Abstract
Brassinosteroids (BRs) are steroid-like hormones essential for plant growth and development. The most active forms of brassinosteroids are Brassinolide (BL) and Castasterone (CS), which are catalyzed by members of the CYP85A family of cytochrome P450 monooxygenases. In Arabidopsis thaliana there are two CYP85A gene members: CYP85A1 and CYP85A2. Unlike CYP85A1, CYP85A2 mediates the conversion of CS to BL. In contrast to mutations in CYP85A2 that result in severe dwarfism, cyp85a1 mutants do not show any obvious morphological phenotype during vegetative or floral development. By analyzing large-scale transcriptional activity in the ovule of Arabidopsis thaliana (Arabidopsis), we determined that CYP85A1 is abundantly expressed in wild-type, but not in sporocyteless (spl) ovules lacking a female gametophyte. Insertional T-DNA lines defective in the activity of CYP85A1 exhibit a semi-sterile phenotype, suggesting a role for the corresponding enzyme acting at the gametophytic level. The CYP85A1 mRNA is localized in the female gametophyte and its neighboring sporophytic cells; however, translational fusions of the CYP85A1 promoter to uidA (GUS) showed GUS expression restricted to the female gametophyte, suggesting that within the ovule the corresponding protein is mostly active in gametophytic cells. A cytological analysis of heterozygous cyp85a1/+ individuals showed that close to 50% of female gametophytes are arrested before the first nuclear mitotic division of the haploid functional megaspore. Our results indicate that BR biosynthesis is required for the initiation of megagametogenesis in Arabidopsis.
Key words: CYP85A1, brassinosteroids, megagametogenesis, Arabidopsis, ovule, female gametophyte
Introduction
The production of seeds in sexual flowering plants is dependent on the formation of male and female gametophytes that are deeply embedded in reproductive organs. In the ovule of most flowering plants, the female gametophyte (or embryo sac) is composed of seven cells that include two synergids, the egg cell (the precursor of the embryo), a voluminous binucleated central cell (the precursor of the endosperm) and a group of antipodal cells whose role remain elusive. In Arabidopsis thaliana (Arabidopsis), this classical 7-cellular female gametophyte of the Polygonum type is derived from a single meiotically derived haploid cell (the functional megaspore) that undergoes three rounds of mitotic divisions before cellularization and differentiation. After fertilization of the egg and central cell by two sperm cells delivered by a pollen tube, the ovule is progressively transformed into a seed. A general understanding of the developmental mechanisms that regulate female gametogenesis has progressively emerged from genetic studies in Arabidopsis. Several mutants showing defects in cell specification,1–4 cell division or expansion5–9 and cellularization or differentiation,10–14 have been identified. In addition, large-scale expression analysis, have revealed a multitude of genes that are active within gametophytic cells.15–19 Although in general the role of phytohormones in plant gametogenesis has been poorly investigated, it has long been known that auxin and ethylene contribute to the regulation of ovary and ovule development and to the coordination of development of male and female gametophytes.20 Recently, female gamete specification was shown to depend on the establishment of a source-derived auxin gradient directing the establishment of cell identity within the female gametophyte of Arabidopsis;21 however, the mechanisms controlling the establishment of this gradient remain unknown, and the possibility of other plant hormones participating in the control of female gametogenesis has been poorly investigated.
Brassinosteroids (BRs) are a class of polyhydroxysteroids that are recognized as important plant hormones affecting many aspects of growth and development. Their role in germination, cell elongation, pollen tube growth, root growth, vascular differentiation and stress tolerance have been demonstrated.22–26 The BR biosynthetic pathway has been elucidated using BR-deficient and BR-insensitive mutants in pea, tomato, rice and Arabidopsis. The common phenotypic features of Arabidopsis BR mutants include dwarfism, curly leaves, reduced apical dominance, reduced fertility, and delayed senescence.27–29 Most of the BR-deficient mutants are defective in the activity of genes encoding cytochrome P450 (CYP450), a class of enzymes involved in oxidative reactions occurring during BR biosynthesis. CYP450 enzymes of the CYP85A family catalyze the last oxidative reactions that lead to the biosynthesis of BRs. In Arabidopsis, CYP85A1 and CYP85A2 are two CYP85 proteins shown to be involved in the final oxidation steps necessary for the biosynthesis of brassinolide.30 CYP85A1 is necessary to catalyze several essential reactions necessary for the production of castasterone,31 and CYP85A2 catalyzes the final step that converts castasterone into brassinolide.30 Whereas cyp85a2 knockout mutants show identical phenotypic defects as those exhibited by BR-deficient dwarf plants, individuals defective in CYP85A1 activity do not show any obvious phenotype. The cyp85a2 mutant phenotype is exacerbated in cyp85a1 cyp85a2 double mutants, suggesting that CYP85A1 and CYP85A2 have overlapping functions, and that CYP85A2 has a dominant role in vegetative development.30
Here we show that contrary to CYP85A2, CYP85A1 plays a reproductive role during female gametogenesis in Arabidopsis thaliana. A large transcriptional analysis based on Massively Parallel Signature Sequencing (MPSS) revealed that CYP85A1 was a candidate gene to be active within the fully differentiated female gametophyte, a finding confirmed by its pattern of both in situ mRNA localization and reporter gene expression based on promoter-driven translational fusions. The genetic analysis of insertional mutants harboring a T-DNA in the CYP85A1 gene demonstrated that individuals defective in CYP85A1 activity show ovules arrested before the first mitotic division of the functional megaspore. Our results show that CYP85A1 is necessary for megagametophyte development, suggesting that BR biosynthesis plays a role in plant gametogenesis.
Results
CYP85A1 is expressed in the female gametophyte of Arabidopsis.
We used large-scale Massively Parallel Signature Sequencing (MPSS35,36) in wild-type and sporocyteless (spl) ovules (lacking a female gametophyte3,4) to identify a multitude of candidate transcripts to be present within gametophytic (haploid) cells but not within sporophytic (diploid) cells of the ovule (N. Sanchez-León et al. submitted). Transcripts corresponding to CYP85A1 were detected in wild-type but not spl ovules, suggesting that in the ovule CYP85A1 is preferentially or specifically expressed in the female gametophyte. A detailed analysis of additional MPSS results as well as ATH1 microarray transcriptional information (NASC microarray database37) indicated that expression of CYP85A1 in vegetative tissues is residual as compared to levels of expression within female reproductive organs, in particular as compared to the ovule (Table 1).
Table 1.
Expression of CYP85A1 and CYP85A2 genes
| GENE | LESa | AGMa | ROSa | GSEa | OVLa | SPLa | COTb | LEFb | STMb | ROTb |
| CYP85A1 | 0 | 0 | 0 | 0 | 39 | 0 | 0 | 0 | 0 | 0 |
| CYP85A2 | 53 | 19 | 3 | 49 | 8 | 3 | 521 | 527 | 118 | 267 |
Expression in transcripts per million (TPMs) following publically available MPSS data. LES, wild-type leaves 21 days after germination; AGM, agamous inflorescences; ROS, roots 21 days after germination; GSE, germinating seedlings; OVL, fully differentiated wild-type ovules prior to pollination; NZZ, fully differentiated nozzle/sporocyteless ovules prior to pollination.
CYP85A1 mRNA is localized in both sporophytic and gametophytic cells.
A reverse-trascription polymerase chain reaction analysis (RT-PCR) revealed that CYP85A1 is expressed in most vegetative tissues at weak levels (results not shown). To determine the spatial and temporal pattern of expression of CYP85A1 in the ovule, we conducted mRNA in situ hybridization. To avoid the localization of CYP85A2 mRNA, digoxygenin-labeled riboprobes were generated by using specific template sequence of the 3′ UTR of CYP85A1. The CYP85A1 mRNA was initially detected in developing ovules containing a differentiated functional megaspore; the mRNA was abundantly localized in the inner integument and the nucellus, including the degenerated megaspores (Fig. 1a), but also in the functional megaspore, indicating that CYP85A1 is initially expressed in both sporophytic and gametophytic cells. During megagametogenesis, CYP85A1 expression persists in the chalazal region of the ovule and the female gametophyte (Fig. 1b). At maturity, the CYP85A1 mRNA was localized in all gametophytic cells, including the synergids, the egg cell and the central cell (Fig. 1b and c). Control hybridizations using a probe corresponding to the sense orientation did not show a signal (Fig. 1d–f). We also analyzed the expression pattern by generating a reporter gene fusion using a 1.36 Kbp genomic fragment (promCYP85A1) to drive uidA (GUS) reporter gene expression. This genomic region is composed of the first 1,117 bp located upstream of the CYP85A1 transcription initiation site, and the first 243 bp of its transcribed sequence. By floral-dip transformation we generated a total of 19 transformants, all of which showed normal growth and seed set. Although GUS expression was absent from the meiotically derived megaspores (Fig. 1g), ovules from promCYP85A1::uidA transformants showed initial GUS expression at the 1-nuclear to 2-nuclear stage of female gametogenesis (1NFG and 2NFG; Fig. 1h). GUS expression was absent from sporophytic cells covering the developing female gametophyte (Fig. 1i), but remained prevalent in the female gametophyte throughout its development, and at ovule maturity, in the vasculature of the funiculus (Fig. 1j). These results show that CYP85A1 is initially expressed both in sporophytic and gametophytic cells, and later only the female gametophyte, suggesting that during megagametogenesis the CYP85A1 mRNA is transported from maternal companion cells to the female gametophyte.
Figure 1.

CYP85A1 is expressed in adjacent sporophytic and gametophytic cells. (a–f) Localization of CYP85A1 mRNA by in situ hybridization (a–c antisense probe; d–f, sense probe). (a) Developing ovule at the end of megasporogenesis. (b) Transversal section of a fully differentiated ovule. (c) Longitudinal section of a fully differentiated ovule. (d) Developing ovule at the end of megasporogenesis. (e) Transversal section of a fully differentiated ovule. (f) Longitudinal section of a fully differentiated ovule. (g–j) Reporter gene expression in ovules of promCYP85A1::uidA (GUS) transformants. (g) GUS expression at the end of megasporogenesis. (h) GUS expression in a 2-nulceate megagametophyte. (i) GUS expression in a 4-nucleate megagametophyte. (j) GUS expression in a fully differentiated ovule. FM, Functional Megaspore; mFG, mature Female Gametophyte; ii, inner integument; oi, outer integument; dms, degenerating megaspores; FG, Female Gametophyte; Ch, chalaza; Cc, Central Cell; Ec, Egg Cell; Sy, Synergid cells. Scale bar: 10 µm.
CYP85A1 is necessary for the initiation of megagametogenesis.
To elucidate the function of CYP85A1 in Arabidopsis, individuals from two independent insertional lines harboring T-DNA elements within the coding region of the CYP85A1 gene were phenotypically analyzed.39,40 The genomic sequence of CYP85A1 is composed of nine exons, whereas cyp85a1-1 contains a T-DNA insertion in the fifth exon of the genomic sequence, in cyp85a1-2 the T-DNA in inserted in its regulatory region, 182 nucleotides upstream of the translation initiation site (Fig. 2a). For both alleles the location of the T-DNA insertion was confirmed by PCR amplification. As compared to wild-type plants, both heterozygous cyp85a1 insertional lines (cyp85a1-1/+ and cyp85a1-2/+) showed a semi-sterile phenotype in developing siliques generated by self-pollination, with a variable frequency of aborted unfertilized ovules (Fig. 2b). To determine the reproductive nature of this defect, we conducted reciprocal crosses to wild-type plants and scored the frequency of aborted ovules. When heterozygous cyp85a1-1/+ and cyp85a1-2/+ individuals were crossed as males to wild-type plants, no signs of abnormal ovule abortion were observed; however, when these same individuals were crossed to wild-type as females, a significant portion of ovules remained unfertilized and aborted following silique maturation, at frequencies ranging from 40.4% to 44.5% (Fig. 2b and c). These frequencies are close to an expected 50% of ovule abortion prevalent in fully penetrant gametophytic mutations of Arabidopsis.
Figure 2.
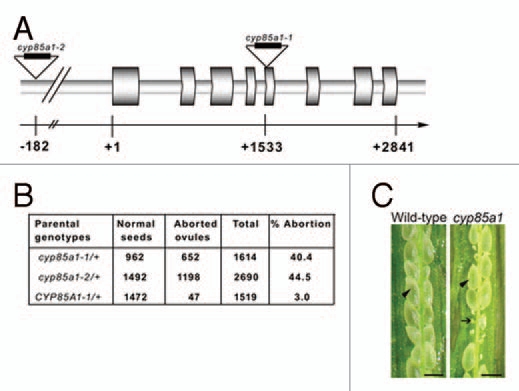
Heterozygus cyp85a1/+ individuals are semi-sterile and exhibit low phenotypic transmission through female gametes. (A) Genomic structure of CYP85A1; triangles indicate the location of the T-DNA analyzed and the corresponding alleles. (B) Inheritance of ovule abortion in self- and cross-pollinated cyp85a1 alleles. (C) Photograph of open wild-type and cyp85a1/+ siliques, the arrowheads show normal seeds and the arrow show aborted ovules. Scale bar: 1 mm.
To determine the cellular nature of the defect, whole-mounted cleared ovules were analyzed throughout their development. As compared to wild-type where a single meiotically derived product (the functional megaspore) survives and divides mitotically to subsequently differentiate a female gametophyte (Figs. 3a–c), heterozygous cyp85a1-1/+ and cyp85a1-2/+ individuals undergo normal and synchronized megasporogenesis; however, shortly after the end of meiosis, in almost half of the ovules (data not shown) the functional megaspore differentiates but does not initiate the first mitotic division (Fig. 3d–g). The largest proportion of defective mature ovules showed a single conspicuous cell with a single nucleus. Additionally, at reduced frequencies, some developing ovules show a premature degeneration of the functional megaspore (Fig. 3h), while in some others the functional megaspore divides mitotically and arrests at the 2-nuclear stage of megagametogenesis (Fig. 3i); these two nuclei remain located at opposite ends of the cell, in agreement with the normal pattern of female gametophyte development.
Figure 3.

cyp85a1 is necessary for the initiation of megagametogenesis. (a) Developing wild-type ovule at the end of megasporogenesis. (b) Developing wild-type ovule at the 4-nuclear stage of megagametogenesis (FG4). (c) Fully differentiated wild-type ovule showing a mature megagametophyte. (d) Developing ovule in a cyp85a1/+ individual at the end of megasporogenesis; the arrowhead shows the functional megaspore. (e) Developing ovule in a cyp85a1/+ individual arrested at the 1-nuclear stage of megagametogenesis; the arrowhead shows a conspicuous aberrant cell. (f) Developing ovule in a cyp85a1/+ individual showing a single cell with a conspicuous nucleus (arrowhead). (g) Fully differentiated ovule in a cyp85a1/+ individual showing a single conspicuous cell similar to those shown in (e and f). (h) Developing ovule in a cyp85a1/+ in which all haploid megaspores have degenerated. (i) Developing ovule in a cyp85a1/+ individual arrested at the 2-nuclear stage of megagametogenesis. (j) pFM1-dependent GUS expression in a wild-type ovule at the end of megasporogenesis. (k) pFM1-dependent GUS expression in a developing ovule of a cyp85a1/+ individual. (l) pFM1-dependent GUS expression in a fully differentiated ovule of a cyp85a1/+ individual. FM, Functional Megaspore; Cc, Central cell; Ec, Egg cell; Sc, Synergid cell. Arrowheads show arrested nuclei. Scale bar, 10 µm.
To determine the identity of the single cell that persists in fully differentiated ovules, we took advantage of pFM1, a molecular marker that is only expressed in the functional megaspore and not in any other meiotically-derived of sporophytic cell of the developing ovule (Fig. 3j).7 pFM1 represents an ideal marker to conduct crosses with cyp85a1/+ individuals showing a significant proportion of aborted ovules, as it marks meiotically derived cells that have acquired a functional identity at the end of megasporogenesis. In defective cyp85a1-1/+ pFM1/+ F1 ovules, GUS expression was restricted to the single large cell that persists in mature ovules (Fig. 3k and l), suggesting that in cyp85a1-1 mutants female gametophyte development is arrested after the differentiation of the functional megaspore. These results indicate that CYP85A1 is necessary for the initiation of megagametogenesis in Arabidopsis, as defective cyp85a1 ovules fail to undergo haploid mitosis after differentiation of the functional megaspore.
Discussion
In flowering plants developing a female gametophyte of the Polygonum type (including Arabidopsis), megagametogenesis initiates with the mitotic division of the functional megaspore nucleus.41,42 Although the interaction between the sporophyte and the developing gametophyte has been suggested to be important for gametogenesis, little is known about specific mechanisms that could be involved in controlling these interactions. While the presence of frequent plasmodesmata connecting diploid sporophytic cells to the functional megaspore (or the developing female gametophyte) has been observed in numerous species,43,44 little evidence exists on the nature of key molecules trafficking between the sporophytic and gametophytic cells. Recently, the asymmetric localization of auxin biosynthesis during the syncytial phase of female gametophyte development was shown to control the determination of gametophytic cell identity.21 Although a highly dynamic and specific pattern of auxin distribution was localized in both the developing sporophytic cells and the female gametophyte, a possible hormonal signal connecting these two closely associated tissues has not been identified.
Here we show that a CYP85A1, a gene encoding a key enzyme for the biosynthesis of brassinosteroids (BRs) in Arabidopsis, is expressed in the developing female gametophyte and its surrounding sporophytic cells, and that it plays an important function in the initial steps of megagametogenesis. A combination of in situ hybridization and reporter translational fusions showed that although CYP85A1 mRNA is expressed in the sporophytic cells surrounding the meiotically derived megaspores, subsequently the CYP85A1 protein has a strong tendency to be only localized in gametophytic cells, including the functional megaspore and the female gametophyte, indicating that the production of BRs is highly coordinated during the sporophyte-to-gametophyte transition. cyp85a1 mutants differentiate a functional megaspore that does not undergo haploid mitosis, suggesting that BR biosynthesis—and the possible action of castasterone or brassinolide—is important to initiate haploid nuclear divisions in the ovule.
Although several BR deficient mutants show reduced fertility due to poor stamen or pollen tube elongation,29 BRs have been recently implicated in the control of male gametogenesis by positively regulating the expression of key genes involved in anther and pollen development. The direct interaction of BES1—a transcription factor involved in BR biosynthesis—with several promoter regions of essential genes for microspore development indicates that BRs are important for male gametogenesis.45 Additionally, the plant receptor kinase SOMATIC EMBRYOGENESIS RECEPTOR KINASE 1 (SERK1), important in microsporogenesis and in the acquisition of embryonic competence, is also involved in the brassinolide sensing pathway,46 demonstrating that BR-dependent signaling plays a key role in seed formation. Although additional experimental work is necessary to determine if either castasterone or brassinolide are necessary for mitotic progression of gametogenesis in the ovule, our findings indicate that the biosynthesis of BRs is necessary for the haploid phase of the life cycle at the onset of megagametogenesis. Our results extend previous findings related to the role of BRs in plant reproduction by showing that BRs biosynthesis is important for female fertility, opening the possibility for a yet unknown mechanism of BRs production in the female gametophyte that could precede the control of embryonic competence following double fertilization.
Materials and Methods
Material and growth conditions.
Seeds of Arabidopsis thaliana (ecotype Columbia or Landsberg erecta) were sterilized and germinated in MS medium under stable short day conditions (12 h light/12 h dark) at 18°C. Seedlings were transplanted and grown on a 3:1:1 mixture of Mix3-Sunshine (SunGro, Bellevue, WA): Vermiculite:Perlite (vol/vol/vol ratio) containing 1.84 kg/m3 of 14-14-14 slow release fertilizer (Osmocote, sierra, Marysville, OH) and grown under controlled greenhouse conditions.
In situ hybridization.
Developing flower buds, developing flowers and isolated gynoecia of wild-type plants were fixed in 4% paraformaldehyde and embedded in Tissueprep2 (Fisher scientific, catalog No.: T555). Sections 12 µm in thickness were cut using a Leica microtome and mounted on ProbeOn Plus slides (Fisher Scientific. Catalog No.: 22-230-900). A fragment of 84 pb was amplified using primers CYP85A1sense (5′-CAA TCT TAG GAT TTC ACC CT-3′) and CYP85A1antisense (5′-CGT CTT CTG TAT CCT CTG C-3′) and cloned in the PCR II TOPO vector (Invitrogen). Plasmid linearization was performed with BamHI and NotI to subsequently synthesize the sense and antisense digoxygenin-labeled probes, respectively. Hybridization was performed as described Vielle-Calzada et al.32
Generation of promCYP85A1::uidA plasmid.
The regulatory sequence of 1,360 pb including the 5′UTR sequence and 132 bp from the first exon was PCR amplified from genomic Arabidopsis DNA. The PCR primers used were as follows: CYP85A1 sense 5′-GGA TCC CAA AGA TTG GCC AGC CCA T, and CYP85A1 antisense 5′-GTC GAC AAC TTG TCG GAC CCC ACA CTT. The amplified product, with artificially generated BamHI and SalI sites, was cloned into plasmid pBI101.3 to generate promCYP85A1::uidA. The plasmid was introduced into Agrobacterium tumefaciens strain LBA-4404 by electroporation. Arabidopsis plants were transformed using the floral dip method.33
Histological analysis.
For whole-mount observation of ovule development, individual gynoecia were dissected longitudinally by using hypodermic needles (1 ml insulin syringes, Becton Dickinson) and processed as described Acosta-Garcia and Vielle-Calzada.5 β-Glucuronidase staining assays were conducted as described Vielle-Calzada et al.34
Acknowledgements
We thank the Genomic Sequencing Services at Langebio for help with plasmid sequencing, and Santos García-Aguilar and Jaime Mendiola for greenhouse support. V.P.E. and N.S.L. are recipients of a scholarship from Consejo Nacional de Ciencia y Tecnología (CONACyT). This research was funded by the UC-MEXUS program, CONACyT, and the Howard Hughes Medical Institute through the International Scholar Program (J.P.V.C.).
References
- 1.Bemer M, Wolters-Arts M, Grossniklaus U, Angenent GC. The MADS domain protein DIANA acts together with AGAMUS-LIKE80 to specify the central cell in Arabidopsis ovules. Plant Cell. 2008;20:2088–2101. doi: 10.1105/tpc.108.058958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olmedo-Monfil V, Durán-Figueroa N, Arteaga-Vázquez M, Demesa-Arévalo E, Autran D, Grimanelli D, et al. Control of female gamete formation by a small RNA pathway in Arabidopsis. Nature. 2010;464:628–632. doi: 10.1038/nature08828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiefthaler U, Balasubramanian S, Sieber P, Chevalier D, Wisman E, Schneitz K. Molecular analysis of NOZZLE, a gene involved in pattern formation and early sporogenesis during sex organ development in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1999;96:11664–11669. doi: 10.1073/pnas.96.20.11664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang WC, Ye D, Xu J, Sundaresan V. The SPOROCYTELESS gene of Arabidopsis is required for initiation of sporogenesis and encodes a novel nuclear protein. Genes Dev. 1999;13:2108–2117. doi: 10.1101/gad.13.16.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Acosta-García G, Vielle-Calzada JP. A classical arabinogalactan protein is essential for the initiation of female gametogenesis in Arabidopsis. Plant Cell. 2004;16:2614–2628. doi: 10.1105/tpc.104.024588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebel C, Mariconti L, Gruissem W. Plant retinoblastoma homologues control nuclear proliferation in the female gametophyte. Nature. 2004;429:776–780. doi: 10.1038/nature02637. [DOI] [PubMed] [Google Scholar]
- 7.Huanca-Mamani W, Garcia-Aguilar M, León-Martínez G, Grossniklaus U, Vielle-Calzada JP. CHR11, a chromatin-remodeling factor essential for nuclear proliferation during female gametogenesis in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2005;102:17231–17236. doi: 10.1073/pnas.0508186102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siddiqi I, Ganesh G, Grossniklaus U, Subbiah V. The dyad gene is required for progression through female meiosis in Arabidopsis. Development. 2000;127:197–207. doi: 10.1242/dev.127.1.197. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka H, Ishikawa M, Kitamura S, Takahashi Y, Soyano T, Machida C, et al. The AtNACK1/HINKEL and STUD/TETRASPORE/AtNACK2 genes, which encode functionally redundant kinesins, are essential for cytokinesis in Arabidopsis. Genes Cell. 2004;9:1199–1211. doi: 10.1111/j.1365-2443.2004.00798.x. [DOI] [PubMed] [Google Scholar]
- 10.Gross-Hardt R, Kägi C, Baumann N, Moore JM, Baskar R, Gagliano WB, et al. LACHESIS restricts gametic cell fate in the female gametophyte of Arabidopsis. PLoS Biol. 2007;5:494–500. doi: 10.1371/journal.pbio.0050047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matias-Hernandez L, Battaglia R, Galbiati F, Rubes M, Eichenberger C, Grossniklaus U, et al. VERDANDI is a direct target of the MADS domain ovule identity complex and affects embryo sac differentiation in Arabidopsis. Plant Cell. 2010;22:1702–1715. doi: 10.1105/tpc.109.068627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moll C, von Lyncker L, Zimmermann S, Kägi C, Baumann N, Twell D, et al. CLO/GFA1 and ATO are novel regulators of gametic cell fate in plants. Plant J. 2008;56:913–921. doi: 10.1111/j.1365-313X.2008.03650.x. [DOI] [PubMed] [Google Scholar]
- 13.Portereiko MF, Lloyd A, Steffen JG, Punwani JA, Otsuga D, Drews GN. AGL80 is required for central cell and endosperm development in Arabidopsis. Plant Cell. 2006;18:1862–1872. doi: 10.1105/tpc.106.040824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Portereiko MF, Sandaklie-Nikolova L, Lloyd A, Dever CA, Otsuga D, Drews GN. NUCLEAR FUSION DEFECTIVE1 encodes the Arabidopsis RPL21M protein and is required for kariogamy during female gametophyte development and fertilization. Plant Physiol. 2006;141:975–985. doi: 10.1104/pp.106.079319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston AJ, Meler P, Gheyselinck J, Wuest SE, Federer M, Schlagenhauf E, et al. Genetic subtraction profiling identifies genes essentials for Arabidopsis reproduction and reveals interactions between the female gametophyte and the maternal sporophyte. Genome Biol. 2007;8:204. doi: 10.1186/gb-2007-8-10-r204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones-Rhoades MW, Borevitz JO, Preuss D. Genome-wide expression profiling of the Arabidopsis female gametophyte identifies families of small, secreted proteins. PloS Genet. 2007;3:1848–1861. doi: 10.1371/journal.pgen.0030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steffen JG, Kang IH, Macfarlane J, Drews GN. Identification of genes expressed in the Arabidopsis female gametophyte. Plant J. 2007;51:281–292. doi: 10.1111/j.1365-313X.2007.03137.x. [DOI] [PubMed] [Google Scholar]
- 18.Wuest SE, Vijverberg K, Schmidt A, Weiss M, Gheyselinck J, Lohr M, et al. Arabidopsis female gametophyte gene expression map reveals similarities between plant and animal gametes. Curr Biol. 2010;20:506–512. doi: 10.1016/j.cub.2010.01.051. [DOI] [PubMed] [Google Scholar]
- 19.Yu HJ, Hogan P, Sundaresan V. Analysis of the female gametophyte transcriptoma of Arabidopsis by comparative expression profiling. Plant Physiol. 2005;139:1853–1869. doi: 10.1104/pp.105.067314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang XS, O'Neill SD. Ovary and gametophyte development are coordinately regulated by auxin and ethylene following pollination. Plant Cell. 1993;5:403–418. doi: 10.1105/tpc.5.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pagnussat GC, Alandete-Saez M, Bowman JL, Sundaresan V. Auxin-dependent patterning and gamete specification in the Arabidopsis female gametophyte. Science. 2009;324:1684–1689. doi: 10.1126/science.1167324. [DOI] [PubMed] [Google Scholar]
- 22.Clouse SD, Sasse JM. Brassinosteroids: essential regulators of plant growth and development. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:427–451. doi: 10.1146/annurev.arplant.49.1.427. [DOI] [PubMed] [Google Scholar]
- 23.Müssing C, Shin GH, Altmann T. Brassinosteroids promote root growth in Arabidopsis. Plant Physiol. 2003;133:1261–1271. doi: 10.1104/pp.103.028662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steber CM, McCourt P. A role for Brassinosteroids in germination in Arabidopsis. Plant Physiol. 2001;125:763–769. doi: 10.1104/pp.125.2.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szekeres M, Németh K, Koncz-Kálman Z, Mathur J, Kauschmann A, Altmann T, et al. Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell. 1996;85:171–182. doi: 10.1016/s0092-8674(00)81094-6. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto R, Fujioka S, Demura T, Takatsuto S, Yoshida S, Fukuda H. Brassionosteroid levels increase drastically prior to morphogenesis of tracheary elements. Plant Physiol. 2001;125:556–563. doi: 10.1104/pp.125.2.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bishop GJ, Koncz C. Brassinosteroids and Plant steroid hormone signaling. Plant Cell. 2002;14:97–110. doi: 10.1105/tpc.001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clouse SD, Langford M, McMorris TC. A brassinosteroid-insensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiol. 1996;111:671–678. doi: 10.1104/pp.111.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim TW, Hwang JY, Kim YS, Joo SH, Chang SC, Lee JS, et al. Arabidopsis CYP85A2, a cytochrome P450, mediates the Baeyer-Villiger oxidation of Castasterone to Brassinolide in Brassinosteroid biosynthesis. Plant Cell. 2005;17:2397–2412. doi: 10.1105/tpc.105.033738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nomura T, Kushiro T, Yokota T, Kamiya Y, Bishop GJ, Yamaguchi S. The last reaction producing brassinolide is catalyzed by cytochrome P-450s, CYP85A3 in tomato and CYP85A2 in Arabidopsis. J Biol Chem. 2005;280:17873–17879. doi: 10.1074/jbc.M414592200. [DOI] [PubMed] [Google Scholar]
- 31.Shimada Y, Fujioka S, Miyauchi N, Kushiro M, Takatsuto S, Nomura T, et al. Brassinosteroid-6-oxidases from Arabidopsis and Tomato catalyze multiple C-6 oxidations in Brassinosteroid biosynthesis. Plant Physiol. 2001;126:770–779. doi: 10.1104/pp.126.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vielle-Calzada JP, Thomas J, Spillane C, Coluccio A, Hoeppner MA, Grossniklaus U. Maintenance of genomic imprinting at the Arabidopsis MEDEA locus requires zygotic DDM1 activity. Genes Dev. 1999;13:2971–2982. doi: 10.1101/gad.13.22.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 34.Vielle-Calzada JP, Baskar R, Grossniklaus U. Delayed activation of the paternal genome during seed development. Nature. 2000;404:91–94. doi: 10.1038/35003595. [DOI] [PubMed] [Google Scholar]
- 35.Meyers BC, Lee DK, Vu TH, Tej SS, Edberg SB, Matvienko M, et al. Arabidopsis MPSS. An online resource for quantitative expression analysis. Plant Physiol. 2004;135:801–813. doi: 10.1104/pp.104.039495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyers BC, Tej SS, Vu TH, Haudenschild CD, Agrawal V, Edberg SB, et al. The use of MPSS for whole-genome transcriptional analysis in Arabidopsis. Genome Res. 2004;14:1641–1653. doi: 10.1101/gr.2275604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Honys D, Twell D. Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genom Biol. 2004;5:85. doi: 10.1186/gb-2004-5-11-r85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boyes DC, Zayed AM, Ascenzi R, McCaskill AJ, Hoffman NE, Davis KR, et al. Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. Plant Cell. 2001;13:1499–1510. doi: 10.1105/TPC.010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science. 2003;301:653–657. doi: 10.1126/science.1086391. [DOI] [PubMed] [Google Scholar]
- 40.Sessions A, Burke E, Presting G, Aux G, McElver J, Patton D, et al. A high-throughput Arabidopsis reverse genetics system. Plant Cell. 2002;14:2985–2994. doi: 10.1105/tpc.004630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneitz K, Hüiskamp M, Pruitt RE. Wild-type ovule development in Arabidopsis thaliana: a light microscope study of cleared whole-mount tissue. Plant J. 1995;7:731–749. [Google Scholar]
- 42.Christensen CA, King EJ, Jordan JR, Drews GN. Megagametogenesis in Arabidopsis wild type and the Gf mutant. Sex Plant Reprod. 1997;10:49–64. [Google Scholar]
- 43.Russell SD. Fine structure of megagametophyte development in Zea mays. Can J Bot. 1979;57:1093–1110. [Google Scholar]
- 44.Bajon C, Horlow C, Motamayor JC, Sauvanet A, Robert D. Megasporogenesis in Arabidopsis thaliana L.: an ultrastructural study. Sex Plant Reprod. 1999;12:99–109. [Google Scholar]
- 45.Ye Q, Zhu W, Li L, Zhang S, Yin Y, Ma H, et al. Brassinosteroids control male fertility by regulation the expression of key genes involved in Arabidopsis anthers and pollen development. Proc Natl Acad Sci USA. 2010;107:6100–6105. doi: 10.1073/pnas.0912333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karlova R, Boeren S, Russinova E, Aker J, Vervoort J, de Vries S. The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE1 protein complex includes BRASSINOSTEROID-INSENSITIVE1. Plant Cell. 2006;18:626–638. doi: 10.1105/tpc.105.039412. [DOI] [PMC free article] [PubMed] [Google Scholar]


