Abstract
Suspension cultured cells of Capsicum chinense secrete proteins to the culture medium in both control conditions and under methyl jasmonate treatment. The exogenous application of methyl jasmonate induced the accumulation of putative pathogenesis-related proteins, class I chitinase, leucin-rich repeat protein, NtPRp27-like protein and pectinesterase which were also found in suspension cultured cells of C. annuum elicited with methyl jasmonate. However, a germin-like protein, which has never been described in methyl jasmonate-elicited C. chinense suspension cultured cells, was found. The different effects described as being the result of exogenous application of signalling molecules like methyl jasmonate on the expression of germin-like protein suggest that germin-like proteins may play a variety of roles in protecting plants against pathogen attacks and different stresses. Further studies will be necessary to characterize the differential expression of these pathogenesis-related proteins and to throw light on the complexity of their regulation.
Key words: capsicum, cell cultures, methyl jasmonate, pathogenesis-related proteins
When plants are attacked by pathogens, they defend themselves with an arsenal of both passive and active defence mechanisms. The passive or pre-existing defence mechanisms involve structural barriers or strategically positioned reservoirs of antimicrobial compounds which prevent colonization of the tissue. The active or induced defence responses include the hypersensitive response, the production of phytoalexins, lignification and the reinforcement of the cell wall, as well as the biosynthesis of pathogenesis-related proteins (PR-proteins).1 PR-proteins have been described in plant species from at least 13 families and new members of PR-proteins have been recognized and classified into 17 structurally and functionally distinct families.2 Some of them, such as β-1,3-glucanases, chitinases, endochitinases and peroxidases possess antifungal or antibacterial activity.2 In addition to the well-known PR-proteins induced by biotic molecules, some other naturally occurring substances are also involved in the induction of these proteins.3 Among these, jasmonates and its more active derivative, methyl jasmonate (MJ), coordinate the activation of a large set of defence responses and their exogenous application induces resistance in plants. In this way, the addition of MJ to tomato, tobacco and pepper induces genes that encode PR-proteins, such as chitinases, β-1,3-glucanases and peroxidases.4–6 Likewise, although PR-proteins are considered inducible proteins by elicitors, the number of data indicating their occurrence as constitutive proteins in both seeds and different plant organs, irrespective of stress conditions, is suggesting a possible role in passive defence mechanisms.6
In a previous work, we analyzed the inductor effect of MJ on PR-proteins in C. annuum suspension cultured cells (SCCs).6 Analysis of the extracellular proteome showed the presence of amino acid sequences homologous to PR1 and 4, NtPRp27-like proteins (NtPRp27), Class I chitinases, peroxidases, the hydrolytic enzymes LEXYL1 and 2, arabinosidases, pectinases, nectarin IV and leucin-rich repeat protein (LRR), which suggested that MJ plays a role in mediating defenserelated gene product expression in C. annuum. Apart from these MJ-induced proteins, other PR-proteins were found in both the control and elicited cell cultures of C. annuum. These included class IV chitinases, β-1,3-glucanases, thaumatin-like proteins and peroxidases, suggesting that their expression is mainly constitutive since they are involved in growth, development and defence processes.
Here, we study the effect of MJ in its role as inducer of PR-proteins on C. chinense SCCs. For this, extracellular proteins obtained from elicited C. chinense SCCs were separated by SDS-PAGE (Fig. 1). Figure 1 shows the protein pattern of the extracellular medium of C. chinense corresponding to the MJ-treatment (lane MJ) that contained two specific protein bands (2 and 4), and whose expression was MJ-dependent. As shown in Table 1, microsequencing of protein band 2, which has an apparent molecular weight of 24 kDa, identified peptides with homologous amino acid sequences to a Solanum tuberosum NtPRp27-like protein (NtPRp27, accession no. AAO22065) and three C. annuum PR-proteins (class I chitinase, LRR and germin-like proteins [GLP] whose accession numbers are AAR90844, AAN62015 and AAR28997, respectively). Tryptic peptides obtained by digestion of MJ-induced protein band 4 (with an apparent molecular weight of 90 kDa) (Table 1) contained amino acid sequences that showed homology with a C. annuum pectinesterase (accession number ABN08348). These results show that the exogenous application of MJ induces the accumulation of extracellular PR proteins in C. chinense SCCs, suggesting a role for MJ in mediating the defense-related gene product expression in Capsicum. Apart from these MJ-inducible proteins, we observed the presence of other PR-proteins in protein bands 1 and 3 of both the control (lane C in Fig. 1) and MJ-treated cells of C. chinense (lane MJ in Fig. 1). These PR-proteins were putative endochitinases and peroxidases (Table 1), which suggests that their expression is mainly constitutive. Indeed, others studies have confirmed the constitutive expression of these PR-proteins in the extracellular media of non-treated SCCs from Zinnia elegans, Cycas revolute and Taxus baccata or C. annuum.6,7
Figure 1.
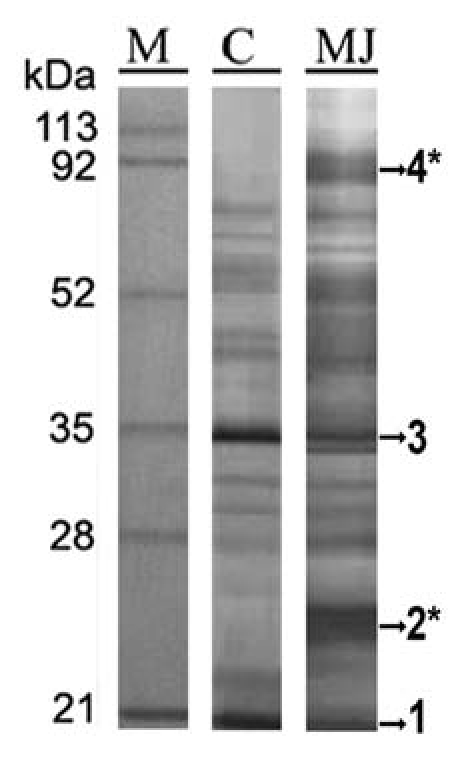
SDS-PAGE followed by Coomassie protein staining of the elicited spent media of C. chinense. Lane M, Molecular weight markers in kDa; lane C, control spent media; lane MJ, spent media from treated cells with MJ. *MJ-induced protein bands.
Table 1.
Tryptic peptides obtained by the digestion with trypsin of protein bands from C. chinense SCCs
| Protein bands | Tryptic peptide | Accession number | Protein | Nominal mass | Scores |
| 20 kDa (band 1) | GPI QIS YNY NYG PCG R YCG ILG VSP GDN LDC GNQ R |
AAY90154 | Endochitinase Capsicum annuum |
19,591 | 219 |
| 24 kDa (band 2)* | YIQ GYS GDV R | AAO22065 | NtPRp27-like protein Solanum tuberosum |
25,172 | 62 |
| NNF YSY NA FIT AAK SFP GFG TTG DTA VR GPI QIS YNY NYG PCG R |
AAR90844 | Chitinase class I Capsicum annuum |
13,175 | 291 | |
| LAG TVP LAN K LSG EIP ISV LK |
AAN62015 | Leucine-rich repeat protein Capsicum annuum |
21,557 | 114 | |
| GEV FAF PR GLV HFQ QN NGD VPA AVV A |
AAR28997 | Germin-like protein Capsicum annuum |
23,295 | 144 | |
| 33 kDa (band 3) | GFE VIA QAK LGG QTY TVA LGR EMV ALA GAH TVG FAR |
CAI48071 | Anionic peroxidase Capsicum chinense |
31,471 | 114 |
| 90 kDa (band 4)* | IDA FQD TLY THT LR TYL GRP WK |
ABN08348 | Pectinesterase Capsicum annuum |
60,079 | 133 |
MJ-induced protein bands.
As regards MJ-inducible PR-proteins, class I chitinases, LRR proteins and pectinesterases have been extensively described by various authors.6,8–10 However, NtPRp27 and GLP have never been described in C. chinense SCCs elicited with MJ. The amino acid sequence related to NtPRp27 showed homology with both Nicotiana tabacum and S. tuberosum NtPRp27, which clearly responded to fungal or virus infection, mechanical wounding, salicylic acid (SA), ethylene (ET) and MJ, which is consistent with many PR-proteins that accumulate transcripts in response to these last signalling molecules.11–13 As regards GLP, these constitute a large and heterogeneous group of proteins with amino acid identities ranging from 25 to 100%, which have different patterns of expression.14,15 Noted by Park et al.4, a C. annuum GLP (CaGLP1) was involved in the defense response to a wide range of pathogenic microbes, including viruses and bacteria. Moreover, when hot pepper plants were treated with SA or ET, they showed a rapid accumulation of CaGLP1 transcripts. In contrast to our results, the expression of CaGLP1 was not affected by MJ.17 Therefore, the different effects on GLP expression observed as the result of exogenous application of signalling molecules like SA, ET and MJ suggest that GLP may play a variety of roles in protecting plants against pathogen attacks and different stresses. In conclusion, it has been determined that in C. chinense SCCs, as well as in C. annuum SCCs, the exogenous application of MJ induces a set of putative proteins which are considered PR-proteins. Although a wide range of functions suggests their involvement in defence reactions, further studies are necessary to characterize their differential expression and the complexity of their regulation.
Acknowledgements
We thank M.M. Santiago Marín for help in maintaining cell cultures. This work was supported by the Ministerio de Ciencia e Innovación [BIO2008-02941], and Fundación Séneca [08799/PI/08]. Sabater-Jara, A.B. and Almagro, L. held grants from the Ministerio de Ciencia e Innovación.
Abbreviations
- CaGLP1
C. annuum germin-like protein
- ET
ethylene
- GLP
germin-like proteins
- LRR
leucin-rich repeat protein
- MJ
methyl jasmonate
- NtPRp27
NtPRp27-like proteins
- PR-proteins
pathogenesis-related proteins
- SA
salicylic acid
- SCCs
suspension cultured cells
References
- 1.Almagro L, Gómez Ros LV, Belchi-Navarro S, Bru R, Ros Barceló A, Pedreño MA. Class III peroxidases in plant defence reactions. J Exp Bot. 2009;60:377–390. doi: 10.1093/jxb/ern277. [DOI] [PubMed] [Google Scholar]
- 2.van Loon LC, Rep M, Pietersen CMJ. Significance of inducible defense-related proteins in infected plants. Annu Rev Phytopathol. 2006;44:135–162. doi: 10.1146/annurev.phyto.44.070505.143425. [DOI] [PubMed] [Google Scholar]
- 3.Edreva A. Pathogenesis-related proteins. Research progress in the last 15 years. General and Applied Plant Physiology. 2005;31:105–124. [Google Scholar]
- 4.Lee YK, Hwang BK. Differential induction and accumulation of β-1,3-glucanase and chitinase isoforms in the extracellular space and leaf tissues of pepper by Xanthomonas campestris pv. vesicatoria infection. J Phytopathol. 1996;144:79–87. [Google Scholar]
- 5.Hwang BK, Sunwoo JY, Kim YJ, Kim BS. Accumulation of β-1,3-glucanase and chitinase isoforms, and salicylic acid in DL-β-amino-n-butyric acid-induced resistance response of pepper stems to Phytophthora capsici. Physiol Mol Plant Pathol. 1997;51:305–322. [Google Scholar]
- 6.Sabater-Jara AB, Almagro A, Belchí-Navarro S, Ferrer MA, Ros-Barceló A, Pedreño MA. Induction of sesquiterpenes, phytoesterols and extracellular pathogenesis-related proteins in elicited cell cultures of Capsicum annuum. J Plant Physiol. 2010;167:1273–1281. doi: 10.1016/j.jplph.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Novo Uzal E, Gómez-Ros LV, Hernández JA, Pedreño MA, Cuello J, Ros Barceló A. Analysis of the soluble cell wall proteome of gymnosperms. J Plant Physiol. 2009;166:831–843. doi: 10.1016/j.jplph.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Wu CT, Bradford KJ. Class I chitinase and β-1,3-glucanase are differentially regulated by wounding, methyl jasmonate, ethylene and gibberellins in tomato seeds and leaves. Plant Physiol. 2003;133:263–273. doi: 10.1104/pp.103.024687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung EH, Jung HW, Lee SC, Han SW, Heu S, Hwang K. Identification of a novel pathogen-induced gene encoding a leucine-rich repeat protein expressed in phloem cells of Capsicum annuum. Biochim Biophys Acta. 2004;1676:211–222. doi: 10.1016/S0167-4781(03)00120-9. [DOI] [PubMed] [Google Scholar]
- 10.Yamagami A, Nakazawa M, Matsui M, Tujimoto M, Sakuta M, Asami T, et al. Chemical genetics reveal the novel trans-membrane protein BIL4, which mediates plant cell elongation in brassinosteroid signalling. Biosci Biotechnol Biochem. 2009;73:415–421. doi: 10.1271/bbb.80752. [DOI] [PubMed] [Google Scholar]
- 11.Okushima Y, Koizumi N, Kusano T, Sano H. Secreted proteins of tobacco cultured BY2 cells, identification of a new member of pathogenesis-related proteins. Plant Mol Biol. 2000;42:479–488. doi: 10.1023/a:1006393326985. [DOI] [PubMed] [Google Scholar]
- 12.Zhen Dong T, Jun L, Cong Hua X. Cloning of a pathogenesis-related protein gene cDNA of potato using RACE methods combined with cDNA Library. Acta Genetica Sinica. 2003;30:996–1002. [Google Scholar]
- 13.Elvira MI, Galdeano MM, Gilardi P, Garcia-Luque I, Serra MT. Proteomic analysis of pathogenesis-related proteins (PRs) induced by compatible and incompatible interactions of pepper mild mottle virus (PMMoV) in Capsicum chinense L3 plants. J Exp Bot. 2008;59:1253–1265. doi: 10.1093/jxb/ern032. [DOI] [PubMed] [Google Scholar]
- 14.Bernier F, Berna A. Germins and germin-like proteins, plant do-all proteins. But what do they exactly? Plant Physiol Biochem. 2001;39:545–554. [Google Scholar]
- 15.Godfrey D, Able AJ, Dry IB. Induction of a grapevine germin-like protein VvGLP3 gene is closely linked to the site of Erysiphe necator infection. A possible role in defense? Mol Plant Microbe Interac. 2007;20:1112–1125. doi: 10.1094/MPMI-20-9-1112. [DOI] [PubMed] [Google Scholar]
- 16.Park CJ, An JM, Shin YC, Kim KJ, Lee BJ, Paek KH. Molecular characterization of pepper germin-like protein as the novel PR-16 family of pathogenesis-related proteins isolated during the resistance response to viral and bacterial infection. Planta. 2004;219:797–806. doi: 10.1007/s00425-004-1290-x. [DOI] [PubMed] [Google Scholar]
- 17.Hurkman AJ, Tanaka CK. Effect of salt stress on germin gene expression in barley roots. Plant Physiol. 1996;110:971–977. doi: 10.1104/pp.110.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]


