Abstract
Pharmacological studies, using Ca2+ channel blockers (LaCl3 and verapamil) and calmodulin (CaM) antagonists (CPZ and W7), were carried out to understand the role of Ca2+/CaM in the regulation of heat shock-induced expression of Hsp90 (Hsp87 and Hsp85) and Hsp70 (Hsp75 and Hsp73) members in sorghum. It was observed that the expression of both Hsp87 and Hsp85 proteins was decreased in presence of Ca2+ channel blockers and CaM antagonists, under both control and heat stress conditions, as contrary to the steady state levels of Hsp75 and Hsp73, which were not affected significantly under similar conditions. Further, the exposure of sorghum seedlings to geldanamycin, a specific inhibitor of Hsp90, resulted in induction of Hsp87 and Hsp85 in the absence of heat shock also. This study provides the first evidence suggesting that in plants, the in vivo expression of Hsp90 (Hsp87 and Hsp85) is likely to be modulated by Ca2+/CaM under normal and thermal stress conditions. The likely implications of these findings are discussed.
Key words: calmodulin, calmodulin-binding proteins, heat shock proteins, sorghum
Introduction
One of the adaptive responses in plants to high temperature stress involves accumulation of proteins, called heat shock proteins (Hsps). Heat shock proteins perform chaperonic functions and protect cellular proteins from heat-induced damage beside assisting in refolding of the damaged proteins.1 Hsps accumulated in plants can be broadly categorized into five different families, viz. small Hsps (12–40 kDa), Hsp60 (chaperonin), Hsp70, Hsp90 and Hsp100. In mammals, besides being implicated in refolding of newly synthesized and denatured proteins, the members of Hsp90 family also play an important role in regulating key cellular signal molecules like glucocorticoid receptors. Recent studies suggest that in plants, Hsp90 buffers the wild type morphology against developmental or environmental perturbations.2 In vitro studies have shown that Hsp90 is a major repressor of heat shock transcription factor (HSF1), which is responsible for the induction of Hsp genes upon heat shock.3 In vivo evidence for the role of Hsp90 in heat shock response and heat acclimation of Arabidopsis has also been provided recently.4 Although the biochemical activity of Hsp90 is conserved between plants and animals, the molecular mechanisms involved in the regulation of Hsp90 are poorly understood in plants.
Recent studies carried out in our laboratory demonstrated that an 85 kDa heat-modulated protein (Hsp85) from sorghum, identified as a member of Hsp90 family, exhibited calmodulin (CaM)-binding property.5 Heat shock signals are transduced through changes in cytosolic free Ca2+ levels, which are detected by Ca2+ sensors, with CaM being the most extensively characterized.6 The active form of CaM (Ca2+-bonded CaM) binds and regulates the activity of diverse range of proteins, which are called CaM-binding proteins (CaMBPs).7 Although calmodulin was reported to regulate the expression of Hsp70 gene in wheat,8 the role of CaM in regulation of Hsp90 has not been studied as yet in plants. Since Hsp90 has been shown to act as a key regulator of heat shock response3,4 as well as normal development in plants,2 it is imperative that the physiological significance of CaM-Hsp90 interaction be elucidated. Therefore, in the present study we analysed the role of Ca2+-CaM pathway in heat shock-induced regulation of Hsp85 in sorghum seedlings by analyzing the effects of Ca2+ channel blockers and calmodulin antagonists. Further, the effect of these pharmacological agents was also investigated on the steady state levels of Hsp70 (Hsp75 and Hsp73) in sorghum since earlier studies9 showed that maize Hsp70 binds to CaM and its expression at transcript level in wheat is regulated through Ca2+/CaM pathway.8 However, the changes in steady state levels of Hsp70 protein were not analyzed in the latter study.
Results and Discussion
Of the several Hsps synthesized by the cell in response to heat stress, Hsp90 is emerging as a central interface between organism, development and environment.2,15,16 Members of Hsp90 family have been implicated in controlling heat shock response in plants. Recent study has shown that geldanamycin and radicicol, two specific inhibitors of Hsp90, induce heat shock genes and heat acclimation in Arabidopsis.4 These studies provide further evidence that as proposed earlier,3 the Hsp90 keeps the heat shock factor (HSF) in an inactive state thus repressing the heat shock response under non-heat stress conditions. Studies in our lab demonstrated that a constitutively expressed Hsp85 in sorghum, which was identified as a member of Hsp90 family, binds to CaM.5 Besides Hsp85, another protein of 87 kDa (Hsp87), which was inducible by heat stress, was also detected by these antibodies in our studies. However, the biological significance of interaction of CaM with Hsp85 in sorghum is not known. Since CaM is a key sensor of changes in intracellular Ca2+ we, therefore, employed Ca2+ channel blockers, LaCl3 and verapamil, and CaM antagonists, W7 and CPZ for understanding the role of CaM in the regulation of Hsp85 under heat stress in the sorghum seedlings. The concentrations of the various inhibitors, which were comparable to that used in the previous studying reference 8, did not affect the viability of seedlings till the end of experiment (data not shown).
LaCl3 and verapamil prevent Ca2+ influx across the plasma membrane in plants17 and block a voltage-dependent Ca2+ selective channel18 thus disturbing the Ca2+ homeostat of cells. The effect of these compounds on the steady state levels of Hsp90 (Hsp87 and Hsp85) and Hsp70 (Hsp75 and Hsp73) at 37°C was determined at different concentrations. Our previous studies demonstrated that Hsp85 is constitutively expressed, whereas, Hsp87, is inducible by heat stress.5 It was observed that as compared to control (without inhibitor treatment), the steady state levels of Hsp85 at 37°C were decreased significantly by LaCl3 and verapamil, with the latter at 1 mM resulting in almost complete disappearance (Fig. 1A), thus implicating the role of Ca2+ in the regulation of this protein. The role of Ca2+ as a positive regulator of Hsp85 was further supported by the fact that the expression of Hsp85 at 37°C was enhanced significantly at both concentrations of exogenous Ca2+ (Fig. 2A). On the contrary, the steady state levels of Hsp73 at 37°C were neither decreased by LaCl3 or verapamil (Fig. 1B) nor increased by Ca2+ (Fig. 2B) in a statistically significant manner.
Figure 1.
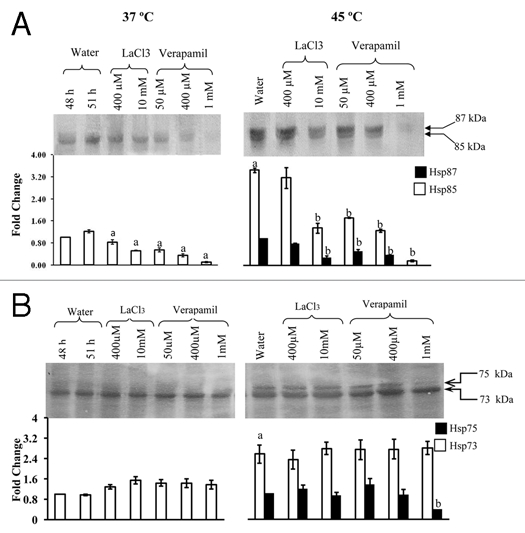
Immunoblot analysis of Hsp87 and Hsp85 (A), and Hsp75 and Hsp73 (B) in sorghum. The 24-h-old seedlings were treated for 24 h with LaCl3 and verapamil at 37°C and exposed to heat shock at 45°C for 3 h. 25 µg of total proteins were resolved on 8% SDS-PAGE, transferred on to Hybond C membrane and probed with anti-N. crassa Hsp80 and anti-S. bicolor Hsp70 polyclonal antibodies. Densitometer scanning shows the fold change in intensity of Hsp85 (□) and Hsp73 (□) compared to the pre-treatment stage (48 h), whereas the intensity of Hsp87(▪) and Hsp75 (▪) is relative to the water grown heat-stressed seedlings (45°C) in this and subsequent frames. The data were subjected to one way analysis of variance. (A): Significant difference at p ≤ 0.05 with respect to 37°C water-grown control at 51 h. (B): Significant difference at p ≤ 0.05 with respect to 45°C water-grown seedlings.
Figure 2.
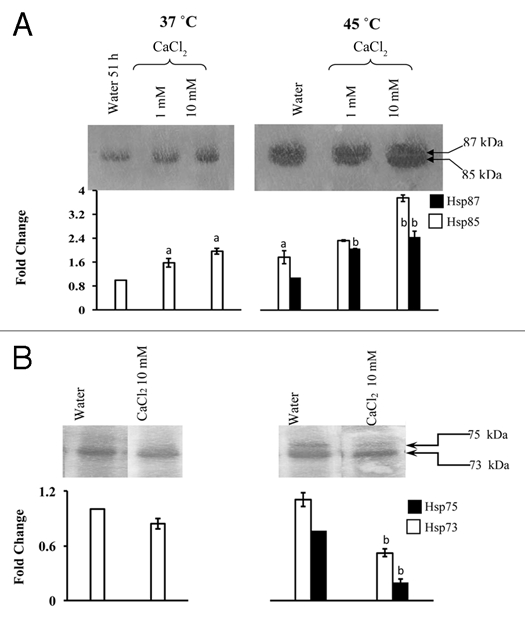
Accumulation of Hsp87 and Hsp85 (A), and Hsp75 and Hsp73 (B) in presence of Ca2+ at 37°C and 45°C. Immunoblotting and densitometric scanning was carried out as described for Figure 1. The data were subjected to one way analysis of variance. (A): Significant difference with respect to 37°C water-grown control at p ≤ 0.05. (B): Significant difference with respect to 45°C water-grown seedlings at p ≤ 0.05.
Calmodulin is one of the most well characterized sensors of intracellular Ca2+. To determine whether the Ca2+-mediated changes in Hsp85 under normal temperature (37°C) are regulated through CaM, sorghum seedlings were exposed to CaM antagonists CPZ and W7. The accumulation of Hsp85 in the sorghum seedlings grown at 37°C was significantly lower in response to 200 µM CPZ as compared to the water grown control, whereas, CPZ at 50 µM did not show any significant effect (Fig. 3A). The W7 at 500 µM, another CaM antagonist, also reduced the levels of Hsp85 significantly, whereas, W5, an inactive analogue of W7, had no apparent effect thus implying that the expression of Hsp85 under normal growth conditions is likely to be mediated specifically through CaM. The steady state levels of Hsp70 protein at 37°C, however, were not affected significantly by CPZ or W7 (Fig. 3B) thus signifying that steady state levels of Hsp70 proteins in sorghum may not be regulated through Ca2+/CaM pathway.
Figure 3.
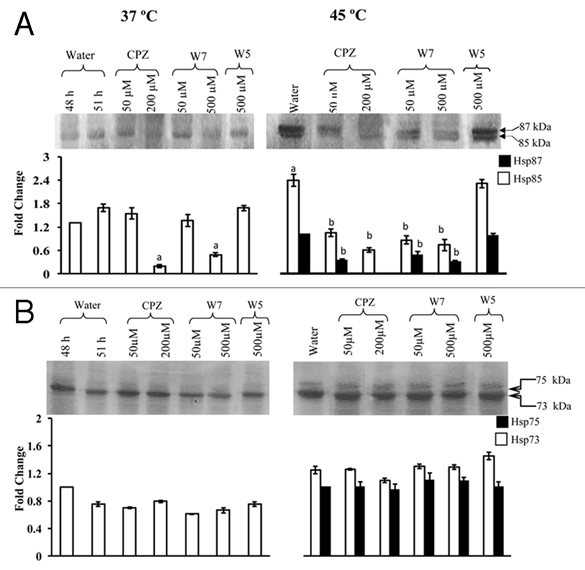
Effect of calmodulin antagonists, CPZ and W7, on accumulation of Hsp87 and Hsp85 (A), and Hsp75 and Hsp73 (B) in the 24 h-old seedlings at 37°C and in response to heat shock at 45°C for 3 h. W5 was used as an inactive analogue of W7. Immunoblotting and densitometric scanning was carried out as described for Figure 1. The data were subjected to one way analysis of variance. (A): significant difference at p ≤ 0.05 with respect to 37°C water-grown control at 51 h. (B): significant difference at p = 0.05 with respect to 45°C water-grown seedlings.
The effect of heat stress on steady state levels of Hsp87, Hsp85, Hsp75 and Hsp73 was studied at sublethal (45°C) and lethal (50°C) temperatures for 3 h. In response to heat shock at 50°C for 3 h, the plants died after 24 h of transfer to 37°C, whereas, plants exposed to 45°C for the same duration continued to grow but at rates significantly lower than the seedlings maintained at 37°C (data not shown). Although, compared to 37°C control, imposition of stress at both 45°C (Fig. 1A) and 50°C (Fig. 4A) resulted in significantly enhanced levels of Hsp85 and Hsp87 but enhancement in Hsp87 was several folds higher at 45°C which is consistent with our earlier observations.5 Temperature stress at 45°C (Fig. 1B) and 50°C (Fig. 4B) also resulted in significant increase in the steady state levels of Hsp73. An additional protein of 75 kDa (shown by arrowhead), which was induced only at 45°C, was also detected by anti-Hsp73 antibodies (Fig. 1B). The Hsp75 appears to be the inducible isoform of Hsp70 family, as reported earlier in Arabidopsis.19 However, the possibility that Hsp75 represents a post-translational modification, as observed for bean Hsp70,20 can't be ruled out and hence warrants further investigations. As compared to 45°C, lesser induction of Hsp85 and Hsp87 and the absence of Hsp75 at 50°C may be due to general decrease in protein synthesis at higher temperature, as observed earlier also in sorghum.21
Figure 4.
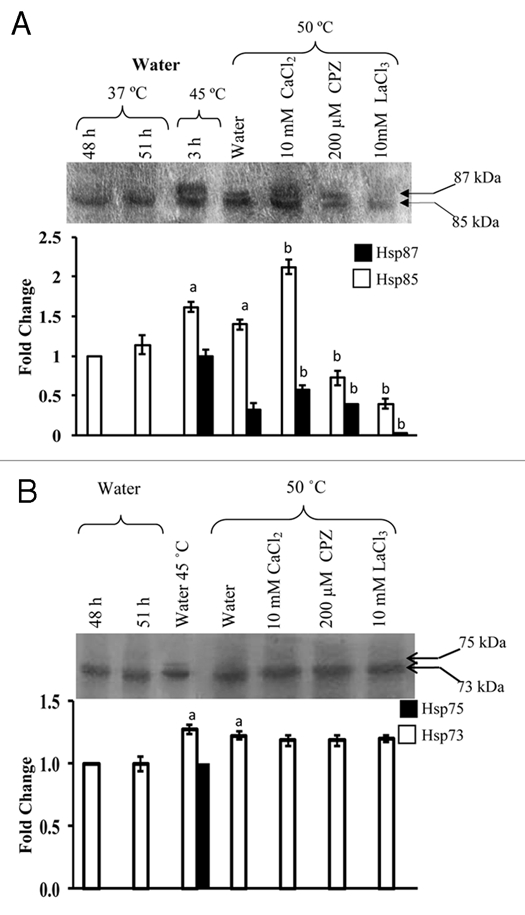
Effect of heat shock at lethal temperature (50°C) on expression of Hsp87 and Hsp85 (A), and Hsp75 and Hsp73 (B) in 24 h-old sorghum seedlings treated for 24 h with CaCl2, CPZ and LaCl3. Immunoblotting and densitometric scanning was carried out as described for Figure 1. The data were subjected to one way analysis of variance. (A): significant difference at p ≤ 0.05 with respect to 37°C water-grown control at 51 h. (B): significant difference at p ≤ 0.05 with respect to 50°C water-grown seedlings.
Role of Ca2+/CaM pathway in heat-shock regulation of Hsp90 and Hsp70 members in sorghum at 45°C was studied using various concentrations of the different inhibitors. For studies at 50°C, only selected inhibitors at a single concentration were used. The heat stress-induced increase in Hsp85 and Hsp87 at 45°C was lower in the LaCl3-treated plants as compared to water-grown seedlings, with higher concentration (10 mM),22 being more effective as compared to 400 µM (Fig. 1A). The heat stress-induced increase in Hsp85 and Hsp87 at 50°C was also inhibited significantly by exogenous LaCl3 (Fig. 4A). Verapamil at higher concentration (1 mM) resulted in almost complete inhibition in the accumulation of Hsp85 and Hsp87 at 45°C, whereas, at lower concentrations (50 and 400 µM) both the proteins were detected in the heat shocked seedlings, albeit at a significantly lower level than the water grown heat-shocked seedlings (Fig. 1A). The expression of Hsp73 at both 45°C (Fig. 1B) and 50°C (Fig. 4B) was not affected significantly by LaCl3 and verapamil. These results imply that the presence of LaCl3 and verapamil attenuated the heat stress-induced increase only in Hsp85 and Hsp87. Intracellular levels of Ca2+ are elevated in response to heat shock23 which is attributed to depolarization of the plasma membrane and the influx of extracellular Ca2+ into the cells.24 La3+ and verapamil block the Ca2+-channels thus preventing the Ca2+ influx. Inhibition of heat shock-induced synthesis of Hsp85 and 87 by LaCl3 and verapamil, but not of Hsp73, therefore, implies the role of Ca2+ as a transducing signal in the regulation of Hsp90 in sorghum. The role of Ca2+ in regulation of Hsp90 members was also evident from the fact that application of exogenous Ca2+ at 10 mM resulted in a significant enhancement in the heat stress-induced increase in Hsp85 and Hsp87 at both 45°C (Fig. 2A) and 50°C (Fig. 4A). The effect of Ca2+ on Hsp70 proteins was temperature dependent. Whereas, the steady state level of Hsp73 was not affected significantly by exogenous Ca2+ at 50°C (Fig. 4B), the levels of both Hsp75 and Hsp73 at 45°C were significantly decreased in the presence of Ca2+ (Fig. 2B). It is likely that Ca2+ may be mitigating the effect of heat stress in sorghum plants through increase in Hsp90s (Hsp87 and Hsp85) instead of Hsp70s (Hsp75 and Hsp73). Further, application of Ca2+ did not result in detectable induction of Hsp87 at 37°C in the absence of heat shock (Fig. 2A) thus further implying the inducible nature of this isoform. Four cytosolic isoforms of Hsp90, which are immunologically homologous, have been reported in Arabidopsis, out of which three are constitutive and one inducible.2,25 It is likely that Hsp87 may be a homologue of inducible Hsp90 of Arabidopsis,25 which needs confirmation by cloning of the sorghum Hsp87 gene. Alternatively, it is also possible that Hsp87 may be the result of a post-translational modification, as has been reported for small Hsps in A. thaliana26 and Hsp70 in bean.20
To determine as to whether the heat shock-induced regulation of Hsp90 and Hsp70 in sorghum is mediated through CaM, the seedlings were treated with CaM antagonists CPZ, W7 and inactive analogue, W5, for studies at 45°C. However, only CPZ (200 µM) was used for studies at 50°C as it was more effective than W7 in inhibiting Hsp87 and Hsp85. The heat shockinduced increase in Hsp87 and Hsp85 at 45°C was significantly decreased by both 50 and 200 µM CPZ, with the two proteins being barely detectable at higher concentration (Fig. 3A). The increase in Hsp85 at 50°C was also inhibited by CPZ (Fig. 4A). The W7, another CaM antagonist, also downregulated the levels of Hsp85 and Hsp87 significantly at 45°C, the inhibition being marginally higher at 500 µM as compared to 50 µM (Fig. 3A), whereas W5 had no apparent effect. The attenuation of the increase in Hsp87 and Hsp85 at 45°C by CPZ and W7, and at 50°C in the presence of CPZ implies that the accumulation of these proteins under heat shock is also being regulated by Ca2+/CaM pathway. On the contrary, the heat-shock induced increase in the steady-state levels of Hsp75/Hsp73 at 45°C (Fig. 3B) and 50°C (Fig. 4B) was not affected significantly by CPZ or W7. The lack of apparent effect of Ca2+ channel blockers and CaM antagonists on sorghum Hsp70 members (Hsp75 and Hsp73), observed in this study, is at variance with the earlier findings, which reported inhibition of HSP70 expression at transcript level by these inhibitors in wheat.8 This variability in response of Hsp70 between sorghum and wheat may either be due to genotypic differences and/or due to regulation of Hsp70 at post-transcriptional level.27 This study is, therefore, the first to provide evidence that instead of Hsp70, as reported in wheat,8 it is the expression of Hsp90 (Hsp87 and Hsp85) in sorghum, which appears to be modulated by Ca2+/CaM. We, however, acknowledge that the CaM antagonists, W7 and CPZ, are reported to bind to plant Ca2+-dependent protein kinases28,29 and also affect the plants in a non-specific manner30 but the binding of Hsp90 with CaM in Ca2+-dependent manner5 supports its regulation by CaM. It is also pertinent to mention that despite the possible non-specific effects of these antagonists, several studies carried out recently have used this system and demonstrated CaM-specific inhibition.8,31–36 Furthermore, the differential changes in steady state levels of Hsp70 and Hsp90 in response to Ca2+ channel blockers and CaM, as observed in the present study, also imply that the effect of these compounds on the two classes of proteins was specific and was not the result of a general effect on all proteins.
In order to gain insight into the role of Hsp90 in regulation of heat shock response in sorghum, the seedlings were treated with geldanamycin (GDA), a specific inhibitor of Hsp90. Incubation with GDA for a period of 6 h and 9 h, which was decided after time course studies (data not shown), resulted in significantly enhanced levels of Hsp87 and Hsp85 even in the absence of heat shock also (Fig. 5A), which is in confirmation with the earlier observations in Arabidopsis.4 On the contrary, steady state levels of Hsp73 showed a consistent but non-significant decline in the presence of GDA. Induction under non-heat stress conditions by geldanamycin, therefore, provides further evidence that Hsp90 is likely to be one of the key regulators of heat shock response in sorghum. As proposed earlier for Arabidopsis,4 the geldanamycin-induced heat shock response in sorghum (this study) may be due to the dissociation of Hsp90 from the heat shock factor (HSF), which is maintained in an inactive state by Hsp90 under optimal temperature conditions.3 Though information on molecular mechanisms that inhibit the binding of Hsp90 to HSF in vivo is scanty, a hypothetical model proposed by Yamada et al.37 suggested that unfolded proteins, produced under heat shock, may be interacting with Hsp90, thereby, releasing the HSF, which is responsible for activation of heat shock responsible genes. Based on the present study it is proposed that besides unfolded proteins, the CaM, whose steady state levels are enhanced within 5–10 min under heat stress in plants23,38 including sorghum (our unpublished data), may also be responsible for binding to Hsp90 thus resulting in the release of HSF and consequently the expression of heat shock proteins. We hereby propose a modification to Yamada's model37 incorporating CaM also as one of the regulatory aspects (Fig. 6). Support for the CaM being a natural inhibitor of Hsp90 also comes from the earlier studies which reported the induction of Hsp genes in the transgenic Arabidopsis plants that overexpressed CaM gene.38 These authors, however, attributed the induction of Hsp genes to the modulation of DNA-binding activity of HSF by CaM, which is inconsistent with the fact that inhibition of Hsp90 by specific inhibitor results in induction of heat shock response in both sorghum (this study) and Arabidopsis.4 Further investigations to elucidate the in vivo physical interaction of Hsp85 with CaM, and also the effect of Ca2+ and CaM on biochemical interaction of Hsp85 with HSF are underway for validation of the proposed model.
Figure 5.
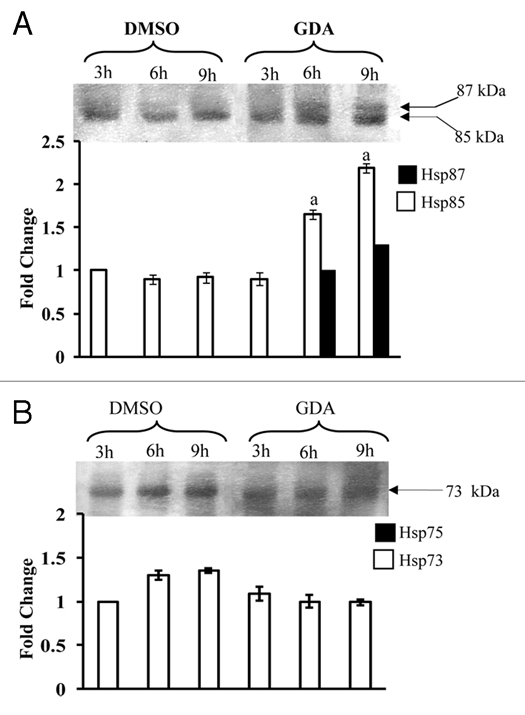
Effect of geldanamycin (5 µM) on expression of Hsp87 and Hsp85 (A), and Hsp75 and Hsp73 (B) at 37°C at different time intervals. 24 hr-old sorghum seedlings were treated with geldanamycin under dark conditions for 3, 6 and 9 h at 37°C. The respective controls were irrigated with water containing equal volume of DMSO. Immunoblotting was carried out as described for Figure 1. The data were subjected to one way analysis of variance. (A): significant difference at p = 0.05 with respect to the respective DMSO controls.
Figure 6.
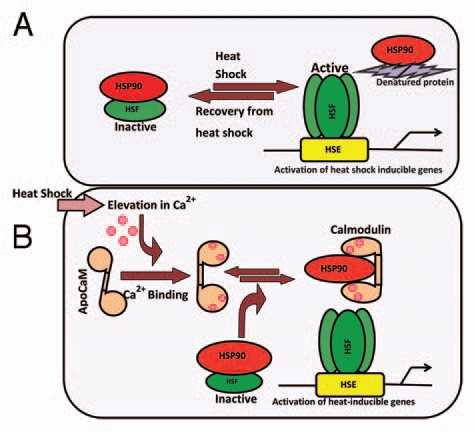
Modification of the hypothetical model (A), proposed by Yamada et al. (2008),37 to understand the molecular mechanisms underlying the regulation of Hsp90 family members (Hsp85 here) in the absence and presence of heat shock. It is proposed that besides denatured proteins, calmodulin (CaM) may also be regulating the heat shock response (B). Heat shock-induced transient increase in intracellular Ca2+ activates apocalmodulin (ApoCaM; inactive form) to its Ca2+-bound active form. The Ca2+-CaM binds to Hsp90 and dissociates the monomeric heat shock factor (HSF), which trimerizes and interacts with heat shock elements (HSE) thus resulting in upregulation of the heat shock-inducible genes.
Materials and Methods
The seeds of sorghum (Sorghum bicolor L. Moench) cultivar GK908 were surface sterilized with 0.1% (m/v) mercuric chloride and 70% ethanol followed by washing with autoclaved double distilled water (ADDW). All chemicals used, except N-(6-Aminohexyl)-1-naphthalenesulfonamide HCl (W5), were of molecular biology grade and purchased from Sigma-Aldrich, USA. W5 was procured from (Enzo Life Sciences, Plymouth Meeting, USA). Seeds were imbibed overnight (12 h) in ADDW at 37°C and sown on autoclaved non-absorbent wet cotton for germination in the dark at 37°C for 24 h. To study the effect of CaCl2, Ca2+ channel blockers [Lanthanum (III) Chloride (LaCl3) and verapamil] and CaM antagonists [chlorpromazine (CPZ), N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide (W7) and W5 (inactive analogue of W7)], the 24 h-old-seedlings were transferred on to sterile, cotton bedded disposable 90 mm petriplates, which were irrigated with 30 ml of these solutions, and incubated at 37°C for further 24 h. Exposure of seedlings to inhibitors for 24 h ensured that the intracellular concentration of these compounds was not a limiting factor. Final working concentrations used for different inhibitors were; LaCl3-400 µM and 10 mM; verapamil-50 µM, 400 µM and 1 mM; CPZ-50 µM and 200 µM; W7-50 µM and 500 µM; W5-500 µM; CaCl2-1 mM and 10 mM. Except for W5 (100 mM in DMSO) and W7 (100 mM in methanol), all stock solutions (100 mM) were prepared in water. The control plants were treated with water in an identical manner, except for W5 and W7 controls, which were irrigated with water containing equal volume of respective solvents. Effect of heat shock was studied after 24 h of inhibitor treatment by incubating the seedlings at 45°C and 50°C for 3 h in an incubator. For studying the effect of geldanamycin (GDA) (stock of 10 mg/ml in DMSO) on Hsps, the seedlings were treated with the inhibitor (5 µM) for different time periods. All experiments were conducted as two or three biological replicates, each of which contained 50–60 seedlings for every treatment. After imposition of treatments, the seedlings were harvested and stored in liquid nitrogen till further analysis.
Purification and identification of Hsp73.
The Hsp73 was identified as a CaM-binding protein by 2-D electrophoresis followed by CaM-gel overlay assay as described earlier in reference 5. The CaMBPs, purified after CaM-affinity chromatography, were resolved on preparative SDS-PAGE gel. Before staining, a lane was sliced from the gel and transferred to Hybond C membrane for CaM-gel overlay assay. Rest of the gel was subjected to Zn2+-reverse staining according to Ortiz et al.10 Briefly, the gel was incubated in 0.2 M imidazole and 0.1% SDS for 15 min at room temperature. After brief washing with ADDW, the gel was soaked in 0.2 M ZnSO4 till the development of transparent bands against a white background. The 73 kDa band, which corresponded to the CaM-binding protein on Hybond C membrane, was excised and the protein was eluted and electrodialyzed against 50 mM Tris-Cl pH 7.5 for 1 h using Electro-Eluter (Model 422, Bio-Rad laboratories, USA), as per the manufacturer's instructions. The identity of the extracted protein (Hsp73), as a member of Hsp70 family, was established by MALDI-TOF/TOF analysis before immunization. The eluted protein was digested with Trypsin and the digested peptides were used for obtaining mass spectrum using MALDI-TOF/TOF (Autoflex II TOF/TOF, Bruker Daltonics, Germany). The obtained spectrum was further used for MASCOT search to get the homology of the peptide fragments with known proteins in the database. This analysis clearly indicated the protein to be a member of Hsp70 family.
Raising of polyclonal antibodies.
The polyclonal antibodies against sorghum Hsp73 were raised in female New Zealand White rabbits (2.0 kg) according to Harlow and Lane.11 100 µg of purified Hsp73 protein was mixed with Freunds complete adjuvant in 1:1 and mixed thoroughly using Luer Lock syringe and injected subcutaneously on dorsal thorax and lumbar area of female rabbits. First and second antigen boosters were given to rabbits after 20 and 35 days of primary immunization. For booster, 100 µg of protein was mixed with Freunds incomplete adjuvant in 1:1 ratio and injected intramuscularly on both the hind legs. Rabbits were bled from marginal ear vein and blood was collected in sterile vial. The whole serum was separated from blood by incubating the vials at 37°C for overnight followed by centrifugation at 14,000 rpm. The serum was transferred to a fresh sterile vial and stored at −20°C for titre determination and immunobloting.
Immunoblotting.
Fifty seedlings for each treatment, in 2 or 3 biological replicates, were homogenized in liquid nitrogen and 0.2 g of the powder was transferred to autoclaved pre-chilled 2.0 ml micro centrifuge tubes and extracted with 1.0 ml of ice cold extraction buffer [50 mM Tris-HCl (pH 7.5), 0.1% Triton X-100, 2 mM DTT, 30 µl protease inhibitor cocktail (Sigma Aldrich, USA)]. Insoluble material was removed by centrifugation at 14,000 rpm for 30 min at 4°C. Total proteins were estimated according to Bradford's method12 using different concentrations of bovine serum albumin (Sigma Aldrich, USA) as standard. Immunoblotting studies were carried out after resolving total proteins (25 µg) on 8% SDS-PAGE13 followed by transfer on to Hybond C membrane, which was probed with anti-Neurospora crassa-Hsp 80 antibodies, as described earlier in reference 5 and 14. The anti-S. bicolor Hsp73 antibody was used at a titre of 1:2,500. Equal loading of total proteins was confirmed by staining parallel gels with Coomassie brilliant blue R-250. For densitometer scanning of the blots, the intensity of Hsp85 and Hsp73 was normalized with respect to the pre-treatment stage (48 h), whereas the intensity of Hsp87 and Hsp75, which were induced only under heat stress, was compared relative to the water grown seedlings heat-stressed at 45°C. Proteins from both 37°C and 45°C seedlings were resolved on the same gel and developed together on the same blot. Therefore, the band intensity is comparable between 37°C and 45°C treatments within each panel of Figures 1–3. Further, the standard curves for quantification, by using densitometric scanning of the blots carrying different amounts of crude protein, were generated (Sup. Fig. 1) to ensure that the crude protein loaded was within the linear range.
The data obtained were subjected to one way analysis of variance using the software SPSS version 11 (SPSS for Windows, Rel. 11.0.1. 2001. Chicago: SPSS Inc.).
Acknowledgements
Thanks are due to Dr. Manju Kapoor, Professor Emeritus, Department of Biological Sciences, University of Calgary, Alberta, Canada and Dr. Sudhir Kumar Sopory, Group Leader, International Centre for Genetic Engineering and Biotechnology, New Delhi, India for the gift of anti-N. crassa Hsp80 and anti-rice calmodulin antibodies, respectively. Funds for this work were provided by the Department of Biotechnology, Government of India, New Delhi.
Supplementary Material
References
- 1.Wang W, Vinocur B, Shoseyov O, Altman A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004;9:244–252. doi: 10.1016/j.tplants.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Sangster TA, Bahrami A, Wilczek A, Watanabe E, Schellenberg K, McLellan C, et al. Phenotypic diversity and altered environmental plasticity in Arabidopsis thaliana with reduced Hsp90 levels. PLoS One. 2007;2:648. doi: 10.1371/journal.pone.0000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell. 1998;94:471–480. doi: 10.1016/s0092-8674(00)81588-3. [DOI] [PubMed] [Google Scholar]
- 4.Yamada K, Fukao Y, Hayashi M, Fukazawa M, Suzuki I, Nishimura M. Cytosolic HSP90 regulates the heat shock response that is responsible for heat acclimation in Arabidopsis thaliana. J Biol Chem. 2007;282:37794–37804. doi: 10.1074/jbc.M707168200. [DOI] [PubMed] [Google Scholar]
- 5.Virdi AS, Thakur A, Dutt S, Kumar S, Singh P. A sorghum 85 kDa heat stress-modulated protein shows calmodulin-binding properties and cross-reactivity to anti-Neurospora crassa Hsp 80 antibodies. FEBS Lett. 2009;583:767–770. doi: 10.1016/j.febslet.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 6.Reddy AS. Calcium: silver bullet in signaling. Plant Sci. 2001;160:381–404. doi: 10.1016/s0168-9452(00)00386-1. [DOI] [PubMed] [Google Scholar]
- 7.Wayne AS, Hillel F. Calmodulin as a versatile calcium signal transducer in plants. New Phytologist. 2001;151:35–66. doi: 10.1046/j.1469-8137.2001.00154.x. [DOI] [PubMed] [Google Scholar]
- 8.Liu HT, Li B, Shang ZL, Li XZ, Mu RL, Sun Dy, et al. Calmodulin is involved in heat shock signal transduction in wheat. Plant Physiol. 2003;132:1186–1195. doi: 10.1104/pp.102.018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun XT, Li B, Zhou GM, Tang WQ, Bai J, Sun DY, et al. Binding of the maize cytosolic Hsp70 to calmodulin, and identification of calmodulin-binding site in Hsp70. Plant Cell Physiol. 2000;41:804–810. doi: 10.1093/pcp/41.6.804. [DOI] [PubMed] [Google Scholar]
- 10.Ortiz ML, Calero M, Fernandez Patron C, Patron CF, Castellanos L, Mendez E. Imidazole-SDS-Zn reverse staining of proteins in gels containing or not SDS and microsequence of individual unmodified electroblotted proteins. FEBS Lett. 1992;296:300–304. doi: 10.1016/0014-5793(92)80309-5. [DOI] [PubMed] [Google Scholar]
- 11.Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor NY: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 12.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 13.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 14.Pareek A, Singla SL, Grover A. Immunological evidence for accumulation of two high-molecular-weight (104 and 90 kDa) HSPs in response to different stresses in rice and in response to high temperature stress in diverse plant genera. Plant Mol Biol. 1995;29:293–301. doi: 10.1007/BF00043653. [DOI] [PubMed] [Google Scholar]
- 15.Queitsch C, Hong SW, Vierling E, Lindquist S. Heat shock protein 101 plays a crucial role in thermotolerance in Arabidopsis. Plant Cell. 2000;12:479–492. doi: 10.1105/tpc.12.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutherford SL, Lindquist S. Hsp90 as a capacitor for morphological evolution. Nature. 1998;396:336–342. doi: 10.1038/24550. [DOI] [PubMed] [Google Scholar]
- 17.Bush DS. Calcium regulation in plant cells and its role in signaling. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:95–122. [Google Scholar]
- 18.Piñeros M, Tester M. Characterization of a voltage-dependent Ca2+-selective channel from wheat roots. Planta. 1995;195:478–488. [Google Scholar]
- 19.Sung DY, Vierling E, Guy CL. Comprehensive expression profile analysis of the Arabidopsis Hsp70 gene family. Plant Physiol. 2001;126:789–800. doi: 10.1104/pp.126.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vidal V, Ranty B, Dillenschneider M, Charpenteau M, Ranjeva R. Molecular characterization of a 70 kDa heat-shock protein of bean mitochondria. Plant J. 1993;3:143–150. doi: 10.1046/j.1365-313x.1993.t01-6-00999.x. [DOI] [PubMed] [Google Scholar]
- 21.Howarth CJ. Heat shock proteins in sorghum and pearl millet; ethanol, sodium arsenite, sodium malonate and the development of thermotolerance. J Exp Bot. 1990;41:877–883. [Google Scholar]
- 22.Phean OPS, Punteeranurak P, Buaboocha T. Calcium signaling-mediated and differential induction of calmodulin gene expression by stress in Oryza sativa L. J Biochem Mol Biol. 2005;38:432–439. doi: 10.5483/bmbrep.2005.38.4.432. [DOI] [PubMed] [Google Scholar]
- 23.Gong M, van der Luit AH, Knight MR, Trewavas AJ. Heat-shock-induced changes in intracellular Ca2+ level in tobacco seedlings in relation to thermotolerance. Plant Physiol. 1998;116:429–437. [Google Scholar]
- 24.Trofimova MS, Andreev IM, Kuznetsov VV. Calcium is involved in regulation of the synthesis of HSPs in suspension-cultured sugar beet cells under hyperthermia. Physiol Plant. 1999;105:67–73. [Google Scholar]
- 25.Krishna P, Gloor G. The Hsp90 family of proteins in Arabidopsis thaliana. Cell Stress Chaperones. 2001;6:238–246. doi: 10.1379/1466-1268(2001)006<0238:thfopi>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wehmeyer N, Hernandez LD, Finkelstein RR, Vierling E. Synthesis of small heat-shock proteins is part of the developmental program of late seed maturation. Plant Physiol. 1996;112:747–757. doi: 10.1104/pp.112.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leborgne-Castel N, Jelitto-Van Dooren EP, Crofts AJ, Denecke J. Overexpression of BiP in tobacco alleviates endoplasmic reticulum stress. Plant Cell. 1999;11:459–470. doi: 10.1105/tpc.11.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polya GM, Micucci V. Interaction of wheat germ Ca2+-dependent protein kinases with calmodulin antagonists and polyamines. Plant Physiol. 1985;79:968–972. doi: 10.1104/pp.79.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osuna L, Coursol S, Pierre JN, Vidal J. A Ca2+-dependent protein kinase with characteristics of protein kinase C in leaves and mesophyll cell protoplasts from Digitaria sanguinalis: possible involvement in the C4-phosphoenolpyruvate carboxylase phosphorylation cascade. Biochem Biophys Res Comm. 2004;314:428–433. doi: 10.1016/j.bbrc.2003.12.103. [DOI] [PubMed] [Google Scholar]
- 30.Avron M, Shavit N. Inhibitors and uncouplers of photophosphorylation. Biochim Biophys Acta. 1965;109:317–331. doi: 10.1016/0926-6585(65)90160-3. [DOI] [PubMed] [Google Scholar]
- 31.Ali R, Ma W, Lemtiri-Chlieh F, Tsaltas D, Leng Q, von Bodman S, et al. Death don't have no mercy and neither does calcium: Arabidopsis CYCLIC NUCLEOTIDE GATED CHANNEL2 and innate immunity. Plant Cell. 2007;19:1081–1095. doi: 10.1105/tpc.106.045096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen T, Wu X, Chen Y, Li X, Huang M, Zheng M, et al. Combined proteomic and cytological analysis of Ca2+-calmodulin regulation in Picea meyeri pollen tube growth. Plant Physiol. 2009;149:1111–1126. doi: 10.1104/pp.108.127514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma W, Smigel A, Tsai YC, Braam J, Berkowitz GA. Innate immunity signaling: Cytosolic Ca2+ elevation is linked to downstream nitric oxide generation through the action of calmodulin or a calmodulin-like protein. Plant Physiol. 2008;148:818–828. doi: 10.1104/pp.108.125104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang T, Poovaiah BW. Molecular and biochemical evidence for the involvement of calcium/calmodulin in auxin action. J Biol Chem. 2000;275:3137–3143. doi: 10.1074/jbc.275.5.3137. [DOI] [PubMed] [Google Scholar]
- 35.Liu HT, Sun DY, Zhou RG. Ca2+ and AtCaM3 are involved in the expression of heat shock protein gene in Arabidopsis. Plant Cell Environ. 2005;28:1276–1284. [Google Scholar]
- 36.Chang-Quan W, Ye-Feng Z, Tao L. Activity changes of calmodulin and Ca2+-ATPase during low-temperature-induced anthocyanin accumulation in Alternanthera bettzickiana. Physiologia Plant. 2005;124:260–266. [Google Scholar]
- 37.Yamada K, Nishimura M. Cytosolic heat shock protein 90 regulates heat shock transcription factor in Arabidopsis thaliana. Plant Signal Behav. 2008;3:660–662. doi: 10.4161/psb.3.9.5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang W, Zhou RG, Gao YJ, Zheng SZ, Xu P, Zhang SQ, et al. Molecular and genetic evidence for the key role of AtCaM3 in heat-shock signal transduction in Arabidopsis. Plant Physiol. 2009;149:1773–1784. doi: 10.1104/pp.108.133744. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


