Abstract
The ubiquitin proteasome system is involved in the regulation of nearly every aspect of plant growth and development. Protein ubiquitination involves the covalent attachment of ubiquitin to target proteins through a cascade catalyzed by three enzymes known as E1, E2 and E3. E3s are of particular interest as they confer substrate specificity during ubiquitination through their diverse substrate recognition domains. Recently, a number of E3s have been identified that actively participate in abscisic acid hormone biology, including regulation of biosynthesis, de-repression or activation of abscisic acid response and degradation of signaling components. In this review, we summarize recent exciting studies of the different types of E3s that target specific mediators of abscisic acid signaling or affect the plants response to the hormone.
Key words: abscisic acid, E3 ubiquitin ligase, proteasome, ubiquitination
Post-translational control of protein degradation by the ubiquitin proteasome system (UPS) is a highly regulated process essential for the proper growth and development of all eukaryotes through removing abnormal proteins and most short-lived regulatory proteins.1,2 Plants utilize the UPS to alter their proteome to mediate cellular changes required for growth, development and responses to biotic and abiotic stress. Plants also rely a great deal on hormones to induce changes in growth and development in response to a wide range of environmental stimuli. Hormone biosynthesis, perception, signaling and response can be exquisitely regulated through modulating protein levels via the UPS. Regulation of the abscisic acid (ABA) signaling pathway, like auxin, gibberellin, jasmonate and ethylene, have been linked to UPS components with the application of biochemical, genetic and genomic approaches.3–5 Although some aspects of ABA signaling have been elucidated, the involvement of the UPS, especially E3 ubiquitin ligases, help us gain further insight into the entire network of ABA signal transduction. In this review we focus on recently identified E3s that play a variety of roles in ABA signaling. A number of articles are available that provide a comprehensive review of the role of E3 ligases in the biosynthesis, perception and signaling by other hormones such as auxin and ethylene.3–5
Ubiquitin-Proteasome System and E3 Ubiquitin Ligases
The UPS regulates a wide spectrum of cellular processes such as gene transcription, signal transduction, metabolism, DNA repair and replication. The outcome of ubiquitination is the covalent attachment of ubiquitin, an evolutionary conserved 76-amino acid protein, to selected proteins by the sequential action of three enzymes. First, the E1 (ubiquitin-activating enzyme) hydrolyzes ATP to form a thiolester bond between the ubiquitin's C-terminal glycine and the E1's cysteine residue. Activated ubiquitin is then transferred from the E1 to a conserved cysteine residue on E2 (ubiquitin conjugating enzyme). Next, the E3 (ubiquitin ligase) mediates the transfer of ubiquitin to a lysine residue in the substrate protein. Attachment of a single ubiquitin molecule to a substrate protein can regulate the localization or activity of the protein.6 The process can be reiterated to form a polyubiquitin chain on the substrate. Ubiquitin contains seven lysines (K6, K11, K27, K29, K31, K48 and K63) that can all be used to form ubiquitin-ubiquitin linkages.7 Which lysine residue of ubiquitin is used to produce the polyubiquitin chain on a target protein can impart diverse consequences to the substrate. To target substrates for degradation by the 26S proteasome, polyubiquitin chains are made using ubiquitin-ubiquitin linkages between the C-terminal glycine and the K48 of the previous ubiquitin moiety.8 A chain of at least four ubiquitin moieties is required to provide an efficient proteasome delivery signal.9 Other types of polyubiquitin chains have been found to serve as non-proteolytic signals. For example, K63 linked polyubiquitin chains are involved in DNA repair, protein trafficking and kinase activation.10 However, recent studies have shown that K63, as well as K11, linked polyubiquitin chains may also target substrates to the proteasome for degradation.11,12
In both proteolytic and non-proteolytic cases, it is the E3 that confers substrate specificity to the pathway and correctly positions the substrate protein for ubiquitin conjugation. It has been postulated that more than 5% (>1,300 genes) of the Arabidopsis thaliana genome encodes for components that function in the UPS. Ninety percent of these genes encode for components of E3 ubiquitin ligases.2 The large number of E3 encoding genes in Arabidopsis and other eukaryotes indicate the importance of the ubiquitin ligases during the highly regulated process of ubiquitination target selection and development of eukaryotes in general.
E3 ubiquitin ligases can be classified into three groups based on the type of interaction domain used to bind the E2-ubiquitin conjugate. The first group, Homology to E6-Associated Carboxy-Terminus (HECT)-type E3s, is the smallest E3 subfamily with seven members in Arabidopsis.13 The HECT domain, a 350 amino acid motif that contains both a ubiquitin-binding site and an E2-binding site, enables the E3 to form a covalent thioester-linked E3-ubiquitin intermediate before the transfer of ubiquitin to the substrate. The second and third groups of E3s are Really Interesting New Gene (RING)-type and U-box-type E3 ligases. Both RING and U-box domains are thought to be structurally and functionally similar, using zinc chelation or hydrogen bonding, respectively, to build an E2 interaction site.14,15 Unlike HECT-type E3s, RING- and U-box-type E3s mediate ubiquitin transfer directly from the E2 to the substrate without forming an E3-ubiquitin intermediate. Sixty-four genes predicted to encode for U-box-type E3s have been identified in the Arabidopsis genome.16 By contrast, RING-type E3s are the most abundant with 469 predicted RING domain-containing proteins found in the Arabidopsis proteome.17
All HECT and U-box-type E3s and most of the RING-type E3s function as a single subunit enzyme that contains both the E2-ubiquitin and substrate binding module within the same protein. RING domain-containing proteins also function as part of multi-subunit E3 complexes. One large group of multi-subunit RING E3s described in Arabidopsis are the Cullin-based RING Ligases (CRLs), which contain the cullin protein as a scaffold, RING Box 1 (RBX1), a E2 binding RING domain-containing protein, and one or more substrate recognition proteins binding directly to the cullin or indirectly via adaptor proteins. In Arabidopsis, five canonical cullin proteins (CUL1, CUL2, CUL3a, CUL3b and CUL4) have been shown to be components of E3 ligase complexes.18 Based on the combination of cullin and substrate recognition subunit, CRLs are divided into three broad categories: SKP-Cullin-F-box (SCF), in which CUL1 or CUL2 interacts with the substrate recognition F-box protein via an adaptor, S-Phase-kinase-Associated Protein 1 (SKP1); BTB-CUL3-RBX1 (BCR), in which CUL3a or CUL3b interacts directly with substrate recognition proteins that contain a Broad-complex, Tramtrack, Bric-a-Brac/Poxvirus and Zinc Finger (BTB/POZ) domain; CUL4-based ligases which utilize DNADamage Binding 1 (DDB1) as an adaptor to incorporate substrate recognition proteins DDB1-BINDING WD40 (DWD) or DDB1 and CUL4-ASSOCIATED Factor (DCAF) into the complex.18 Although only two Arabidopsis RING domain-containing proteins (RBX1a and RBX1b) have been discovered which serve as the E2 binding subunit of CRLs, the substrate recognition subunits are highly versatile, which greatly increases the number of potential CRLs. For example, over 700 F-box proteins are found in the predicted Arabidopsis proteome.19 Another type of multi-subunit E3 identified within the Arabidopsis proteome is the Anaphase Promoting Complex (APC).20 The APC2 subunit shares homology to cullin and the APC11 subunit is a RING domain-containing protein.
Generally, the activity of E3 ligases can be controlled post-translationally by covalent modifications such as phosphorylation or ubiquitination, by noncovalent binding of proteins or small molecules, or by competition among substrates.21 Plant hormones, jasmonoyl-isoleucine (JA-Ile) and auxin, have been reported to have a similar way of regulating SCF-type E3 ligase activity. Both JA-Ile and auxin bind to E3 ligases directly, which promote the interaction between transcriptional repressors [Jasmonate Zim-domain (JAZ) or Auxin/indole-3-acetic acid (Aux/IAA0]) and F-box proteins [Coronatine Insensitive10 (COI10) or Transport Inhibitor Response 1/Auxin-Binding F-Box Protein (TIR1/AFB)] in the SCF complex, leading to the proteasomal degradation of the repressors, release of the transcriptional activators [MYC2 or Auxin Response Factors (ARFs)] and activation of gene transcription.22,23 In our recent paper, we demonstrated that the RING-type E3 ligase, Keep On Going (KEG), which is involved in ABA signaling, is posttranslationally modified by ubiquitination and phosphorylation in vivo. KEG regulates its own abundance via self-ubiquitination in response to ABA.24 Our data also suggests that autoubiquitination and subsequent 26S proteasome-dependent turn-over of KEG is mediated by changes in KEG phosphorylation status.24
E3 Ligases and ABA Signaling
The plant hormone ABA is an important growth regulator that impacts a number of developmental processes, such as embryo and seed development, seed dormancy, germination, seedling establishment, vegetative development and reproduction.25 In addition, ABA also plays important roles in plant responses to abiotic and biotic stress, including salt, drought, cold and pathogen attack.25 Although the key physiological roles of ABA have been established for some time the network map of ABA signaling is still not fully understood. Recent remarkable progress in deciphering the ABA signaling pathway includes the identification of two types of ABA receptors, a pair of G-protein coupled receptors (GPCR) named GPCR-type G protein 1 (GTG1) and GTG2 and members of the START domain superfamily called Pyrabactin Resistance 1(PYR1)/PYR-like (PYL)/Regulatory Component of ABA Receptor 1 (RCAR1).26,27 The intermediate signaling steps between ABA perception and response are complex and involve a variety of kinases, phosphatases, transcription factors and ubiquitin ligases.
A number of E3 ligases with known and potential roles in regulating ABA signaling have been identified via various methods (Fig. 1). The abundance of the B3-domain transcription factor Abscisic Acid Insensitive 3 (ABI3), which functions as a downstream component of ABA signaling, is regulated by ABI3-interacting protein (AIP2), a RING-type E3 ligase.28 AIP2 can polyubiquitinate ABI3 in vitro. aip2 mutants show higher ABI3 protein levels compared to wild type and are hypersensitive to ABA, whereas AIP2-overexpressing plants contain lower levels of ABI3 protein than wild type and are less sensitive to ABA. Together, these results indicate that AIP2 is a negative regulator of ABA response and that it executes this function through modulating the stability of ABI3.28
Figure 1.
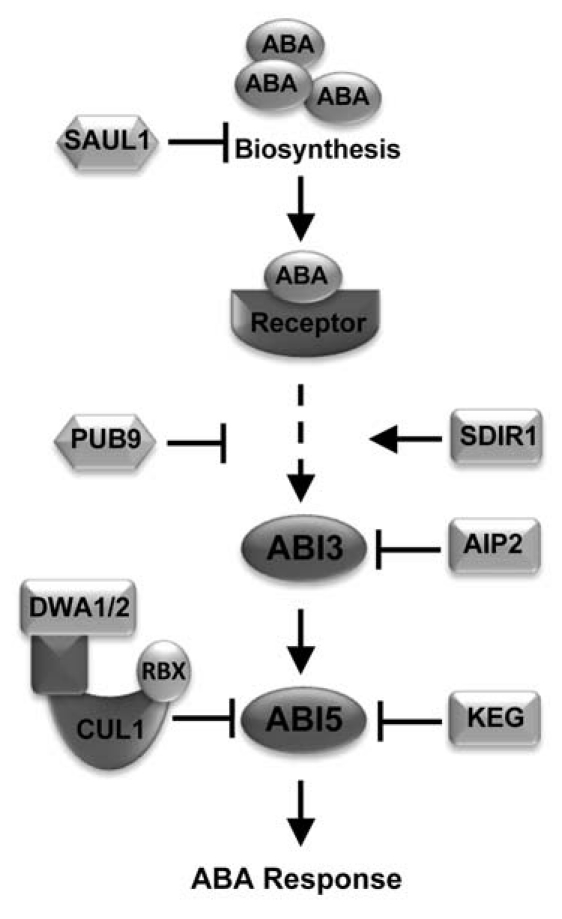
Summary of E3 ligases in the ABA Signaling. U-box type E3s: SAUL1 inhibits ABA biosynthesis; PUB9 is a negative regulator of ABA signaling and function at/or upstream of the ABI3 transcription factor. RING-type E3s: SDIR1 is a positive regulator of ABA signaling that acts upstream of ABI5-like bZIP transcription factors; AIP2 promotes ABI3 degradation; KEG is a negative regulator that modulates ABI5 protein abundance. CRLs: DWA1 and DWA2 are also required for maintaining appropriate levels of ABI5.
ABI5, a member of the Arabidopsis basic leucine zipper (bZIP) transcription factor family, functions downstream of ABI3 to execute ABA-dependent postgermination growth arrest.29,30 The efficiency of the ABA mediated growth arrest is directly dependent on ABI5 protein accumulation through transcriptional activation and enhanced protein stability.30,31 Accumulation of ABI5 protein in seedlings treated with 26S proteasome inhibitors and in RPN10 (a subunit of the 26S proteasome) mutant plants demonstrates that ABI5 turn-over is dependent on the UPS.30,32 KEG, a multi-domain RING-type E3 ligase, has been shown to be required for maintaining low levels of ABI5 in the absence of ABA.24,33 Disruption of KEG gene expression results in growth arrest immediately after germination, accumulation of extremely high levels of ABI5 protein and hypersensitivity to ABA.33 Whereas overexpression of KEG leads to ABA insensitivity.24 Loss of ABI5 in keg-1 background only partially rescues the keg mutant phenotype suggesting that KEG may also regulate other ABA-responsive transcription factors, such as the ABI5-related bZIP proteins.33 We have begun to unravel the mechanism by which ABA protects ABI5 from degradation by KEG.24 In the presence of ABA, KEG protein levels are reduced via ABA-induced selfubiquitination and subsequent degradation by the 26S proteasome, thus allowing ABI5 levels to rise. Mutations within KEG's kinase domain or treatments with kinase inhibitors prohibit the ABA-induced self-ubiquitination and degradation of KEG, suggesting that KEG phosphorylation may be involved in this process.24 Other factors, such as ABI five binding protein (AFP), phosphorylation and sumoylation of ABI5 may also play a role in KEG-mediated ABI5 degradation, however the mechanism involved is still unclear.30,34,35
Several other RING-type E3 ligases have been shown to function during ABA signaling. However, substrates for these E3 ligases remain to be identified. A member of the Arabidopsis Toxico para Levadura (ATL) subfamily of RING proteins, ATL43, may be involved in the ABA response since the T-DNA insertional mutants display an ABA-insensitive phenotype during seed germination assays. The expression of ATL43 has been reported to be induced with ABA treatment, suggesting that ATL43 may be a positive effector of ABA signaling.36 Salt- and Drought-Induced Ring Finger 1 (SDIR1), was also shown to positively regulate ABA signaling.37 Overexpression of SDIR1 leads to ABA hypersensitivity and ABA-associated phenotypes, such as salt hypersensitivity during seed germination, enhanced ABA-induced stomatal closure and enhanced drought tolerance. Overexpression of bZIP transcription factors ABI5, ABRE Binding Factor 3 (ABF3) and ABF4 can rescue the ABA-insensitive phenotype of sdir1, whereas overexpression of SDIR1 could not rescue the abi5 mutation, suggesting that SDIR1 acts upstream of these bZIP transcription factors.37 Similar to SDIR1, RHA2a positively regulates ABAmediated control of seed germination and early seedling development. The RHA2a mutant is less sensitive to ABA than wild type during seed germination and early seedling development, whereas transgenic plants overexpressing RHA2a are hypersensitive. However, RHA2a-mediated ABA response is independent of the ABA-responsive transcription factors ABI3, ABI4 and ABI5.38 The XERICO gene encodes a small RING-H2 domain-containing protein. XERICO-overexpressing plants exhibited hypersensitivity to ABA as well as salt and osmotic stress during seeds germination and early seedling growth. Adult XERICO-overexpressing plants display increased tolerance to drought stress. It is suggested that the XERICO mutant phenotypes are due to an increase in the levels of endogenous ABA biosynthesis but not ABA signaling.39
At least three U-box-type E3 ligases have been linked to specific ABA responses. AtPUB9 is responsive to ABA treatment at the transcript level and the protein is found to relocalize from the nucleus to the plasma membrane in tobacco BY-2 cells following ABA treatment. Atpub9 knockout lines are hypersensitivity to ABA during seed germination assays which can be rescued by loss of ABI3, indicating that AtPUB9 is involved in mediating ABA responses.40 AtPUB44, also known as Senescence-Associated E3 Ubiquitin Ligase 1 (SAUL1), is suggested to function during ABA biosynthesis. SAUL1 mutants exhibit increased ABA production which coincides with enhanced activity and accumulation of Arabidopsis Aldehyde Oxidase 3 (AAO3) that converts abscisic aldehyde to ABA. A direct interaction between SAUL1 and AAO3 was demonstrated, suggesting that SAUL1 targets AAO3 for ubiquitin-dependent proteasomal degradation to prevent premature senescence.41 AtCHIP is another U-box E3 ligase thought to have a role in ABA signaling. AtCHIP can interact with and ubiquitinate the A subunit of protein phosphatase 2A (PP2A), which is a ubiquitous protein phosphatase with links to broad functions in plants including ABA and stress responses. Overexpression of AtCHIP leads to increased PP2A activity and hypersensitivity to the effects of ABA during germination and stomatal aperture opening.42
CRLs have also been reported to be involved in ABA signaling. F-box protein AtTLP9, a member of the Arabidopsis TUBBY-LIKE family, interacts with Arabidopsis Skp1-like 1 (ASK1), a cullin binding protein, suggesting that AtTLP9 acts as a substrate recognition subunit in a SCF E3 ligase complex.43 AtTLP9 mutant lines display an ABA insensitive phenotype during seed germination assays, whereas transgenic plants overexpressing AtTLP9 are hypersensitive to ABA. These results suggest that AtTLP9 is involved in modulating ABA-mediated responses, probably by targeting a negative regulator of ABA signaling for degradation.43 The involvement of another SCF E3 complex in ABA signaling was uncovered through characterization of Drought Tolerance Repressor (DOR), a putative F-box protein, which interacts with ASK14 and CUL1.44 A null mutation in DOR results in a hypersensitive response to ABA during stomatal closing and a substantial increase in drought tolerance. In contrast, transgenic plants overexpressing DOR were more susceptible to drought stress. These results indicate that DOR acts as a negative regulator of the guard cell ABA response.44 Arm Repeat Protein Interacting with ABF2 (ARIA) contains a conserved BTB/POZ domain in its C-terminal region which indicates that ARIA is a potential substrate-specific adaptor for a CUL3-based CRL.45 Overexpression or knockout of ARIA affects a subset of ABA-regulated processes, suggesting that ARIA is a positive regulator of ABA signaling. Although ARIA interacts with ABF2, a transcriptional regulator of ABA responses and both genes display similar expression pattern and subcellular localization, the physiological relevance of the interaction is unknown and the biochemical mechanism of ARIA is unclear.45 DWD hypersensitive to ABA 1 (DWA1) and DWA2, which function as components of CUL4 based CRLs, have been implicated in regulating ABI5 protein levels.46 Both DWA1 and DWA2 interact with ABI5 and both dwa1 and dwa2 accumulate higher levels of ABI5 protein after ABA treatment compared to wild type plants. As a result, both mutants exhibit ABA hypersensitive phenotypes. Double mutant, dwa1 dwa2, shows even stronger ABA hypersensitivity and higher levels of ABI5 accumulation following ABA treatment.46 As mentioned above, KEG has also been shown to target ABI5 for degradation.24,33 The fact that KEG, DWA1 and DWA2 all target the same substrate for degradation is quite interesting. The regulation of a single substrate by multiple E3 ligases has been well documented in animal systems. For example, many E3s promote the ubiquitination of the transcription factor p53 which function to inhibit the proliferation of DNA damaged cells.47 The different E3s may regulate p53 abundance under certain circumstances or within specific cellular compartments. One striking difference between keg and dwa1 or dwa2 is that, in the absence of ABA, keg accumulates extremely high levels of ABI5 whereas ABI5 is only detectable in dwa1, dwa2 or dwa1 dwa2 following treatment with exogenous ABA. Therefore, similar to the p53 scenario, KEG and DWA1 or DWA2 may target ABI5 for degradation under different circumstances, for example at different stages of ABA signaling.
Conclusions and Perspective
Considering the large number and diversity of E3 ubiquitin ligases in plant genomes, it's not surprising that more and more E3s have been identified as regulators of hormone biosynthesis, perception and signaling. As detailed in this review, single subunit RING-type and U-box-type as well as multi-subunit CRLs have all been implicated in ABA biosynthesis and signaling. Figure 1 summarizes known and potential E3 ligases involved in ABA signaling. However, we did not include all E3s (such as RHA2a, ATL43, CHIP and TLP9) discussed in this review into the model due to the unclear roles of these ubiquitin ligases during ABA signaling. Identifying target substrates for these E3 ligases will not only unravel complex patterns of interactions between E3 ligases and ABA signaling components, but also help in expanding our understanding of the ABA signal transduction network. Furthermore, another important question that remains to be addressed is how these E3 ligases are regulated. With clues from KEG and E3s in other hormone signaling pathways, such as auxin and JA-Ile, this question will definitely be resolved gradually.22–24
Acknowledgements
S.L.S. is supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC) and funds from a Human Frontier Science Program Organization (HFSPO) Career Development Award.
References
- 1.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 2.Smalle J, Vierstra RD. The ubiquitin 26S proteasome proteolytic pathway. Annu Rev Plant Biol. 2004;55:555–590. doi: 10.1146/annurev.arplant.55.031903.141801. [DOI] [PubMed] [Google Scholar]
- 3.Stone SL, Callis J. Ubiquitin ligases mediate growth and development by promoting protein death. Curr Opin Plant Biol. 2007;10:624–632. doi: 10.1016/j.pbi.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Dreher K, Callis J. Ubiquitin, hormones and biotic stress in plants. Ann Bot. 2007;99:787–822. doi: 10.1093/aob/mcl255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santner A, Estelle M. The ubiquitin-proteasome system regulates plant hormone signalling. Plant J. 2010;61:1029–1040. doi: 10.1111/j.1365-313X.2010.04112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hicke L. Protein regulation by monoubiquitin. Nat Rev Molecr Cell Biol. 2001;2:195–201. doi: 10.1038/35056583. [DOI] [PubMed] [Google Scholar]
- 7.Peng J, Schwartz D, Elias JE, Thoreen CC, Cheng D, Marsischky G, et al. A proteomics approach to understanding protein ubiquitination. Nature Biotechnol. 2003;21:921–926. doi: 10.1038/nbt849. [DOI] [PubMed] [Google Scholar]
- 8.Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–205. doi: 10.1126/science.1127085. [DOI] [PubMed] [Google Scholar]
- 9.Thrower JS, Hoffman L, Rechsteiner M, Pickart CM. Recognition of the polyubiquitin proteolytic signal. EMBO J. 2000;19:94–102. doi: 10.1093/emboj/19.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fushman D, Pickart CM. Polyubiquitin chains: polymeric protein signals. Curr Opin Chem Biol. 2004;8:610–616. doi: 10.1016/j.cbpa.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson AD, Zhang NY, Xu P, Han KJ, Noone S, Peng J, et al. The lysine 48 and lysine 63 ubiquitin conjugates are processed differently by the 26S proteasome. J Biol Chem. 2009;284:35485–35494. doi: 10.1074/jbc.M109.052928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin L, Williamson A, Banerjee S, Philipp I, Rape M. Mechanism of ubiquitin-chain formation by the human anaphase-promoting complex. Cell. 2008;16:653–665. doi: 10.1016/j.cell.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Downes BP, Stupar RM, Gingerich DJ, Vierstra RD. HECT ubiquitin-protein ligase (UPL) family in Arabidopsis: UPL3 has a specific role in trichome development. Plant J. 2003;35:729–742. doi: 10.1046/j.1365-313x.2003.01844.x. [DOI] [PubMed] [Google Scholar]
- 14.Lorick KL, Jensen JP, Fang S, Ong AM, Hatakeyama S, Weissman AM. RING fingers mediate ubiquitin-conjugating enzyme (E2)-dependent ubiquitination. Proc Natl Acad Sci USA. 1999;96:11364–11369. doi: 10.1073/pnas.96.20.11364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koegl M, Hoppe T, Schlenker S, Ulrich HD, Mayer TU, Jentsch S. A novel ubiquitination factor, E4, is involved in multiubiquitin chain assembly. Cell. 1999;96:635–644. doi: 10.1016/s0092-8674(00)80574-7. [DOI] [PubMed] [Google Scholar]
- 16.Yee D, Goring DR. The diversity of plant U-box E3 ubiquitin ligases: from upstream activators to downstream target substrates. J Exp Bot. 2009;60:1109–1121. doi: 10.1093/jxb/ern369. [DOI] [PubMed] [Google Scholar]
- 17.Stone SL, Hauksdottir H, Troy A, Herschleb J, Kraft E, Callis J. Functional analysis of the RING-type ubiquitin ligase family of Arabidopsis. Plant Physiol. 2005;137:13–30. doi: 10.1104/pp.104.052423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hotton SK, Callis J. Regulation of Cullin RING Ligases. Ann Rev Plant Biol. 2008;59:67–89. doi: 10.1146/annurev.arplant.58.032806.104011. [DOI] [PubMed] [Google Scholar]
- 19.Gagne JM, Downes BP, Shiu S, Durski AM, Vierstra RD. The F-box subunit of the SCF E3 complex is encoded by a diverse superfamily of genes in Arabidopsis. Proc Natl Acad Sci USA. 2002;99:11519–11524. doi: 10.1073/pnas.162339999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capron A, Okresz L, Genschik P. First glance at the plant APC/C, a highly conserved ubiquitin-protein ligase. Trends Plant Sci. 2003;8:83–89. doi: 10.1016/S1360-1385(02)00028-6. [DOI] [PubMed] [Google Scholar]
- 21.Deshaies RJ, Joazeiro CA. Ring domain E3 ubiquitin ligases. Annu Rev Biochem. 2009;78:399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- 22.Tan X, Calderon-Villalobos LI, Sharon M, Zheng C, Robinson CV, Estelle M, et al. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature. 2007;446:640–645. doi: 10.1038/nature05731. [DOI] [PubMed] [Google Scholar]
- 23.Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, et al. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature. 2007;448:661–665. doi: 10.1038/nature05960. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Stone SL. Abscisic acid increases Arabidopsis ABI5 transcription factor levels by promoting KEG E3 ligase self-ubiquitination and proteasomal degradation. Plant Cell. 2010;22:2630–2641. doi: 10.1105/tpc.110.076075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol. 2010;61:651–679. doi: 10.1146/annurev-arplant-042809-112122. [DOI] [PubMed] [Google Scholar]
- 26.Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, et al. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science. 2009;324:1064–1068. doi: 10.1126/science.1172408. [DOI] [PubMed] [Google Scholar]
- 27.Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science. 2009;324:1068–1071. doi: 10.1126/science.1173041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Garreton V, Chua NH. The AIP2 E3 ligase acts as a novel negative regulator of ABA signaling by promoting ABI3 degradation. Gen Dev. 2005;19:1535–1543. doi: 10.1101/gad.1318705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez-Molina L, Mongrand S, McLachlin DT, Chait BT, Chua NH. ABI5 acts downstream of ABI3 to execute an ABA-dependent growth arrest during germination. Plant J. 2002;32:317–328. doi: 10.1046/j.1365-313x.2002.01430.x. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Molina L, Mongrand S, Chua NH. A postgermination developmental arrest checkpoint is mediated by abscisic acid and requires the AB15 transcription factor in Arabidopsis. Proc Natl Acad Sci USA. 2001;98:4782–4787. doi: 10.1073/pnas.081594298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brocard IM, Lynch TJ, Finkelstein RR. Regulation and role of the Arabidopsis abscisic acid-insensitive 5 gene in abscisic acid, sugar and stress response. Plant Physiol. 2002;129:1533–1543. doi: 10.1104/pp.005793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smalle J, Kurepa J, Yang P, Emborg TJ, Babiychuk E, Kushnir S, et al. Pleiotropic role of the 26S proteasome subunit RPN10 in Arabidopsis growth and development supports a substrate-specific function in Abscisic Acid signaling. Plant Cell. 2003;15:965–980. doi: 10.1105/tpc.009217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stone SL, Williams LA, Farmer LM, Vierstra RD, Callis J. Keep on going: A RING E3 ligase essential for Arabidopsis growth and development, is involved in abscisic acid signaling. Plant Cell. 2006;18:3415–3428. doi: 10.1105/tpc.106.046532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez-Molina L, Mongrand S, Kinoshita N, Chua NH. AFP is a novel negative regulator of ABA signaling that promotes ABI5 protein degradation. Genes Dev. 2003;17:410–418. doi: 10.1101/gad.1055803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miura K, Lee J, Jin JB, Yoo CY, Miura T, Hasegawa PM. Sumoylation of ABI5 by the Arabidopsis SUMO E3 ligase SIZ1 negatively regulates abscisic acid signaling. Proc Natl Acad Sci USA. 2009;106:5418–5423. doi: 10.1073/pnas.0811088106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serrano M, Parra S, Alcaraz LD, Guzman P. The ATL gene family from Arabidopsis thaliana and Oryza sativa comprises a large number of putative ubiquitin ligases of the RING-H2 type. J Molec Evol. 2006;62:434–445. doi: 10.1007/s00239-005-0038-y. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Yang C, Li Y, Zheng N, Chen H, Zhao Q, et al. SDIR1 is a RING finger E3 ligase that positively regulates stress-responsive abscisic acid signaling in Arabidopsis. Plant Cell. 2007;19:1912–1929. doi: 10.1105/tpc.106.048488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bu Q, Li H, Zhao Q, Jiang H, Zhai Q, Zhang J, et al. Arabidopsis RING finger E3 ligase RHA2a is a novel positive regulator of abscisic acid signalling during seed germination and early seedling development. Plant Physiol. 2009;150:463–481. doi: 10.1104/pp.109.135269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ko J, Yang SH, Han K. Upregulation of an Arabidopsis RING-H2 gene, XERICO, confers drought tolerance through increased abscisic acid biosynthesis. Plant J. 2006;47:343–355. doi: 10.1111/j.1365-313X.2006.02782.x. [DOI] [PubMed] [Google Scholar]
- 40.Samuel MA, Mudgil Y, Salt JN, Delmas F, Ramachandran S, Chilelli A, et al. Interactions between the S-domain receptor kinases and AtPUB-ARM E3 ubiquitin ligases suggest a conserved signaling pathway in Arabidopsis. Plant Physiol. 2008;147:2084–2095. doi: 10.1104/pp.108.123380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raab S, Drechsel G, Zarepour M, Hartung W, Koshiba T, Bittner F, et al. Identification of a novel E3 ubiquitin ligase that is required for suppression of premature senescence in Arabidopsis. Plant J. 2009;59:39–51. doi: 10.1111/j.1365-313X.2009.03846.x. [DOI] [PubMed] [Google Scholar]
- 42.Luo J, Shen G, Yan J, He C, Zhang H. AtCHIP functions as an E3 ubiquitin ligase of protein phosphatase 2A subunits and alters plant response to abscisic acid treatment. Plant J. 2006;46:649–657. doi: 10.1111/j.1365-313X.2006.02730.x. [DOI] [PubMed] [Google Scholar]
- 43.Lai CP, Lee CL, Chen PH, Wu SH, Yang CC, Shaw JF. Molecular analyses of the Arabidopsis TUBBY-like protein gene family. Plant Physiol. 2004;134:1586–1597. doi: 10.1104/pp.103.037820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Xu W, Li Z, Deng XW, Wu W, Xue Y. F-box protein DOR functions as a novel inhibitory factor for abscisic acid-induced stomatal closure under drought stress in Arabidopsis. Plant Physiol. 2008;148:2121–2133. doi: 10.1104/pp.108.126912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim S, Choi HI, Ryu HJ, Park JH, Kim MD, Kim SY. ARIA, an arabidopsis Arm repeat protein interacting with a transcriptional regulator of abscisic acid-responsive gene expression, is a novel abscisic acid signaling component. Plant Physiol. 2004;136:3639–3648. doi: 10.1104/pp.104.049189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JH, Yoon HJ, Terzaghi W, Martinez C, Dai M, Li J, et al. DWA1 and DWA2, two Arabidopsis DWD protein components of CUL4-based E3 ligases, act together as negative regulators in ABA signal transduction. Plant Cell. 2010;22:1716–1732. doi: 10.1105/tpc.109.073783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benkirane M, Sardet C, Coux O. Lessons from interconnected ubiquitylation and acetylation of p53: think metastable networks. Biochem Soc Trans. 2010;38:98–103. doi: 10.1042/BST0380098. [DOI] [PubMed] [Google Scholar]


