Abstract
Establishment of adaxial-abaxial polarity is essential for lateral organ development. A stamen consists of a bilaterally symmetrical anther and a radial filament. Using a rice mutant, rod-like lemma, in which establishment of adaxial-abaxial polarity is compromised, we found that stamen patterning is likely to be achieved by a unique regulatory mechanism: rearrangement of adaxial-abaxial polarity in the anther, and abaxialization in the filament. These regulations are not found in leaf development. Here, we discuss similarities and differences between the stamen and the leaf in the mechanisms underlying the establishment of adaxialabaxial polarity. In addition, we propose the idea that the process of establishing adaxial-abaxial polarity in lateral organs is likely to be divided into two phases: a meristem-dependent, followed by a meristem-independent phase. In stamen development, the transition between these two phases is clearly observed as the rearrangement of expression patterns of the adaxial and abaxial marker genes.
Key words: adaxial and abaxial polarity, anther, ETTIN, filament, HD-ZIPIII, leaf, meristem, rice (Oryza sativa), stamen, ta-siRNA
Establishment of polarity along developmental axes is a fundamental process in lateral organ development in plants. Regulation of adaxial-abaxial polarity is well understood in leaf development.1,2 In both Arabidopsis and maize, several transcription factors and small RNAs are known to constitute a complex genetic network that regulates establishment of the polarity in the leaf. By contrast, the mechanism controlling adaxial-abaxial polarity in stamen development is not so well understood.
We have analyzed the rod-like lemma (rol) mutant of rice, in which the stamen and lemma have defects in establishment of adaxial-abaxial polarity.3 In rol, some stamens and lemmas develop into pin-like or rod-like structures, respectively, that are associated with loss of polarity. The rol phenotypes are caused by a weak mutation in the SHOOTLESS2 gene that encodes a protein similar to Arabidopsis RNA-DEPENDENT RNA POLYMERASE6, which is required for the biosynthesis of trans-acting small interfering RNA (ta-siRNA).4 By analyzing the rol mutant and the spatial expression patterns of adaxial and abaxial markers, we proposed a model of the regulatory mechanism underlying the establishment of adaxial-abaxial polarity in the stamen in rice.3 In this model, adaxial-abaxial polarity is rearranged at an early stage during anther development, whereas the adaxial identity is completely lost in the filament. Although this model is inferred from studies of rice, published Arabidopsis research tells us that the mechanism that we proposed is applicable to stamen development in the angiosperms in general.5–8
In this addendum, we discuss similarities and differences between the stamen and the leaf in the mechanisms underlying the establishment of adaxial-abaxial polarity. In addition, we also discuss two phases of polarity establishment, namely meristem-dependent and meristem-independent phases, in lateral organ development.
Similarities and Differences in Polarity Establishment between the Stamen and the Leaf
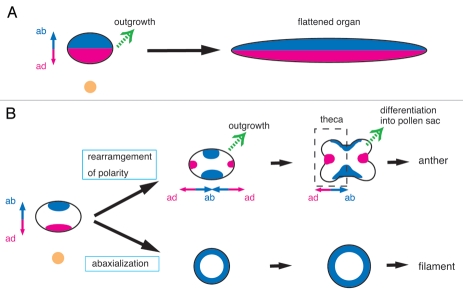
In leaf development, adaxial-abaxial polarity is established early, at a stage when the leaf primordium does not yet exhibit any morphological differences between the adaxial and abaxial domains. The polarity can, however, be recognized by the expression patterns of adaxial or abaxial identity genes (Fig. 1A).6,9–11 Once the adaxial-abaxial polarity is established, the polarity does not change and the developmental fates of the cells, such as epidermal cells and vascular tissue cells, are specified depending on this polarity. In addition, establishment of adaxial-abaxial polarity is required for planar growth of the leaf: that is, laminar outgrowth occurs at the junction region between the adaxial and abaxial domains.12–15 Failure in polarity establishment results in radialized leaves, which are either adaxialized or abaxialized depending on the genes that are compromised.
Figure 1.
Model of the developmental pattern of the leaf (A) and the stamen (B). (A) Lamina outgrowth occurs at the junction of the adaxial and abaxial domains in the leaf. (B) In anther development, the adaxial-abaxial polarity is rearranged. The boundary regions between the adaxial and abaxial domains outgrow and differentiate into pollen sacs. In the filament, a radially symmetrical structure is formed by abaxialization. The adaxial and abaxial domains are shown in red and blue, respectively. Double-headed arrows represent the axis of adaxial-abaxial polarity. Green arrows indicate the direction of formation of the outgrowth. ab, abaxial side; ad, adaxial side.
In stamen development in rice, adaxial-abaxial polarity initially seems to be established as it is in the leaf: OsPHB3, an adaxial identity gene (an ortholog of Arabidopsis PHBULOSA), is expressed in the domain adjacent to the meristem; and an abaxial identity gene, OsETTIN1 (OsETT1), is expressed in the domain opposite to it (Fig. 1B).3 In anther development, however, a marked rearrangement of the spatial expression patterns of the adaxial and abaxial genes takes place. After this rearrangement, OsPHB3 is expressed in the lateral regions of the anther primordium, whereas OsETT1 is expressed in a region near the meristem, in addition to its original abaxial region. The new polarity seems to be established in a new developmental unit, the theca primordium, and subsequent development is likely to be associated with the new polarity. Four outgrowths are formed at boundary regions between the two domains that express the adaxial or abaxial identity genes, and these outgrowths are subsequently differentiated into the pollen sacs. The stomium forms in the adaxial domain, whereas the connective tissue is differentiated in the abaxial domain of the theca. By contrast, the expression of OsPHB3 is lost in the outer layers of the filament, suggesting that the filament is abaxialized (Fig. 1B). Thus, the anther and filament are formed and differentiated depending on distinct polarities.
Accordingly, as compared to leaf development, the establishment of adaxial-abaxial polarity is markedly different in stamen development: namely, independent establishment of polarity in the distal and proximal regions, followed by rearrangement of polarity in the anther and complete abaxialization of the filament. Despite these differences, stamen development is likely to be regulated by a mechanism similar to that underlying leaf development. In the leaf, the lamina outgrowths are formed in the adaxial-abaxial boundaries. Similarly in the anther, the outgrowths that are differentiated into the pollen sacs emerge in the boundary regions between the adaxial and abaxial identities. Consistently, the pollen sacs are partially or completely lost in the rol mutant.3 In filament development, the filament primordium is completely abaxialized and develops into a radialized structure. This developmental pattern closely resembles the formation of a radialized leaf caused by the failure of laminar outgrowth in mutants in which adaxial or abaxial identity is completely compromised.7,12,13
Floral organs including the stamen evolved from the leaf.16,17 Therefore, these similarities in the establishment of adaxial-abaxial polarity are consistent with the evolutionary origin of the stamen. To make a filamentous structure from the flattened leaf, abaxialization might have been introduced during evolution of the stamen. The rearrangement of the adaxial-abaxial polarity might have also been adopted to elaborate the complex structure of the anther, which is composed of two thecae and the connective.
Two Distinct Phases in the Establishment of Adaxial-abaxial Polarity in the Stamen and Leaf
Surgical experiments suggest that a signal from the meristem is required for acquisition of adaxial identity in leaf development.18,19 When an incipient primordium (I1 or P0) was isolated by incision from the meristem, the primordium formed a radially symmetrical structure with abaxialization (Fig. 2A). By contrast, when the incision was made at the P2 stage of leaf development, the leaf developed normally without any perturbation of adaxial-abaxial patterning.18–20 Thus, the signal from the meristem is required for a short developmental window, suggesting that leaf development can be divided into two phases: a meristem-dependent and a meristem-independent phase. That is, after transition from the meristem-dependent phase, the establishment of adaxial-abaxial polarity is likely to be regulated in an organ-autonomous manner.
Figure 2.
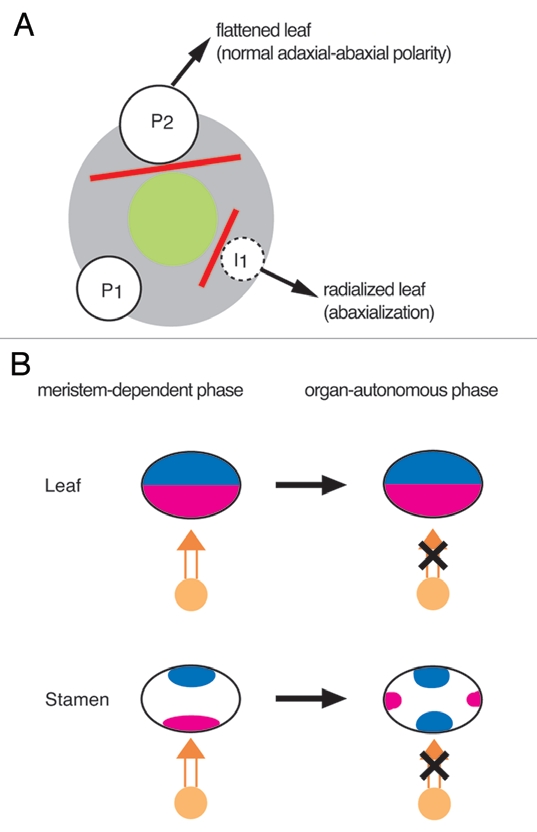
Detection of the meristem-dependent and organ-autonomous phases. (A) Schematic representation of surgical experiments in leaf development. The green circle represents the center of the shoot apical meristem. Red solid lines represent incisions to isolate the primordium from the center of the meristem. (B) Schematic representations of the phase transition in the development of the leaf (upper part) and the stamen (lower part). A clear phase transition is detected in stamen development by the rearrangement of the expression patterns of the adaxial and abaxial markers, whereas no apparent change is observed in leaf development. Orange circles and orange arrows represent the meristem and a putative signal from the meristem, respectively. The adaxial and abaxial domains are shown in red and blue, respectively.
At an early stage of stamen development, the adaxial marker OsPHB3 is expressed in the domain adjacent to the meristem, whereas the abaxial marker OsETT1 is expressed in the domain opposite to it.3 This stage would correspond to the meristem-dependent phase described above. Subsequently, the expression patterns of OsPHB3 and OsETT1 drastically change. For example, the abaxial marker OsETT1 becomes expressed in the domain adjacent to the meristem. This probably indicates that development of the stamen primordia is likely to be free from a putative meristem signal. Therefore, the rearrangement of the polarity in the anther is likely to be associated with the transition from the meristem-dependent phase to the meristem-independent (organ-autonomous) phase. Abaxialization in filament development also seems to be an indication of an organ-autonomous phase.
Once it is established in the leaf, the adaxial-abaxial polarity is maintained and does not change further. Therefore, the transition between the two phases would be hard to distinguish by the expression patterns of the marker genes (Fig. 2B). By contrast, the marked rearrangement of adaxial-abaxial polarity can be detected by changes in the gene expression pattern in stamen development. This rearrangement provides visible evidence that the lateral organ probably has two distinct phases in establishment of the adaxial-abaxial polarity whereby it shifts from a meristem-dependent phase to an organ-autonomous phase.
References
- 1.Chitwood DH, Nogueira FTS, Howell MD, Montgomery TA, Carrington JC, Timmermans MCP. Pattern formation via small RNA mobility. Gene Dev. 2009;23:549–554. doi: 10.1101/gad.1770009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Husbands AY, Chitwood DH, Plavskin Y, Timmermans MCP. Signals and prepatterns: New insights into organ polarity in plants. Gene Dev. 2009;23:1986–1997. doi: 10.1101/gad.1819909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toriba T, Suzaki T, Yamaguchi T, Ohmori Y, Tsukaya H, Hirano HY. Distinct regulation of adaxial-abaxial polarity in anther patterning in rice. Plant Cell. 2010;22:1452–1462. doi: 10.1105/tpc.110.075291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagasaki H, Itoh J, Hayashi K, Hibara K, Satoh-Nagasawa N, Nosaka M, et al. The small interfering RNA production pathway is required for shoot meristem initiation in rice. Proc Natl Acad Sci USA. 2007;104:14867–14871. doi: 10.1073/pnas.0704339104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sessions A, Nemhauser JL, McColl A, Roe JL, Feldmann KA, Zambryski PC. ETTIN patterns the Arabidopsis floral meristem and reproductive organs. Development. 1997;124:4481–4491. doi: 10.1242/dev.124.22.4481. [DOI] [PubMed] [Google Scholar]
- 6.Sawa S, Watanabe K, Goto K, Kanaya E, Morita EH, Okada K. FILAMENTOUS FLOWER, a meristem and organ identity gene of Arabidopsis, encodes a protein with a zinc finger and HMG-related domains. Gene Dev. 1999;13:1079–1088. doi: 10.1101/gad.13.9.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eshed Y, Baum SF, Perea JV, Bowman JL. Establishment of polarity in lateral organs of plants. Curr Biol. 2001;11:1251–1260. doi: 10.1016/s0960-9822(01)00392-x. [DOI] [PubMed] [Google Scholar]
- 8.Dinneny JR, Weigel D, Yanofsky MF. NUBBIN and JAGGED define stamen and carpel shape in Arabidopsis. Development. 2006;133:1645–1655. doi: 10.1242/dev.02335. [DOI] [PubMed] [Google Scholar]
- 9.Siegfried KR, Eshed Y, Baum SF, Otsuga D, Drews GN, Bowman JL. Members of the YABBY gene family specify abaxial cell fate in Arabidopsis. Development. 1999;126:4117–4128. doi: 10.1242/dev.126.18.4117. [DOI] [PubMed] [Google Scholar]
- 10.Kerstetter RA, Bollman K, Taylor RA, Bomblies K, Poethig RS. KANADI regulates organ polarity in Arabidopsis. Nature. 2001;411:706–709. doi: 10.1038/35079629. [DOI] [PubMed] [Google Scholar]
- 11.McConnell JR, Emery J, Eshed Y, Bao N, Bowman J, Barton MK. Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature. 2001;411:709–713. doi: 10.1038/35079635. [DOI] [PubMed] [Google Scholar]
- 12.Waites R, Hudson A. phantastica: A gene required for dorsoventrality of leaves in Antirrhinum majus. Development. 1995;121:2143–2154. [Google Scholar]
- 13.McConnell JR, Barton MK. Leaf polarity and meristem formation in Arabidopsis. Development. 1998;125:2935–2942. doi: 10.1242/dev.125.15.2935. [DOI] [PubMed] [Google Scholar]
- 14.Timmermans MCP, Schultes NP, Jankovsky JP, Nelson T. Leafbladeless1 is required for dorsoventrality of lateral organs in maize. Development. 1998;125:2813–2823. doi: 10.1242/dev.125.15.2813. [DOI] [PubMed] [Google Scholar]
- 15.Bowman JL. The YABBY gene family and abaxial cell fate. Curr Op Plant Biol. 2000;3:17–22. doi: 10.1016/s1369-5266(99)00035-7. [DOI] [PubMed] [Google Scholar]
- 16.Bowman JL, Smyth DR, Meyerowitz EM. Genetic interactions among f loral homeotic genes of Arabidopsis. Development. 1991;112:1–20. doi: 10.1242/dev.112.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Coen ES, Meyerowitz EM. The war of the whorls: genetic interactions controlling flower development. Nature. 1991;353:31–37. doi: 10.1038/353031a0. [DOI] [PubMed] [Google Scholar]
- 18.Sussex IM. Experiments on the cause of dorsiventrality in leaves. Nature. 1951;167:651–652. doi: 10.1038/167651a0. [DOI] [PubMed] [Google Scholar]
- 19.Sussex IM. Morphogenesis in Solanum tuberosum L.: experimental investigation of leaf dorsiventrality and orientation in the juvenile shoot. Phytomorphology. 1954;5:286–300. [Google Scholar]
- 20.Reinhardt D, Frenz M, Mandel T, Kuhlemeier C. Microsurgical and laser ablation analysis of leaf positioning and dorsoventral patterning in tomato. Development. 2005;132:15–26. doi: 10.1242/dev.01544. [DOI] [PubMed] [Google Scholar]