GH3-3, -5, -6 and -12) and 9 to group II (GH3-1, -2, -4, -7, -8, -9, -10, -11 and -13); group III GH3 is absent in rice.10 Rice GH3-1, -2, -8 and -13 paralogs have been biochemically confirmed to have IAA-amido synthetase activity by in vivo or in vitro assays.6–9 It is believed that other GH3 group II paralogs in rice may also possess this enzymatic activity. But why does rice have such a functionally redundant group of GH3 proteins, which disobeys the economic principle? The explanation could be based on the different temporal and spatial expression of the genes encoding these proteins.
Expression Profile Analysis of GH3 Genes in the Life Cycle of Rice
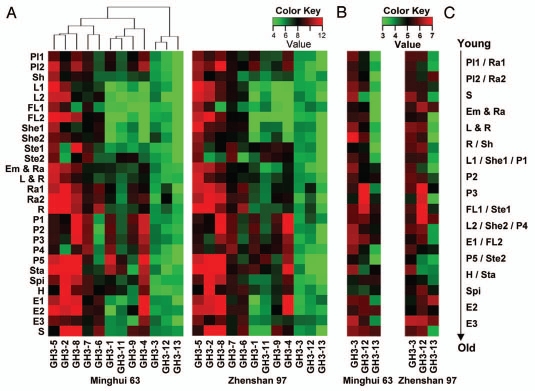
We performed a comprehensive expression analysis of the GH3 family paralogs in rice by processing the genome-wide microarray data from 28 tissues collected throughout the life cycle of two rice varieties, Minghui 63 and Zhenshan 97, in the Collections of Rice Expression Profiling (CREP; crep.ncpgr.cn) database.13 The sequences of rice GH3 genes used in quarrying were obtained from the Rice Genome Annotation Project database (rice.plantbiology.msu.edu). Probes for 12 of the 13 GH3 family genes were identified in the microarray database, except for GH3-10. The logarithm of average signal values for the 12 GH3 genes in 28 tissues are shown in Figure 1.
Figure 1.
Expression profiles of rice GH3 genes in 28 tissues covering the whole life cycle of Minghui 63 and Zhenshan 97 from microarray data. The tissues are displayed at the left side: Pl1, plumule at 48 hours after emergence under light; Pl2, plumule at 48 h after emergence under dark; Sh, shoot of seedling with 2 tillers; L1, leaf at secondary-branch primordium stage; L2, leaf at 4–5-cm young panicle stage; FL1, flag leaf at 5 days before heading; FL2, flag leaf at 14 days after heading; She1, sheath at secondary-branch primordium stage; She2, sheath at 4–5-cm young panicle stage; Ste1, stem at 5 days before heading; Ste2, stem at heading stage; Em & Ra, embryo and radicle at 3 days after germination; L & R, leaf and root at 3-leaf stage; Ra1, radicle at 48 h after emergence under light; Ra2, radicle at 48 h after emergence under dark; R, root of seedling with 2 tillers; P1, panicle at secondary-branch primordium stage; P2, panicle at pistil and stamen primordium differentiation stage; P3, panicle at pollen-mother cell formation stage; P4, panicle at 4–5-cm young panicle stage; P5, panicle at heading stage; Sta, stamen at 1 day before flowering; Spi, spikelet at 3 days after pollination; H, hull at 1 day before flowering; E1, endosperm at 14 days after heading; E2, endosperm at 7 days after pollination; E3, endosperm at 21 days after pollination; S, germinating seed at 72 h of imbibitions. The color key means the log2 transformation of the average signal values. Red and green denote high and low expression. (A) Hierarchical cluster of expression profile for 12 rice GH3 genes. (B) Increased resolution of color key (3–7 color key) for three genes with weak expression levels. (C) Simplified representation of the appearance order for 28 tissues along the time axis of whole life cycle of rice.
In general, GH3-2, -5 and -8 had extremely high expression levels in most tissues, compared with GH3-3, -12 and -13, which had extremely low expression levels in all tissues examined. GH3-1, -4, -6, -7, -9 and -11 showed moderately high expression levels in specific tissues or developmental stages (Fig. 1).
During four successive young panicle development stages (P1–P4), the expression levels of GH3-2, -4, -8 and -9 declined from early to late periods in both Minghui 63 and Zhenshan 97 and one more gene (GH3-12) showed the similar diminished expression pattern only in Zhenshan 97 (Fig. 1). GH3-2, -5 and -6 sustained lower expression levels in young panicles than in other tissues. In light of the effect of some GH3 on inactivation of auxin, these expression patterns of GH3 genes are in agreement with the observation that the expression of many genes related to auxin signaling decreased continuously during panicle development in the two rice varieties.13 In the mature panicle (P5) and stamen stages connected with spermatogenesis, the expression levels of GH3-2, -4, -5 and -8 were fairly high in both varieties. The similar high expression level in stamen for GH3-8 was also detected in another study.6 GH3-1 had the highest expression level in the mature panicle stage (P5) compared with other tissues in Minghui 63, but its expression dropped sharply in Zhenshan 97. The transcripts of a large number of GH3 genes, such as GH3-2, -4, -5, -6, -8 and -9, were enriched in at least one stage of endosperm and germinating seed.
GH3-5 seemed to be the only gene in this family to be expressed highly and homogeneously in green vegetative tissues like leaf, sheath and stem in both Minghui 63 and Zhenshan 97 (Fig. 1). In two stem tissues (Ste1 and Ste2), GH3-2, -3, -12 and -13 had distinctively low expression levels, but GH3-5 and GH3-7 were expressed at high levels in both varieties. In addition, GH3-8 had an expression preference in the stem tissues of Minghui 63. Interestingly, GH3-2, -6 and -8 showed greater expression levels in older leaf (L2) or flag leaf (FL2) than in younger leaves (L1 and FL1) in both varieties. GH3-2 and -5 occupied the top two spots in GH3s with the highest expression levels in radicles (Ra1 and Ra2) and root (R). Subsequently, GH3-6 and -12 showed relatively higher expression levels in the three root tissues (Ra1, Ra2 and R) than in other tissues. Although all the signal values for GH3-13 were quite low, after increasing the resolution of color key of the data, the relatively high expression level of GH3-13 could be discerned in root tissues of both varieties. This expression feature of GH3-13 is consistent with the results analyzed by reverse transcription-polymerase chain reaction reported previously in reference 8.
Taken together, the rice GH3 family paralogs may be redundant in biochemical functions but diverse in expression patterns with each individual in charge of certain life processes.
GH3-2 in Rice Root Development
According to the microarray data, GH3-2 had the highest expression level in root among the GH3s at 48 h after emergence (Ra1 and Ra2) and seedling at 2-tiller (R) stages and it had a low expression level in stem at 5 days before heading and heading stages (Ste1 and Ste2) in both rice varieties (Fig. 1). To further examine this expression feature, we analyzed GH3-2 expression in root and stem from rice variety Minghui 63 at the booting (panicle development) stage. Compared with its expression in the other five tissues, GH3-2 showed the highest expression level in root and the lowest expression level in stem (Fig. 2). These results led us to associate this expression feature with the curious root phenotype of GH3-2-overexpressing plants (D176UM). The T1 families, D176UM11, D176UM14 and D176UM34, presented segregation in root phenotype 1 week after germination. The positive transgenic plants showed filamentous root systems with a fewer number of lateral roots compared with the negative transgenic plants and the wild-type plants (Fig. 3). Five Arabidopsis GH3 group II paralogs, GH3.1, GH3.2, GH3.5, GH3.9 and GH3.17, have been identified to have influence on root development.14–16 Few rice GH3s have so far been experimentally examined to be involved in root development, except for GH3-13.8 Our results point to a potential role of GH3-2 in rice root development.
Figure 2.
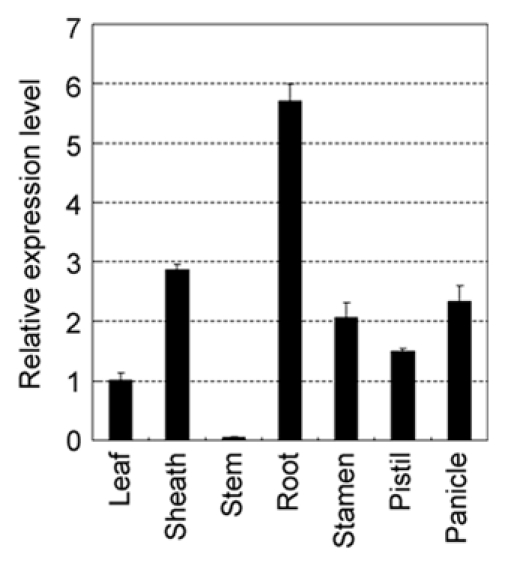
Relative expression level of GH3-2 in different tissues of Minghui 63. All tissue samples were collected at booting stage. Bars represent mean (three replicates) ± standard deviation.
Figure 3.
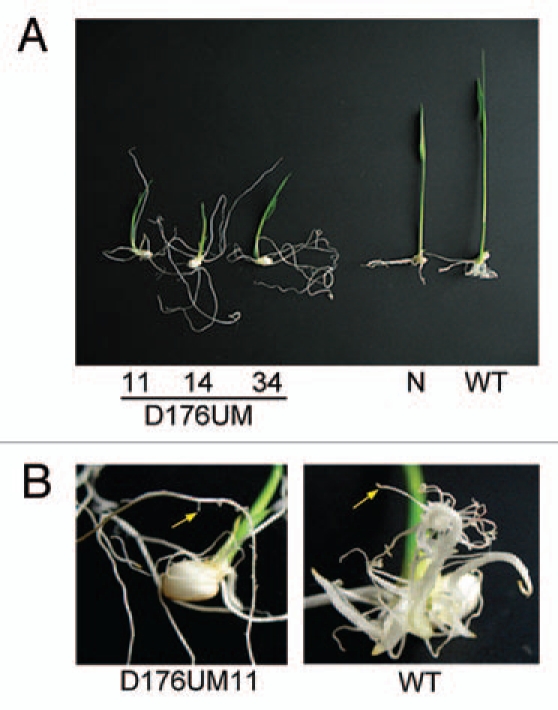
Different root phenotypes of GH3-2-overexpressing plants and wild-type (WT) Mudanjiang 8. (A) GH3-2-overexpressing T1 plants (D176UM 11, D176UM 14 and D176UM 34) at 1 week after germination. N, negative transgenic plant. (B) A close-up photograph of the roots of GH3-2-overexpressing T1 (D176UM 11) and WT plants. Yellow arrows indicate lateral roots.
To further test the inference that GH3-2 regulates root development, we isolated GH3-2 promoter (PGH3-2), which consisted of 1,834 nucleotides and localized on the −1,804 to +30 region (Fig. 4A). The PGH3-2 was fused with the reporter gene β-glucuronidase (GUS). The PGH3-2:GUS construct was transferred into rice variety Zhonghua 11 by Agrobacterium-mediated transformation.17 The roots of positive transgenic plants carrying PGH3-2:GUS were stained for GUS activity. Strong GUS activity in lateral roots and the pericycle where the lateral roots initiated was clearly observed (Fig. 4B–D). In addition, our previous work found an induction of GH3-2 in rice leaf tissues after IAA stimulation.9 Exogenous IAA treatment also caused slightly increased GUS activity in roots of the transgenic plants carrying PGH3-2:GUS (Fig. 4E). It is reported that an Arabidopsis transcription factor MYB96-mediated drought induced lateral root meristem activation via an abscisic acid and auxin signaling crosstalk.18 MYB96 positively regulated a subset of GH3 genes in roots under drought stress and coincidently, MYB96 expression was also initiated in the pericycle. These results give us a hint and impetus for further analysis of the GH3-2-involved signaling pathway regulating rice lateral root development.
Figure 4.
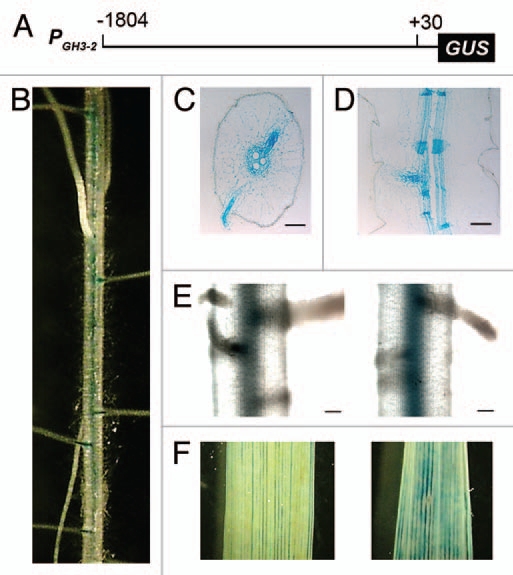
The GUS activity in root of PGH3-2:GUS transgenic plants. (A) The native GH3-2 promoter construct. The DNA fragment located on the −1,804 to +30 region of the GH3-2 gene was fused with the reporter gene β-glucuronidase (GUS). The transcription initiation site is assigned as +1. (B) A photograph of the root of PGH3-2:GUS transgenic plant at seedling stage after GUS staining. (C) A transverse section of the root elongation zone of PGH3-2:GUS transgenic plant. (D) A longitudinal section of the root elongation zone of PGH3-2:GUS transgenic plant. (E) The enhanced GUS activity in root of PGH3-2:GUS transgenic plant after treatment with 100 µM IAA for 2 h. Left is the root of an untreated plant, right is the root of an IAA-treated plant. (F) The enhanced GUS activity in leaf of PGH3-2:GUS transgenic plant after treatment with 100 µM IAA for 2 h. Left is the leaf of an untreated plant, right is the leaf of an IAA-treated plant. Bars = 100 µm in (C) through (E).
Two major rice bacterial pathogens Xanthomonas oryzae pv. oryzae and Xanthomonas oryzae pv. oryzicola infect rice leaf by colonizing xylem vessels and intercellular spaces of the parenchyma cells, respectively.19 Rice fungal pathogen Magnaporthe oryzae is also a foliar pathogen commencing its disease cycle on the surface of leaf cuticle cells.20 We recently demonstrated that X. oryzae pv. oryzae, X. oryzae pv. oryzicola and M. oryzae could secrete IAA as a putative virulence factor during infection and GH3-2 inactivated IAA to mediate a broad-spectrum disease resistance to these pathogens in rice.9 In this work, we used high concentration of IAA to treat rice leaves of PGH3-2:GUS transgenic plants, mimicking and exaggerating the role of IAA secreted by pathogens. An obviously elevated GUS activity in the IAA-treated leaf was confirmed (Fig. 4F). To some extent, this rapid and intensive response of GH3-2 promoter to exogenous IAA in leaf could deepen our understanding of the function of GH3-2 in disease resistance.
Conclusion
In general, the rice GH3 gene family is essential to plant growth and development. A few genes of this family have been confirmed to have IAA amido synthetase biochemical activity. We made a comprehensive whole-life expression profile analysis of 12 rice GH3 genes and pointed out characteristic development stage- and tissue-specific expression patterns of most rice GH3 genes. We also provided an opportunity to obtain more insight into the functions of rice GH3 genes with physiologic significance. In addition, we took GH3-2 as an example to integrate the expression profile message into the concrete gene function exploitation.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (30930063, 30921091).
Abbreviations
- IAA
indole-3-acetic acid
- AuxRE
auxin-responsive cis-acting elements
References
- 1.Woodward AW, Bartel B. Auxin: Regulation, action and interaction. Ann Bot. 2005;95:707–735. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamins R, Scheres B. Auxin: The looping star in plant development. Annu Rev Plant Biol. 2008;59:443–465. doi: 10.1146/annurev.arplant.58.032806.103805. [DOI] [PubMed] [Google Scholar]
- 3.King JJ, Stimart DP, Fisher RH, Bleecker AB. A mutation altering auxin homeostasis and plant morphology in Arabidopsis. Plant Cell. 1995;7:2023–2037. doi: 10.1105/tpc.7.12.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng Y, Dai X, Zhao Y. Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev. 2006;20:1790–1799. doi: 10.1101/gad.1415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park JE, Park JY, Kim YS, Staswick PE, Jeon J, Yun J, et al. GH3-mediated auxin homeostasis links growth regulation with stress adaptation response in Arabidopsis. J Biol Chem. 2007;282:10036–10046. doi: 10.1074/jbc.M610524200. [DOI] [PubMed] [Google Scholar]
- 6.Ding X, Cao Y, Huang L, Zhao J, Xu C, Li X, et al. Activation of the indole-3-acetic acid-amido synthetase GH3-8 suppresses expansin expression and promotes salicylate- and jasmonate-independent basal immunity in rice. Plant Cell. 2008;20:228–240. doi: 10.1105/tpc.107.055657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Domingo C, Andrés F, Tharreau D, Iglesias DJ, Talón M. Constitutive expression of OsGH3.1 reduces auxin content and enhances defense response and resistance to a fungal pathogen in rice. Mol Plant Microbe Interact. 2009;22:201–210. doi: 10.1094/MPMI-22-2-0201. [DOI] [PubMed] [Google Scholar]
- 8.Zhang SW, Li CH, Cao J, Zhang YC, Zhang SQ, Xia YF, et al. Altered architecture and enhanced drought tolerance in rice via the downregulation of indole-3-acetic acid by TLD1/OsGH3.13 activation. Plant Physiol. 2009;151:1889–1901. doi: 10.1104/pp.109.146803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu J, Liu H, Li Y, Yu H, Li X, Xiao J, et al. Manipulating broad-spectrum disease resistance by suppressing pathogen-induced auxin accumulation in rice. Plant Physiol. 2011;155:589–602. doi: 10.1104/pp.110.163774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terol J, Domingo C, Talón M. The GH3 family in plants: genome wide analysis in rice and evolutionary history based on EST analysis. Gene. 2006;371:279–290. doi: 10.1016/j.gene.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Staswick PE, Tiryaki I, Rowe M. Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic and indole-3-acetic acids in an assay for adenylation. Plant Cell. 2002;14:1405–1415. doi: 10.1105/tpc.000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westfall CS, Herrmann J, Chen Q, Wang S, Jez JM. Modulating plant hormones by enzyme action: The GH3 family of acyl acid amido synthetases. Plant Signal Behav. 2010;5:1597–1602. doi: 10.4161/psb.5.12.13941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Xie W, Chen Y, Tang W, Yang J, Ye R, et al. A dynamic gene expression atlas covering the entire life cycle of rice. Plant J. 2010;61:752–766. doi: 10.1111/j.1365-313X.2009.04100.x. [DOI] [PubMed] [Google Scholar]
- 14.Takase T, Nakazawa M, Ishikawa A, Kawashima M, Ichikawa T, Takahashi N, et al. ydk1-D, an auxin-responsive GH3 mutant that is involved in hypocotyl and root elongation. Plant J. 2004;37:471–483. doi: 10.1046/j.1365-313x.2003.01973.x. [DOI] [PubMed] [Google Scholar]
- 15.Staswick PE, Serban B, Rowe M, Tiryaki I, Maldonado MT, Maldonado MC, et al. Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell. 2005;17:616–627. doi: 10.1105/tpc.104.026690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan S, Stone JM. Arabidopsis thaliana GH3.9 influences primary root growth. Planta. 2007;226:21–34. doi: 10.1007/s00425-006-0462-2. [DOI] [PubMed] [Google Scholar]
- 17.Lin Y, Zhang Q. Optimising the tissue culture conditions for high efficiency transformation of indica rice. Plant Cell Rep. 2005;23:540–547. doi: 10.1007/s00299-004-0843-6. [DOI] [PubMed] [Google Scholar]
- 18.Seo PJ, Park CM. Auxin homeostasis during lateral root development under drought condition. Plant Signal Behav. 2009;4:1002–1004. doi: 10.4161/psb.4.10.9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niño-Liu DO, Ronald PC, Bogdanove AJ. Xanthomonas oryzae pathovars: Model pathogens of a model crop. Mol Plant Pathol. 2006;7:303–324. doi: 10.1111/j.1364-3703.2006.00344.x. [DOI] [PubMed] [Google Scholar]
- 20.Ebbole DJ. Magnaporthe as a model for understanding host-pathogen interactions. Annu Rev Phytopathol. 2007;45:437–456. doi: 10.1146/annurev.phyto.45.062806.094346. [DOI] [PubMed] [Google Scholar]