Abstract
Jasmonates (JAs) induce leaf senescence in many plant species. The Arabidopsis F-box protein coronatine insensitive 1 (COI1) is required for various JA-regulated plant responses including plant fertility, defense responses and leaf senescence. However, the molecular basis for COI1-dependent JA-induced leaf senescence remains unknown. In our Plant Physiology paper, we identified a COI1-dependent JA-repressed protein, Rubisco activase (RCA) in Arabidopsis. Further genetic and physiological analyses showed that the COI1-dependent JA repression of RCA correlated with JA-induced leaf senescence, and that loss of RCA led to typical senescence-associated features. Therefore, we suggested that the COI1-dependent JA repression of RCA played an important role in JA-induced leaf senescence. In this addendum, we made a relatively deep discussion on RCA function in JA-induced leaf senescence and JA-mediated defense responses. We also discussed the possible role of JA in plant natural senescence.
Key words: arabidopsis, COI1, jasmonate, leaf senescence, RCA
Jasmonates (JAs), as a plant signal, function in induction of leaf senescence. Exogenous application of JA has been shown to stimulate leaf senescence1–5 and to control a serious of senescence-related genes expression.6,7 The Arabidopsis F-box protein coronatine insensitive 1 (COI1),8 as a JA receptor,9 is essential for JA-induced leaf senescence. Upon JA treatment, the senescence phenotype was observed in the leaves of wild type (WT) but not in the coi1 mutants.3,5
We recently identified a COI1-dependent JA-repressed protein, Rubisco activase (RCA) in Arabidopsis5 (Fig. 1A and B). The transcript level of RCA was also decreased under JA treatment in a COI1-dependent manner, which preceded the reduction of RCA protein5 (Fig. 1A and C). In addition, we found that the levels of RCA transcript and protein were unchanged in the WT leaves treated with synthetic auxin 2,4-dichlorophenoxyacetic acid (2,4-D) (Fig. 1D), confirming that the repressed RCA/RCA expression is rather a specific response to the JA signal than general response to hormone overexposure.
Figure 1.
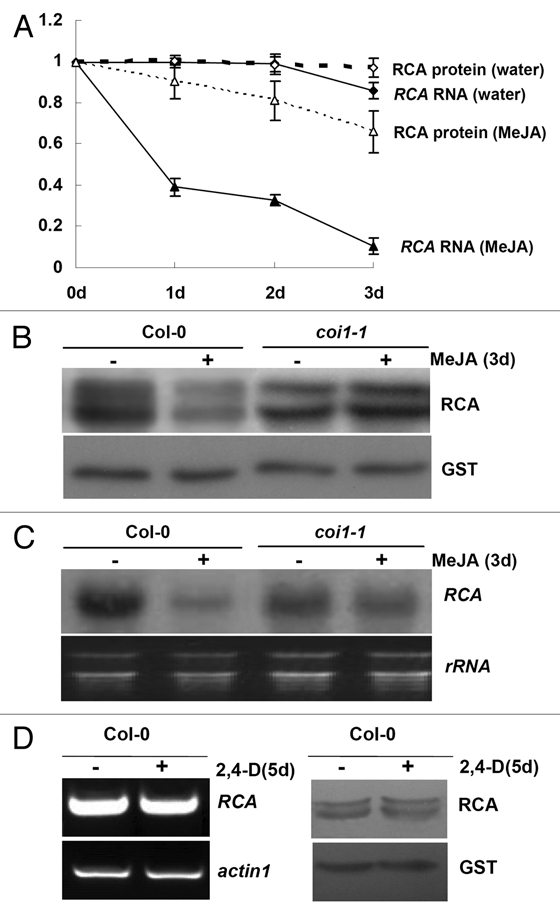
RCA was downregulated at the levels of transcript and protein abundance by JA in a COI1 dependent manner. (A) Quantitative analysis of RCA RNA levels and RCA protein levels in 6-week-old WT and coi1-1 mutant leaves treated with methl jasmonate (MeJA) or water for indicated time-periods. The RCA RNA level and RCA protein level in WT upon water-treatment for 0 days were set to 1 respectively, and the relative RCA RNA levels and RCA protein levels in other samples were calculated accordingly. This figure was the modification to the Figures 3A and 4B in reference 5. (B) Western blot for RCA in 6-week-old WT and coi1-1 mutant leaves treated with MeJA (+) or water (−) for 3 days. The immunoblot was detected with GST antibody as a protein loading control. This figure was the supplementation to the Figure 3A in reference 5. (C) Northern blot for RCA in 6-week-old WT and coi1-1 mutant leaves treated with MeJA (+) or water (−) for 3 days. The EB staining of rRNA was used as loading control. This figure was the supplementation to the Figure 4B in reference 5. (D) Semi-quantitative RT -PCR (left) and western blot for RCA (right) in 6-week-old WT leaves treated with 2,4-D (+) or water (−) for 5 days. The amplified actin1 was shown as an internal control (left). The immunoblot was detected with GST antibody as a protein loading control (right).
We further observed that the COI1-dependent JA-repression of RCA correlated with JA-induced leaf senescence. Upon JA treatment for 5 days, severe senescence-associated features were induced in the WT leaves compared to that in the coi1-1 and coi1-2 mutants5 (Fig. 2A). Simultaneously, the RCA protein level was dramatically reduced in the WT leaves, but not in the coi1 mutants5 (Fig. 2B). Furthermore, we isolated the null mutant rca-1 and the leaky mutant rca-2, and found that these mutants showed typical senescence-related symptoms such as yellowing leaf, lower chlorophyll content, increased expression of senescence-induced genes and decreased expression of senescence-reduced genes at different degrees.5 Thus, we suggested that the COI1-dependent JA repression of RCA played an important role in JA-induced leaf senescence.
Figure 2.
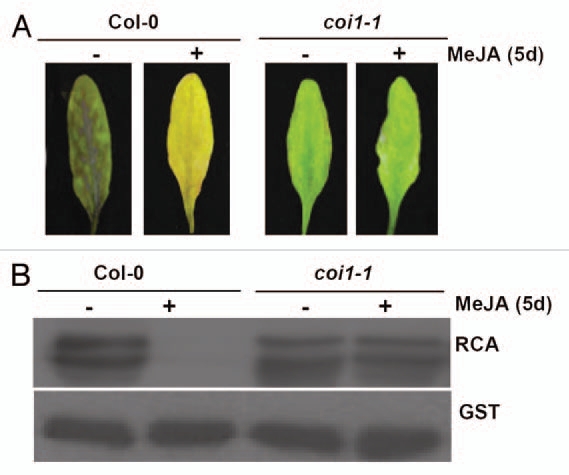
The COI1-dependent JA-repression of RCA correlated with JA-induced leaf senescence. (A) Phenotype of 6-week-old WT and coi1-1 mutant leaves treated with MeJA (+) or water (−) for 5 days. (B) Western blot for RCA in 6-week-old WT and coi1-1 mutant leaves treated with MeJA (+) or water (−) for 5 days. The immunoblot was detected with GST antibody as a protein loading control.
It has been reported that the RCA-deficient plants had a lower CO2 assimilation rate correlated with their defects in growth and photosynthesis, and that exogenous application of high CO2 could restore these deficiency.10,11 It remains to be elucidated that whether the senescence symptoms in these rca mutants could be rescued by growth at high CO2.
Many types of senescence usually associate with upregulation of JA content and JA biosynthetic genes expression.3,12 We examined the expression pattern of JA biosynthetic gene lipoxygenase 2 (LOX2) in the rca-1 and rca-2 mutants. RT-PCR analysis showed that the expression levels of LOX2 were not induced in these rca mutants (Fig. 3), indicating that the leaf senescence in these rca mutants might not associate with upregulation of JA content.
Figure 3.
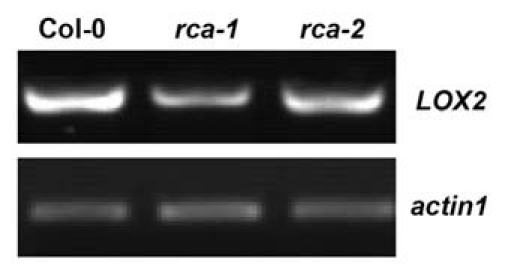
Semi-quantitative RT -PCR for LOX2 in 3-week-old WT, rca-1 and rca-2 mutant plants. The amplified actin1 was shown as an internal control.
To investigate whether the decrease in RCA is a specific JA-related effect or a common feature of other senescence types promoted by various developmental signals and environmental stresses,13,14 we detected the RCA expression pattern in dark-induced senescent WT plants, and found that downregulation of RCA was also involved in dark-induced senescence.5
In conclusion, we set up a model for JA-induced leaf senescence: JA signal is perceived by COI1, subsequently triggers the COI1-dependent degradation of jasmonate ZIM-domain proteins (JAZs), then releases the JAZs-interacting proteins to activate (or repress) the JA-responsive transcription repressors (or activators) essential for the expression of RCA, which thereby downregulates RCA resulting in JA-induced leaf senescence. It is possible that other types of senescence including dark-induced senescence might also accompanied by the reduction of RCA RNA and RCA protein.
As a member of the ATPases associated with a variety of cellular activities (AAA+) protein family, RCA functions in diverse stress-related processes including UV-B exposure, ozone, heat stress, drought and herbivore resistance in different plant systems.15–21 The rca-1 and rca-2 mutants displayed obviously decrease in the JA-induced expression of two defense-responsive genes plant defensin 1.2 (PDF1.2) and thionin 2.1 (Thi2.1),5,22,23 suggesting that RCA may also play a role in JA-mediated defense responses. The multi-function of RCA in defense responses indicates that there might be some common elements in these processes, which is worthy to be identified.
There was no sufficient evidence to support the possible role of JA in natural senescence except that the JA-signal deficient coi1 mutant plants exhibited relatively delayed natural senescence phenotypes including elongated flowering time and higher chlorophyll content.24 It deserves a more thorough analysis to study the role of JA in plant natural senescence: do the natural senescence-associated phenotypes also occur in the JA-biosynthesis deficient mutants fad3-2 fad7-2 fad8, dad1, aos, dde1 and opr3?25–29
Acknowledgments
We thank Dr. Dean Jiang for providing RCA antibody. This work was funded by Ministry of Science and Technology (973 Program 2011CB915404), Ministry of Agriculture (National Key Program for Transgenic Breeding 2008ZX08009-003) and National Natural Science Foundation of China (91017012 and 30800593).
References
- 1.Ueda J, Kato J. Identification of a senescence-promoting substance from wormwood (Artemisia absinthum L.) Plant Physiol. 1980;66:246–249. doi: 10.1104/pp.66.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weidhase RA, Kramell HM, Lehmann J, Liebisch HW, Lerbs W, Parthier B. Methyl jasmonate-induced changes in the polypeptide pattern of senescing barley leaf segments. Plant Science. 1987;51:177–186. [Google Scholar]
- 3.He Y, Fukushige H, Hildebrand D, Gan S. Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiol. 2002;128:876–884. doi: 10.1104/pp.010843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reinbothe C, Springer A, Samol I, Reinbothe S. Plant oxylipins: role of jasmonic acid during programmed cell death, defence and leaf senescence. FEBS J. 2009;276:4666–4681. doi: 10.1111/j.1742-4658.2009.07193.x. [DOI] [PubMed] [Google Scholar]
- 5.Shan X, Wang J, Chua L, Jiang D, Peng W, Xie D. The role of Arabidopsis Rubsico activase in jamonate-induced leaf senescence. Plant Physiol. 2011;155:751–764. doi: 10.1104/pp.110.166595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchanan-Wollaston V, Page T, Harrison E, Breeze E, Lim P, Nam H, et al. Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J. 2005;42:567–585. doi: 10.1111/j.1365-313X.2005.02399.x. [DOI] [PubMed] [Google Scholar]
- 7.Jung C, Lyou SH, Yeu S, Kim MA, Rhee S, Kim M. Microarray-based screening of jasmonate-responsive genes in Arabidopsis thaliana. Plant Cell Rep. 2007;26:1053–1063. doi: 10.1007/s00299-007-0311-1. [DOI] [PubMed] [Google Scholar]
- 8.Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG. COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science. 1998;280:1091–1094. doi: 10.1126/science.280.5366.1091. [DOI] [PubMed] [Google Scholar]
- 9.Yan J, Zhang C, Gu M, Bai Z, Zhang W, Qi T, et al. The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell. 2009;21:2220–2236. doi: 10.1105/tpc.109.065730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salvucci ME, Portis AR, Ogren WL. Light and CO2 response of ribulose-1,5-bisphosphate carboxylase/oxygenase activation in Arabidopsis leaves. Plant Physiol. 1986;80:655–659. doi: 10.1104/pp.80.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mate CJ, Hudson GS, von Caemmerer S, Evans JR, Andrews TJ. Reduction of ribulose bisphosphate carboxylase activase levels in tobacco (Nicotiana tabacum) by antisense RNA reduces ribulose bisphosphate carboxylase carbamylation and impairs photosynthesis. Plant Physiol. 1993;102:1119–1128. doi: 10.1104/pp.102.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seltmann MA, Stingl NE, Lautenschlaeger JK, Krischke M, Mueller MJ, Berger S. Differential impact of lipoxygenase2 and jasmonates on natural and stress-induced senescence in Arabidopsis thaliana. Plant Physiol. 2010;152:1940–1950. doi: 10.1104/pp.110.153114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schippers J, Jing H, Hille J, Dijkwel P. Developmental and hormonal control of leaf senescence. In: Gan S, editor. Senescence processes in plants. Oxford: Blackwell Publishing; 2007. pp. 145–170. [Google Scholar]
- 14.Balazadeh S, Riaño-Pachón D, Mueller-Roeber B. Transcription factors regulating leaf senescence in Arabidopsis thaliana. Plant Biol. 2008:63–75. doi: 10.1111/j.1438-8677.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- 15.Ogura T, Wilkinson AJ. AAA+ superfamily ATPases; common structure—diverse function. Genes Cells. 2001;6:575–597. doi: 10.1046/j.1365-2443.2001.00447.x. [DOI] [PubMed] [Google Scholar]
- 16.Pelloux J, Jolivet Y, Fontaine V, Banvoy J, Dizengremel P. Changes in Rubisco and Rubisco activase gene expression and polypeptide content in Pinus halepensis M. subjected to ozone and drought. Plant Cell and Environ. 2001;24:123–131. [Google Scholar]
- 17.Rokka A, Zhang L, Aro EM. Rubisco activase: an enzyme with a temperature-dependent dual function? Plant J. 2001;25:463–471. doi: 10.1046/j.1365-313x.2001.00981.x. [DOI] [PubMed] [Google Scholar]
- 18.Liu L, White MJ, MacRae TH. Identification of ultraviolet-B responsive genes in the pea, Pisum sativum L. Plant Cell Rep. 2002;20:1067–1074. [Google Scholar]
- 19.Bota J, Medrano H, Flexas J. Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytol. 2004;162:671–681. doi: 10.1111/j.1469-8137.2004.01056.x. [DOI] [PubMed] [Google Scholar]
- 20.Demirevska-Kepova K, Holzer R, Simova-Stoilova L, Feller U. Heat stress effects on ribulose-1,5-bisphosphate carboxylase/oxygenase, Rubisco binding protein and Rubisco activase in wheat leaves. Biol Plant. 2005;49:521–525. [Google Scholar]
- 21.Mitra S, Baldwin IT. Independently silencing two photosynthetic proteins in Nicotiana attenuata has different effects on herbivore resistance. Plant Physiol. 2008;148:1128–1138. doi: 10.1104/pp.108.124354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Penninckx IA, Eggermont K, Terras FR, Thomma BP, De Samblanx GW, Buchala A, et al. Pathogen-induced systemic activation of a plant defensin gene in Arabidopsis follows a salicylic acid-independent pathway. Plant Cell. 1996;8:2309–2323. doi: 10.1105/tpc.8.12.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vignutelli A, Wasternack C, Apel K, Bohlmann H. Systemic and local induction of an Arabidopsis thionin gene by wounding and pathogens. Plant J. 1998;14:285–295. doi: 10.1046/j.1365-313x.1998.00117.x. [DOI] [PubMed] [Google Scholar]
- 24.Xiao S, Dai L, Liu F, Wang Z, Peng W, Xie D. COS1: An Arabidopsis coronatine insensitive1 suppressor essential for regulation of jasmonate-mediated plant defense and senescence. Plant Cell. 2004;16:1132–1142. doi: 10.1105/tpc.020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McConn M, Browse J. The critical requirement for linolenic acid is pollen development, not photosynthesis, in an Arabidopsis mutant. Plant Cell. 1996;8:403–416. doi: 10.1105/tpc.8.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanders PM, Lee PY, Biesgen C, Boone JD, Beals TP, Weiler EW, et al. The Arabidopsis delayed dehiscence1 gene encodesan enzyme in the jasmonic acid synthesis pathway. Plant Cell. 2000;12:1041–1061. doi: 10.1105/tpc.12.7.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stintzi A, Browse J. The Arabidopsis male-sterile mutant, opr3, lacks the 2-oxophytodienoic acid reductase required for asmonate synthesis. Proc Natl Acad Sci USA. 2000;97:10625–10630. doi: 10.1073/pnas.190264497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishiguro S, Kawai-Oda A, Ueda K, Nishida I, Okada K. The DEFECTIVE IN ANTHER DEHISCENCE1 gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence and flower opening in Arabidopsis. Plant Cell. 2001;13:2191–2209. doi: 10.1105/tpc.010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park JH, Halitschke R, Kim HB, Baldwin IT, Feldmann KA, Feyereisen R. A knockout mutation in allene oxide synthase results in male sterility and defective wound signal transduction in Arabidopsis due to a block in jasmonic acid biosynthesis. Plant J. 2002;31:1–12. doi: 10.1046/j.1365-313x.2002.01328.x. [DOI] [PubMed] [Google Scholar]


