Abstract
The receptors for the fungal elicitor EIX (LeEix1 and LeEix2) belong to a class of leucine-rich repeat cell-surface glycoproteins with a signal for receptor-mediated endocytosis. Both receptors are able to bind the EIX elicitor while only the LeEix2 receptor mediates defense responses. We show that LeEix1 acts as a decoy receptor and attenuates EIX induced internalization and signaling of the LeEix2 receptor. We demonstrate that BAK1 binds LeEix1 but not LeEix2. In plants where BAK1 was silenced, LeEix1 was no longer able to attenuate plant responses to EIX, indicating that BAK1 is required for this attenuation. We suggest that LeEix1 functions as a decoy receptor for LeEix2, a function which requires the kinase activity of BAK1.
Key words: LRR-RLP, LeEix, Bak1, decoy receptor, endocytosis
Leucine-rich-repeat receptor proteins (LRR-RLPs) have been linked with defense response signaling in plants.1–5 The tomato Cf genes which mediate resistance to Cladosporium fulvum encode LRR-RLPs. Additional LRR-RLPs include the tomato Verticillium (Ve) resistant proteins6,7 and the LeEix proteins.8 The Eix receptors (LeEix1 and LeEix2) contain a signal for receptor-mediated endocytosis, which we have previously shown to be essential for proper induction of defense responses.9,10 Both receptors are able to bind Eix, but only LeEix2 mediates EIX-induced defense.8 In a recent work we demonstrate that LeEix1 attenuates Eix-induced internalization and signaling, and heterodimerizes with LeEix2 upon application of Eix.11 Our work further shows that the brassinosteroid co-receptor Bri-Associated Kinase 1 (BAK1) binds LeEix1 but not LeEix2. In BAK1-silenced plants, LeEix1 was no longer able to attenuate plant responses to Eix, indicating that BAK1 is required for this attenuation and leading to the hypothesis that LeEix1 functions as a decoy receptor for LeEix2.11
BAK1 in Defense Signaling
BAK1 has been reported to be involved in the defense responses mediated by plant receptors.6,12–14 BAK1 is required for proper defense receptor signaling by the Flagellin sensitive 2 (FLS2) and Ve1 receptors.6,12,13 Lack of BAK1 leads to inhibition of endocytosis and a decrease in signaling in the case of flagellin.12 FLS2-BAK1 hetero-dimerization occurs almost instantaneously after perception of the ligand flg22, and kinase activity of BAK1 is essential for FLS2 signaling. De novo phosphorylation of both FLS2 and BAK1 was found to occur within 15 seconds of flg22 application. Additional plant defense elicitors, such as bacterial EF-Tu, also induce rapid formation of complexes with de novo phosphorylated BAK1. Thus, it was proposed that several Leucine-rich-repeat receptor like kinases (LRR-RLKs) form tight complexes with BAK1 almost instantaneously after ligand binding and that the subsequent phosphorylation events are key initial steps in signal transduction.14
Decoy Receptors
Decoy receptors are well known in mammalians.15,16 One of the better characterized mammalian decoy receptor families is the DcR family of Tumor Necrosis Factor (TNF) decoy receptors.17 The decoy receptor DcR3 binds to the ligands of several TNF-superfamily members, resulting in competition for ligand binding and inhibition of TNF signaling.18 Interestingly, several types of tumors have been found to express DcR3, thereby reducing TNF signaling, probably as a mechanism for tumor proliferation.18 Additional decoy receptors related to programmed cell death (PCD) in mammalians were characterized for TRAIL, a cytotoxic protein which induces PCD.19 TRAIL receptors 3 and 4, also termed DcR1 and DcR2, were found to bind TRAIL and block TRAIL-induced apoptosis.19,20 It would seem that the use of decoy receptors for regulation of signaling cascades in mammalian systems was mostly observed in cases where the signaling can result in cell death. We found that LeEix1 acts as a decoy receptor in the LeEix/EIX system. Overexpression of LeEix1 results in attenuation of the induction of HR mediated by EIX.11 Similarly to mammalian systems, the LeEix1 decoy receptor attenuates signaling that normally results in PCD.21
A Possible Model for the Function of BAK1 in the EIX/LeEix System
Based on our results we would like to propose the general model depicted in Figure 1: BAK1 is bound to LeEix1 in the cell membrane under a certain steady state.11 Upon EIX application, BAK1 may phosphorylate or mediate the phosphorylation of LeEix1, (possible due to a conformational change ensuing from the binding of EIX to LeEix1) causing dissociation of this complex and binding of LeEix1 to LeEix2, or precipiting the binding of LeEix1 to LeEix2 in other signaling avenues which do not rely on phosphorylation. This binding serves to prevent the internalization and signaling of LeEix2. In the latter case, BAK1 may remain bound to the LeEix complex, although this binding is not detectable in the BiFC system. After several hours of exposure to EIX, we see a large increase in the expression of LeEix1, an in vivo situation which mimics LeEix1 overexpression creating a new steady state in which LeEix1 greatly attenuates EIX signaling, as we have demonstrated in our recent paper. Greater time frames see a decrease in LeEix1 to its normal endogenous expression level, upon which LeEix2 is free to internalize and transmit the EIX induced signal, and HR is induced, albeit to a lower level. In the absence of BAK1, LeEix1 does not bind BAK1, does not undergo BAK1 modification and cannot attenuate EIX signaling. Additional experimentation is needed to validate this hypothesis.
Figure 1.
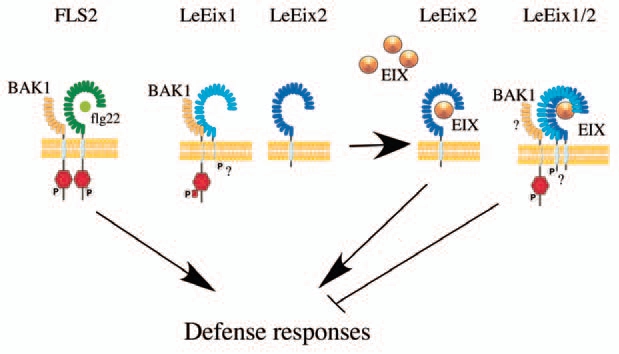
Schematic representation of BAK1 LeEix/EI X function. Arrows point induction or attenuation of plant defense responses.
Acknowledgements
This work was partly supported by the Israel Science Foundation administered by the Israel Academy of Science and Humanities no. 294/07. FYVE-dsRed was a kind gift from Dr. Jozef Samaj. We thank Dr. Russinova and Dr. de Vries for the Arabidopsis BAK1. Dr. Rathjen for BAK1 VIGS vectors and Dr. Jia Li for BAK1 K317E.
References
- 1.Becraft PW. Receptor kinase signaling in plant development. Annu Rev Cell Dev Biol. 2002;18:163–192. doi: 10.1146/annurev.cellbio.18.012502.083431. [DOI] [PubMed] [Google Scholar]
- 2.Bittel P, Robatzek S. Microbe-associated molecular patterns (MAMPs) probe plant immunity. Curr Opin Plant Biol. 2007;10:335–341. doi: 10.1016/j.pbi.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 3.Chakrabarti A, Panter SN, Harrison K, Jones JD, Jones DA. Regions of the Cf-9B disease resistance protein able to cause spontaneous necrosis in Nicotiana benthamiana lie within the region controlling pathogen recognition in tomato. Mol Plant Microbe Interact. 2009;22:1214–1226. doi: 10.1094/MPMI-22-10-1214. [DOI] [PubMed] [Google Scholar]
- 4.Postel S, Kemmerling B. Plant systems for recognition of pathogen-associated molecular patterns. Semin Cell Dev Biol. 2009;20:1025–1031. doi: 10.1016/j.semcdb.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Wang G, Ellendorff U, Kemp B, Mansfield JW, Forsyth A, Mitchell K, et al. A genome-wide functional investigation into the roles of receptor-like proteins in Arabidopsis. Plant Physiol. 2008;147:503–517. doi: 10.1104/pp.108.119487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fradin EF, Zhang Z, Ayala JCJ, Castroverde CDM, Nazar RN, Robb J, et al. Genetic dissection of Verticillium wilt resistance mediated by tomato Ve1. Plant Physiol. 2009;150:320–332. doi: 10.1104/pp.109.136762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawchuk LM, Hachey J, Lynch DR, Kulcsar F, van Rooijen G, Waterer DR, et al. Tomato Ve disease resistance genes encode cell surface-like receptors. Proc Natl Acad Sci USA. 2001;98:6511–6515. doi: 10.1073/pnas.091114198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ron M, Avni A. The receptor for the fungal elicitor ethylene-inducing xylanase is a member of a resistance-like gene family in tomato. Plant Cell. 2004;16:1604–1615. doi: 10.1105/tpc.022475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bar M, Avni A. EHD2 inhibits ligand-induced endocytosis and signaling of the leucine-rich repeat receptor-like protein LeEix2. Plant J. 2009;59:600–611. doi: 10.1111/j.1365-313X.2009.03897.x. [DOI] [PubMed] [Google Scholar]
- 10.Bar M, Avni A. EHD2 inhibits signaling of leucine rich repeat receptor-like proteins. Plant Signal Behav. 2009;4:682–684. doi: 10.4161/psb.4.7.9078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bar M, Sharfman M, Ron M, Avni A. BAK1 is required for the attenuation of ethylene-inducing xylanase (Eix)-induced defense responses by the decoy receptor LeEix1. Plant J. 2010;63:791–800. doi: 10.1111/j.1365-313X.2010.04282.x. [DOI] [PubMed] [Google Scholar]
- 12.Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nurnberger T, Jones JDG, et al. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature. 2007;448:497–500. doi: 10.1038/nature05999. [DOI] [PubMed] [Google Scholar]
- 13.Heese A, Hann DR, Gimenez-Ibanez S, Jones AME, He K, Li J, et al. The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci USA. 2007;104:12217–12222. doi: 10.1073/pnas.0705306104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulze B, Mentzel T, Jehle AK, Mueller K, Beeler S, Boller T, et al. Rapid heteromerization and phosphorylation of ligand-activated plant transmembrane receptors and their associated kinase BAK1. J Biol Chem. 2010;285:9444–9451. doi: 10.1074/jbc.M109.096842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bengtsson AK, Ryan EJ. Immune function of the decoy receptor osteoprotegerin. Crit Rev Immunol. 2002;22:201–215. [PubMed] [Google Scholar]
- 16.Mantovani A, Muzio M, Ghezzi P, Colotta F, Introna M. Negative regulators of the interleukin-1 system: Receptor antagonists and a decoy receptor. Int J Clin Lab Res. 1996;26:7–14. doi: 10.1007/BF02644768. [DOI] [PubMed] [Google Scholar]
- 17.Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999;11:255–260. doi: 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- 18.Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer. 2002;2:420–430. doi: 10.1038/nrc821. [DOI] [PubMed] [Google Scholar]
- 19.Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277:815–818. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- 20.Bisgin A, Terzioglu E, Aydin C, Yoldas B, Yazisiz V, Balci N, et al. TRAIL death receptor-4, decoy receptor-1 and decoy receptor-2 expression on CD8+ T cells correlate with the disease severity in patients with rheumatoid arthritis. BMC Musculoskelet Disord. 2010;11:192. doi: 10.1186/1471-2474-11-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elbaz M, Avni A, Weil M. Constitutive caspase-like machinery executes programmed cell death in plant cells. Cell Death Differ. 2002;9:726–733. doi: 10.1038/sj.cdd.4401030. [DOI] [PubMed] [Google Scholar]


