Abstract
Glutathione (GSH) has widely been known to be a multifunctional molecule especially as an antioxidant up until now, but has found a new role in plant defense signaling. Research from the past three decades indicate that GSH is a player in pathogen defense in plants, but the mechanism underlying this has not been elucidated fully. We have recently shown that GSH acts as a signaling molecule and mitigates biotic stress through non-expressor of PR genes 1 (NPR1)-dependent salicylic acid (SA)-mediated pathway. Transgenic tobacco with enhanced level of GSH (NtGB lines) was found to synthesize more SA, was capable of enhanced expression of genes belonging to NPR1-dependent SA-mediated pathway, were resistant to Pseudomonas syringae, the biotrophic pathogen and many SA-related proteins were upregulated. These results gathered experimental evidence on the mechanism through which GSH combats biotic stress. In continuation with our previous investigation we show here that the expression of glutathione S-transferase (GST), the NPR1-independent SA-mediated gene was unchanged in transgenic tobacco with enhanced level of GSH as compared to wild-type plants. Additionally, the transgenic plants were barely resistant to Botrytis cinerea, the necrotrophic pathogen. SA-treatment led to enhanced level of expression of pathogenesis-related protein gene (PR1) and PR4 as against short-chain dehydrogenase/reductase family protein (SDRLP) and allene oxide synthase (AOS). These data provided significant insight into the involvement of GSH in NPR1-dependent SA-mediated pathway in mitigating biotic stress.
Key words: GSH, signaling molecule, biotrophic pathogen, NPR-1, PR-1, PR-4, transgenic tobacco
Plant responses to different environmental stresses are achieved through integrating shared signaling networks and mediated by the synergistic or antagonistic interactions with the phytohormones viz. SA, jasmonic acid (JA), ethylene (ET), abscisic acid (ABA) and reactive oxygen species (ROS).1 Previous studies have shown that in response to pathogen attack, plants produce a highly specific blend of SA, JA and ET, resulting in the activation of distinct sets of defense-related genes.2,3 Regulatory functions for ROS in defense, with a focus on the response to pathogen infection occur in conjunction with other plant signaling molecules, particularly with SA and nitric oxide (NO).4–6 Till date, numerous physiological functions have been attributed to GSH in plants.7–11 In addition to previous studies, recent study has also shown that GSH acts as a signaling molecule in combating biotic stress through NPR1-dependent SA-mediated pathway.12,13
Our recent investigation involved raising of transgenic tobacco overexpressing gamma-glutamylcysteine synthetase (γ-ECS), the rate-limiting enzyme of the GSH biosynthetic pathway.12 The stable integration and enhanced expression of the transgene at the mRNA as well as protein level was confirmed by Southern blot, quantitative RT-PCR and western blot analysis respectively. The transgenic plants of the T2 generation (Fig. 1), the phenotype of which was similar to that of wild-type plants were found to be capable of synthesizing enhanced amount of GSH as confirmed by HPLC analysis.
Figure 1.
Transgenic tobacco of T2 generation, (A) three-week-old plant, (B) mature plant.
In the present study, the expression profile of GST was analyzed in NtGB lines by quantitative RT-PCR (qRT-PCR) and found that the expression level of this gene is unchanged in NtGB lines as compared to wild-type plants (Fig. 2). GST is known to be a NPR1-independent SA-related gene.14 This suggests that GSH does not follow the NPR1-independent SA-mediated pathway in defense signaling.
Figure 2.
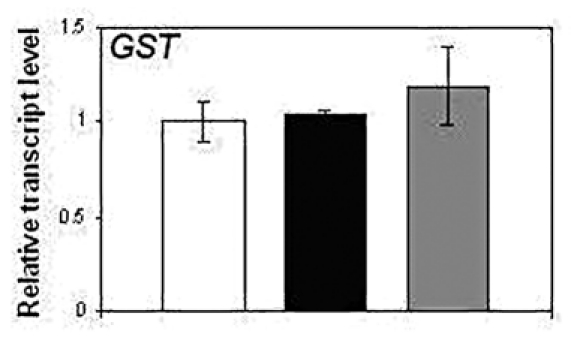
Expression pattern of GST in wild-type and NtGB lines.
Disease test assay with NtGB lines and wild-type plants was performed using B. cinerea and the NtGB lines showed negligible rate of resistance to this necrotrophic pathogen (Fig. 3). SA signaling has been known to control defense against biotrophic pathogen in contrast, JA/ET signaling controls defense against necrotrophic pathogen.1,15 Thus it has again been proved that GSH is not an active member in the crosstalk of JA-mediated pathway, rather it follows the SA-mediated pathway as has been evidenced earlier.12
Figure 3.
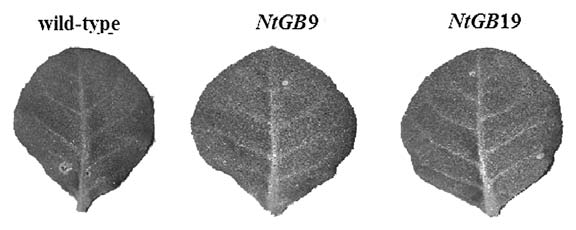
Resistance pattern of wild-type and NtGB lines against Botrytis cinerea.
Additionally, the leaves of wild-type and NtGB lines were treated with 1 mM SA and the expression of PR1, SDRLP, AOS and PR4 genes were analyzed and compared to untreated plants to simulate pathogen infection. The expression of PR1 increased after exogenous application of SA. In case of PR4, the ET marker, the expression level increased in NtGB lines. On the other hand, the level of SDRLP was nearly the same. However, the expression of AOS was absent in SA-treated leaves (Fig. 4). PR1 has been known to be induced by SA-treatment16 which can be corroborated with our results. In addition, ET is known to enhance SA/NPR1-dependent defense responses,17 which was reflected in our study as well. AOS, the biosynthetic pathway gene of JA, further known to be the antagonist of SA, was downregulated in SA-treated plants.
Figure 4.
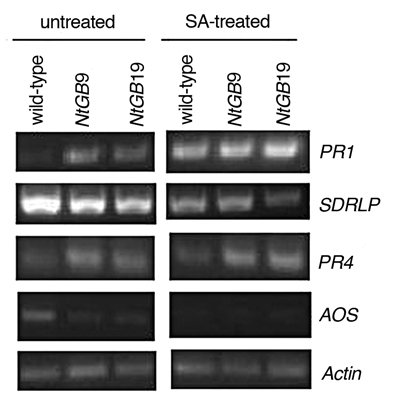
Gene expression pattern of PR1, SDRLP, PR4 and AOS in untreated and SA-treated wildtype and NtGB lines.
Taken together, it can be summarized that this study provided new evidence on the involvement of GSH with SA in NPR1-dependent manner in combating biotic stress. Additionally, it can be claimed that GSH is a signaling molecule which takes an active part in the cross-communication with other established signaling molecules like SA, JA, ET in induced defense responses and has an immense standpoint in plant defense signaling.
Acknowledgments
This work was supported by Department of Science and Technology (DST), New Delhi, India and partly by Council of Scientific and Industrial Research(CSIR), New Delhi, India.
References
- 1.Loake G, Grant M. Salicylic acid in plant defence—the players and protagonists. Curr Opin Plant Biol. 2007;10:466–472. doi: 10.1016/j.pbi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Koornneef A, Pieterse CMJ. Cross talk in defense signaling. Plant Physiol. 2008;146:839–844. doi: 10.1104/pp.107.112029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bari R, Jones J. Role of plant hormones in plant defense responses. Plant Mol Biol. 2009;69:473–488. doi: 10.1007/s11103-008-9435-0. [DOI] [PubMed] [Google Scholar]
- 4.Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:1360–1385. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Torres MA, Jones JDG, Dangl JL. Reactive oxygen species signaling in response to pathogens. Plant Physiol. 2006;141:373–378. doi: 10.1104/pp.106.079467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolwell GP, Daudi A. Reactive oxygen species in plant pathogen interactions. In: del Río LA, Puppo A, editors. Reactive Oxygen Species in Plant Signaling, Signaling and Communication in Plants. Springer: Verlag Berlin Heidelberg; 2009. pp. 113–133. [Google Scholar]
- 7.Rennenberg H. Processes involved in glutathione metabolism. In: Wallsgrove RM, editor. Amino acids and their derivatives in higher plants. Cambridge University Press; 1995. p. 155.p. 171. [Google Scholar]
- 8.Foyer CH, Lopez-Delgado H, Dat JF, Schott IM. Hydrogen peroxide- and glutathione-associated mechanisms of acclamatory stress tolerance and signalling. Physiol Plant. 1997;100:241–254. [Google Scholar]
- 9.Noctor G, Gomez L, Vanacker H, Foyer CH. Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signaling. J Exp Bot. 2002;53:1283–1304. doi: 10.1093/jexbot/53.372.1283. [DOI] [PubMed] [Google Scholar]
- 10.Mhamdi A, Hager J, Chaouch S, Queval G, Han Y, Taconnat L, et al. Arabidopsis GLUTATHIONE REDUCTASE 1 plays a crucial role in leaf responses to intracellular H2O2 and in ensuring appropriate gene expression through both salicylic acid and jasmonic acid signaling pathways. Plant Physiol. 2010;153:1144–1160. doi: 10.1104/pp.110.153767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishikawa K, Yoshimura K, Harada K, Fukusaki E, Ogawa T, Tamoi M, et al. AtNUDX6, an ADP-ribose/NADH pyrophosphohydrolase in Arabidopsis, positively regulates NPR1-dependent salicylic acid signaling. Plant Physiol. 2010;152:2000–2012. doi: 10.1104/pp.110.153569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghanta S, Bhattacharyya D, Sinha R, Banerjee A, Chattopadhyay S. Nicotiana tabacum overexpressing γ-ECS exhibits biotic stress tolerance likely through NPR1-dependent salicylic acid mediated pathway. Planta. 2011;233:895–910. doi: 10.1007/s00425-011-1349-4. [DOI] [PubMed] [Google Scholar]
- 13.Ghanta S, Chattopadhyay S. Glutathione as a signalling molecule-another challenge to pathogens. Plant Signal Behav. 2011 doi: 10.4161/psb.6.6.15147. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanco F, Salinas P, Cecchini NM, Jordana X, Van Hummelen, Alvarez ME, et al. Early genomic responses to salicylic acid in Arabidopsis. Plant Mol Biol. 2009;70:79–102. doi: 10.1007/s11103-009-9458-1. [DOI] [PubMed] [Google Scholar]
- 15.Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol. 2005;43:205–227. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- 16.Ohshima M, Itoh H, Matsuoka M, Murakami T, Ohashi Y. Analysis of stress-induced or salicylic-induced expression of the pathogenesis-related I a protein gene in transgenic tobacco. Plant Cell. 1990;2:95–106. doi: 10.1105/tpc.2.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Van Zaanen W, Koornneef A, Korzelius JP, Dicke M, Van Loon LC, et al. Herbivore-induced resistance against microbial pathogens in Arabidopsis. Plant Physiol. 2006;142:352–363. doi: 10.1104/pp.106.083907. [DOI] [PMC free article] [PubMed] [Google Scholar]


