Abstract
Caffeine functions in the chemical defense against biotic attackers in a few plant species including coffee and tea. Transgenic tobacco plants that endogenously produced caffeine by expressing three N-methyltransferases involved in the caffeine biosynthesis pathway exhibited a strong resistance to pathogens and herbivores. Here we report that transgenic Chrysanthemum, which produced an equivalent level of caffeine as the tobacco plants at approximately 3 µg g−1 fresh tissues, also exhibited a resistance against grey mold fungal attack. Transcripts of PR-2 gene, a marker for pathogen response, were constitutively accumulated in mature leaves without pathogen attack. The levels of salicylic acid and its glucoside conjugate in mature leaves of the transgenic lines were found to be 2.5-fold higher than in the wild type control. It is suggested that endogenous caffeine stimulated production and/or deposition of salicylates, which possibly activated a series of defense reactions even under non-stressed conditions.
Key words: caffeine, chrysanthemum, gray mold, pathogenesis-related gene, salicylic acid
Plants respond to pathogen attacks by producing a diverse array of antimicrobial chemicals, many of which are referred to as secondary metabolites, including alkaloids, terpenoids and phenolic compounds. 1 Among these compounds, alkaloids have been well-studied with respect to chemical defense.2 Caffeine (1,3,7-trimethylxanthine) is a typical purine alkaloid that is produced in more than 80 plant species, such as coffee, tea, cacao and kola.3 In nature, caffeine has been known to confer a toxic effect against pathogens and herbivores.4–6 This suggested that caffeine-producing transgenic plants, which originally do not produce caffeine, may acquire the resistance against natural attackers.
When coffee genes encoding distinct N-methyltransferases, which successively methylate xanthosine derivatives to yield caffeine,7 were introduced and expressed in tobacco plants, caffeine was produced about 3–5 µg g−1 fresh weight of the tissue.8 The resulting plants exhibited a high resistance against herbivores8 and viral and bacterial pathogens.9 A novel feature of this observation was that the caffeine effect appeared not to be direct toxicity, but indirect stimulation of the defense network, or systemic-acquired resistance of the host plant.6,9 The present article reports that transgenic chrysanthemum plants, which produced caffeine at the same level as tobacco plants, simultaneously exhibited a strong resistance against pathogenic fungus and a high level of salicylic acid and its glucoside conjugate. The results suggested that salicylates were over-accumulated and triggered the onset of resistance activity in transgenic chrysanthemum.
Cultured plantlets of chrysanthemum (Dendranthema x grandiflorum cv. Shinba) were obtained from the Nara Prefectural Agricultural Experimental Station in Japan, and cultured on MS medium containing 3% sucrose and 0.35% Gellan Gum (Pure Chemical Co., Japan). Genetic transformation of chrysanthemum was carried out as described in reference 10, using Agrobacterium tumefaciens strain LBA4404 harboring a pBIN-NMT777 which contained three N-methyltransfease genes, CaXMT1, CaMXMT1 and CaDXMT1, each encoding xanthosine methyltransfease (XMT), methylxanthine methyltransferase (MXMT) and dimethylxanthine methyltransferase (DXMT), respectively.8 The resulting transformed plantlets were finally selected on MS medium containing 250 mg l−1 cefotaxime and 10 mg l−1 kanamycin. The wild-type and transgenic plants (lines C#1, C#2 and C#3) were grown in a closed greenhouse at 25°C under natural day conditions. Transcript accumulation was estimated by RT-PCR using appropriate primer sets for each gene.9 PR-2 was isolated by PCR using two degenerated primers (forward; 5′-GAT GCT CTA CAA TCC CTC AAC G-3′ and reward; 5′-CGC TTG ACG TGC ATA GTG AGC A-3′). The products were cloned and analyzed for nucleotide and deduced amino acid sequences, and clones containing the complete coding sequence were selected and used for further analysis. Caffeine content was determined as described using HPLC.8 Infection test of gray mold (Botrytis cinerea) was performed with healthy detached leaves as followings: spores of B. cinerea (1 × 105 spores ml−1) suspended in 5 mm diameter × 3 mm thick agar disks (1% agar, 2.5% glucose) were deposited on the adaxial side of each chrysanthemum leaflets and incubated in a wet chamber at 100% relative humidity for 15 days at 20°C (light period 12 h day−1). Susceptibility was determined by measuring the diameter of necrotic lesions. Data were obtained from at least 3 independent samples, and the standard deviation was calculated by the t test. Salicylic acid (SA) and salicylic acid-β-glucoside (SAG) were extracted and quantified using 1 g of frozen tissues as the starting material.11
Transgenic chrysanthemum plants producing caffeine were successfully constructed by introducing three N-methyltransfease genes CaXMT1, CaMXMT1 and CaDXMT1. After antibiotic selection, eight kanamycin-resistant transgenic plantlets were obtained, among which 6 were confirmed by the RT-PCR to express all three N-methyltransferase genes (Fig. 1A). Three lines (C#1, C#2 and C#3) were then selected and grown to the maturity, and caffeine production in fully matured leaves was examined by the HPLC assay (Fig. 1B). In non-transgenic wild type plants, caffeine was not detectable. In three transgenic lines, caffeine was accumulated at 3 µg g−1 fresh weight of the tissue, being comparable with reported values in transgenic tobacco plants.8,9
Figure 1.
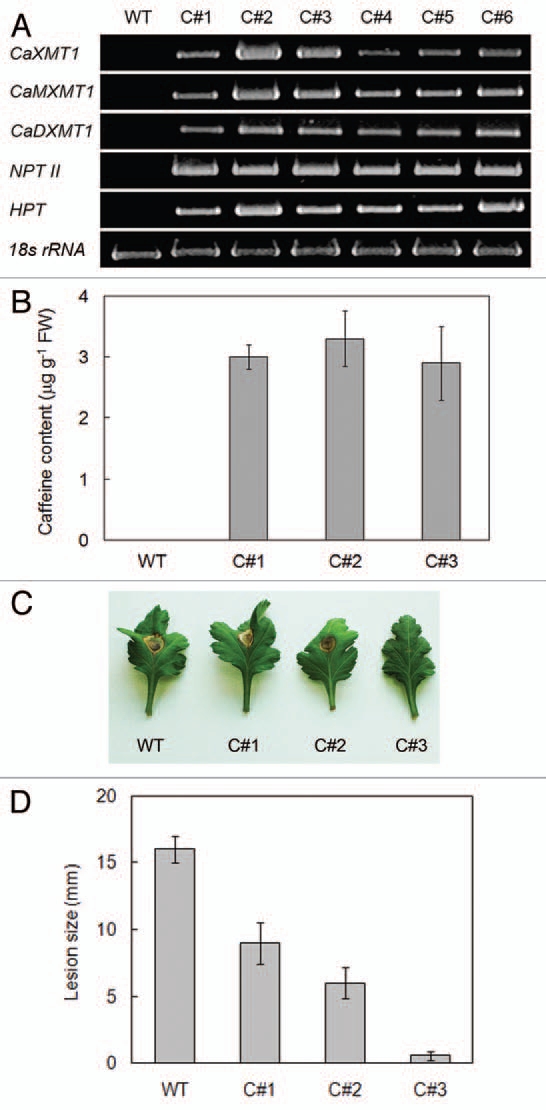
Properties of transgenic chrysanthemum. (A) Expression of transcripts for caffeine biosynthetic genes. Total RNA was isolated from leaves of 1-month transgenic lines indicated and subjected to RT-PCR using the gene specific primers for CaXMT, CaMXMT1 and CaDXMT1. Since the cassette contains NPTII and HPT genes, their expression was also shown. As the internal standard, 18s rRNA was used. (B) Quantification of caffeine content. One g of fresh leaf tissue of indicated lines was processed and subjected to HPLC analysis. Caffeine amount is expressed in mg per g of fresh weight (FW). Three independent samples were measured and the standard deviation is shown by the error bar. (C) Pathogen symptom. Detached healthy mature leaf of indicated lines was inoculated with B. cinerea at the concentration of 1 × 105 spores ml−1, incubated at 20°C for 15 days and photographed. (D) Quantification of lesion. Diameter of the necrotic lesion was measured 15 days after inoculation. More than 12 independent samples were assayed, and values were treated by the t test to show the significance of probability (p < 0.05).
Transgenic and wild type chrysanthemum plants were tested for resistance against Botrytis cinerea, a necrotrophic fungus that affects many plant species. In the wild type plant, lesions appeared 72 h after inoculation, and rapidly developed from the infected site to outward leaves (Fig. 1C). The lesion size exceeded 16 mm 5 days after infection (Fig. 1D). In the transgenic lines, the lesion appeared 90 h after inoculation. The lesion size was dependent on the line, varying between 9 mm and 1 mm (Figs. 1C and D). The line C#3 strongly restricted the fungus growth.
Resistance against pathogens is frequently associated with elevated expression of pathogen-related genes. One of such marker genes is PR-2 encoding β-1,3-glucanase, and chrysanthemum counterpart was isolated (accession number HM045423). A partial PCR product of 598 bp exhibited the highest similarity with the PR-2 (AJ011769) from Cichorium (chicory) at 97%. The similarity to tobacco PR-2 (M60460) was 72%. Its transcripts in transgenic lines were constitutively accumulated under the non-stressed condition while those in the wild type plant were not accumulated (Fig. 2A). The isolated PR-2 from chrysanthemum clearly responded to pathogen infection and related stresses in leaves of wild type plants upon treatments with B. cinerea and mechanical wounding (Fig. 2B). It also responded to SA (Fig. 2B).
Figure 2.
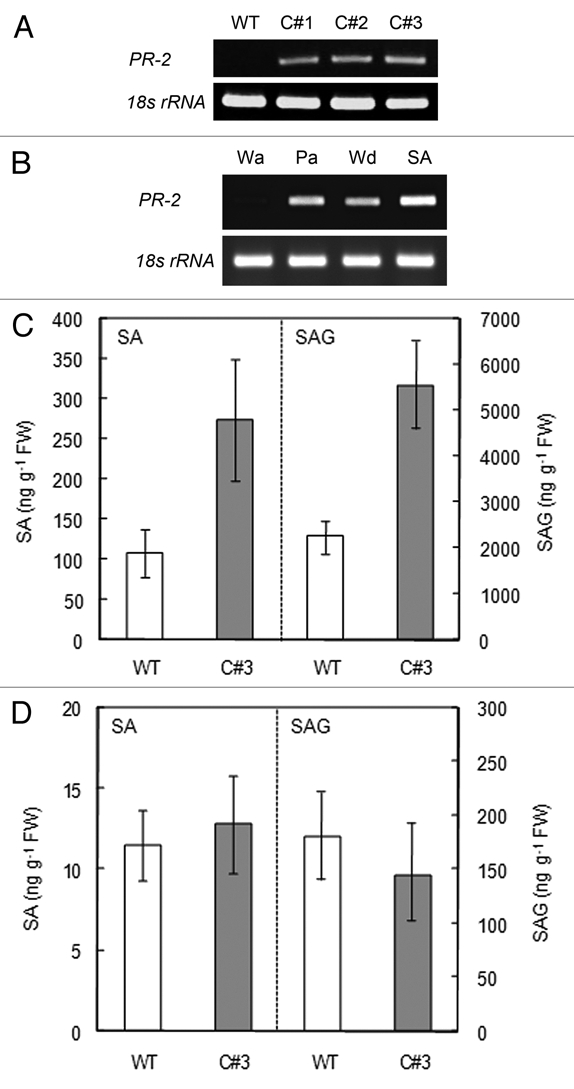
Activation of defense response. (A) Constitutive expression of PR-2. Total RNA was isolated from mature leaves of indicated lines, and assayed for PR-2 transcript accumulation by RT-PCR. Internal standard was 18s rRNA. (B) Stress induction. Detached leaves of the wild type plant were treated with water (Wa), pathogenic B. cinerea (Pa), wounding (Wd) and 2 mM SA (SA). Total RNA was isolated and subjected to RT-PCR. Internal standard was 18s rRNA. (C and D) Quantification of SA and SAG in mature leaves (C) and in vitro leaf tissues (D). One g of fresh leaf was detached from the wild type (open column) and the line C#3 (shaded column) plants, and processed for SA and SAG estimation. At least three independent samples were measured and values are expressed in ng g−1 of fresh weight (FW). The standard deviation is shown by error bars.
One of the hallmarks in plant defense response is SA, which simultaneously activates many defense-related genes including PR-2.12 In mature leaves of wild type plants, the amount of SA and SAG was 100 and 2,300 ng g−1 fresh weight, respectively, without pathogen attack. These values are comparable with those found in tobacco plants.13 In mature leaves of the transgenic C#3, which showed the strongest resistance against B. cinerea (Fig. 1C), levels of SA and SAG were 270 and 5,500 ng g−1 fresh weight, respectively, being constitutively 2.5-fold higher than those in the control (Fig. 2C). The increase in SA is also comparable with that found in tobacco leaves infected with tobacco mosaic virus for two days.14 In contrast, their levels in leaves cultured in vitro were similar in both control and transgenic line (Fig. 2D), showing SA and SAG approximately 10 ng and 150 ng g−1 fresh tissues in both samples. This finding implies that SA production is conditional, responding to growth environment. External stresses during the growth in a greenhouse possibly induced its production, and endogenous caffeine helped or enhanced its overproduction.
The present finding is significant in two aspects: (1) endogenously produced caffeine commonly stimulates the defense response; (2) the activated defense response is closely correlated with the elevated level of SA. Transgenic tobacco plants producing caffeine were previously shown to acquire resistance against pathogen and herbivore attacks.8,9 Molecular analyses suggested that the effect of caffeine was not direct by the toxicity to the attackers, but indirect by the stimulation of a self-defense system.9 It was conceivable that since endogenous caffeine is mildly toxic the host plant counteracted the situation by activating the self-defense.6 Since this is similar to the vaccination in vertebrates, the idea was proposed that plants can also be vaccinated by producing appropriate “antigenic“ compounds in planta.6 The present finding that chrysanthemum is efficiently “vaccinated” as the tobacco plants suggested that the system is commonly utilized among plant species. Its application to important crop plants may practically be useful for sustainable agriculture.
The molecular mechanism of defense activation by caffeine is not clear. The present finding may give a clue to consider it, showing that a low level of endogenous caffeine caused accumulation of endogenous SA and SAG. Two alternative mechanisms are conceivable: direct interaction with or indirect influence on the biosynthesis (and/or deposition) of salicylates. In the former case, caffeine directly interacts with components involved in SA synthesis and constitutively stimulates its production. This idea is less favorable because the level of SA in the in vitro cultured tissues was the same as the control, and only increased after plants were exposed to environmental stresses in a greenhouse. Since SA and SAG levels also increased in the wild type plants during growth, it appears that their 2.5-fold increase in the transgenic lines is the result of amplification or acceleration of ordinary SA/SAG production/deposition by endogenous caffeine. In this context, the latter case is more probable in such a way that caffeine stimulates the stress-signaling pathway, resulting in activation of biosynthesis and/or deposition of SA and SAG. In this case, caffeine may generally amplify normal stress response during the growth, and the host becomes ready to cope with external attackers. Some observations are consistent with this idea. In mammalian cells, caffeine was shown to block the activity of phosphodiesterase, which functions in metabolism of cyclic AMP, a critical factor in phosphorylation signaling cascade.15 Since plants adopt a similar signaling system,16 identification of signaling factors that are affected in transgenic lines is a powerful approach to understand the molecular aspects.
Acknowledgements
The authors thank Korean Agricultural Culture Collection (KACC) for providing Botrytis cinerea (KACC no. 40573). This work was supported by grants from the World Class University Project (Korea) and Japan Society for the Promotion of Science (Japan).
References
- 1.Dixon RA. Natural products and plant disease resistance. Nature. 2001;411:843–847. doi: 10.1038/35081178. [DOI] [PubMed] [Google Scholar]
- 2.Croteau R, Kutchan TM, Lews NG. Natural products (secondary metabolites) In: Buchanan BB, Gruissem W, Jones RL, editors. Biochemistry and Molecular Biology of Plants. Vol. 1250. Rockville, MD: American Society of Plant Physiologists; 2000. p. 318. [Google Scholar]
- 3.Ashihara H, Crozier A. Biosynthesis and metabolism of caffeine and related purine alkaloids in plants. Adv Bot Res. 1999;30:118–205. [Google Scholar]
- 4.Hollingsworth RG, Armstrong JW, Campbell E. Caffeine as a repellent for slugs and snails: at high concentrations this stimulant becomes a lethal neurotoxin to garden pests. Nature. 2002;417:915–916. doi: 10.1038/417915a. [DOI] [PubMed] [Google Scholar]
- 5.Kim YS, Uefuji H, Ogita S, Sano H. Transgenic tobacco plants producing caffeine: A potential new strategy for insect pest control. Transgenic Res. 2006;15:667–672. doi: 10.1007/s11248-006-9006-6. [DOI] [PubMed] [Google Scholar]
- 6.Kim YS, Choi YE, Sano H. Plant vaccination: Stimulation of defense system by caffeine production in planta. Plant Signal Behav. 2010;5:489–493. doi: 10.4161/psb.11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uefuji H, Ogita S, Yamaguchi Y, Koizumi N, Sano H. Molecular cloning and functional characterization of three distinct N-methyltransferases involved in the caffeine biosynthetic pathway in coffee plants. Plant Physiol. 2003;132:372–380. doi: 10.1104/pp.102.019679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uefuji H, Tatsumi Y, Morimoto M, Kaothien-Nakayama P, Ogita S, Sano H. Caffeine production in tobacco plants by simultaneous expression of three coffee N-methyltransferases and its potential as a pest repellant. Plant Mol Biol. 2005;59:221–227. doi: 10.1007/s11103-005-8520-x. [DOI] [PubMed] [Google Scholar]
- 9.Kim YS, Sano H. Pathogen resistance of transgenic tobacco plants producing caffeine. Phytochem. 2008;69:882–888. doi: 10.1016/j.phytochem.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 10.Shinoyama H, Kazuma T, Komano M, Nomura Y, Tsuchiya T. An efficient transformation system in chrysanthemum [Dendranthema x grandiflorum (Ramat.) Kitamura] for stable and non-chimeric expression of foreign genes. Plant Biotech. 2002;19:335–343. [Google Scholar]
- 11.Seo S, Ishizuka K, Ohashi Y. Induction of salicylic acid β-glucosidase in tobacco leaves by exogenous salicylic acid. Plant Cell Physiol. 1995;36:447–453. [Google Scholar]
- 12.Raskin I. Role of salicylic acid in plants. Ann Rev Plant Physiol Plant Mol Biol. 1992;43:439–463. [Google Scholar]
- 13.Seo S, Katou S, Seto H, Gomi K, Ohashi Y. The mitogen-activated protein kinases WIPK and SIPK regulate the levels of jasmonic and salicylic acids in wounded tobacco plants. Plant J. 2007;49:899–909. doi: 10.1111/j.1365-313X.2006.03003.x. [DOI] [PubMed] [Google Scholar]
- 14.Seo S, Seto H, Koshino H, Yoshida S, Ohashi Y. A diterpene as an endogenous signal for the activation of defense response to infection with Tobacco mosaic virus and wounding in tobacco. Plant Cell. 2003;15:863–873. [PMC free article] [PubMed] [Google Scholar]
- 15.Goth R, Cleaver JE. Metabolism of caffeine to nucleic acid precursors in mammalian cells. Mutat Res. 1976;36:105–114. doi: 10.1016/0027-5107(76)90025-7. [DOI] [PubMed] [Google Scholar]
- 16.Nathanson JA. Caffeine and related methylxanthine possible naturally occurring pesticides. Science. 1984;226:184–187. doi: 10.1126/science.6207592. [DOI] [PubMed] [Google Scholar]


