Abstract
Electrostatic nature of plant plasma membrane (PM) plays significant roles in the ion uptake and toxicity. Electrical potential at the PM exterior surface (ψ0o) influences ion distribution at the PM exterior surface, and the depolarization of ψ0o negativity increases the electrical driving force for cation transport, but decreases the driving force for anion transport across the PMs. Assessing environmental risks of toxic ions has been a difficult task because the ion concentration (activity) in medium is not directly corrected to its potential effects. Medium characteristics like the content of major cations have important influences on the bioavailability and toxicity of ions in natural waters and soils. Models such as the Free Ion Activity Model (FIAM) and the Biotic Ligand Model (BLM), as usually employed, neglect the ψ0o and hence often lead to false conclusions about interaction mechanisms between toxic ions and major cations for biology. The neglect of ψ0o is not inconsistent with its importance, and possibly reflects the difficulty in the measurement of ψ0o. Based on the dual effects of the ψ0o, electrostatic models were developed to better predict the uptake and toxicity of metallic and metalloid ions. These results suggest that the electrostatic models provides a more robust mechanistic framework to assess metal(loid) ecotoxicity and predict critical metal(loid) concentrations linked to a biological effect, indicating its potential utility in risk assessment of metal(loid)s in water and terrestrial ecosystems.
Key words: electrostatic models, plasma membrane, surface electric potential, ion uptake, toxicity, risk assessment
GCS Model and Calculation of ψ0o
The Gouy-Chapman-Stern (GCS) model combines a classic electrostatic Gouy-Chapman theory and ion binding (Stern model). The Gouy-Chapman theory describes the electric double layer originated from negative charges and dipoles on the hydrophilic outer surface of PMs. The Stern model developed by Kinraide et al.1 describes the strong interactions for binding of ions to PM sites. Therefore, the GCS model incorporates the intrinsic surface charge density (σ0) of a membrane, the ion composition of the bathing medium, and ion binding to the membrane.
The Gouy-Chapman portion of the model can be expressed in the Müller (Grahame) equation (derivation presented in Barber2). This equation describes the relationships among the PM exterior surface charge density (σPMS), the ion concentrations in bulk-phase medium (BM) ([IZ]b), and the electrical potential at the PM surface (ψ0o).
| Eq. 1 |
Here, εr is the dielectric constant for water; ε0 is the permittivity of a vacuum; and F, R, T are the Faraday constant, the gas constant and temperature, respectively. σPMS is expressed in coulombs per square meter (Cm−2). σPMS depends on σtotal, the surface charge density in the absence of any bound solute ions. σPMS also includes the ions binding to the PM surface.
For the Stern portion of the GCS model, the PM surface was assumed to be composed of two classes of binding sites: one negatively charged (R−) and one neutral (P0). The model takes this binding into account. The model accommodates 1:1 binding of ions to R− and P0. The reactions can be expressed as:
| Eq. 2 |
and
| Eq. 3 |
Binding constants can be expressed as:
| Eq. 4 |
where [R−], [P0], [RIZ-1] and [PIZ] indicate membrane surface density (molm−2). [IZ]0 denotes the concentration of the unbound ion at the PM surface and can be calculated as:
| Eq. 5 |
If the binding constants KR,I and KP,I, RT and PT and the concentrations of all ions in bulk-phase medium are known, the σPMS can be calculated as:
| Eq.6 |
Thus, the electrical potential at the PM surface (ψ0o) can be calculated by combining Equations 1 and 6. The up-to-date parameters for the model are presented in Table 3 of Kinraide and Wang3 and were derived and selected from many sources and adjusted by correlation between the computed values of ψ0o and the measured Zeta potentials (ζ), which are the electric potential at the hydrodynamic plane of shear at a small distance from the PM exterior surface. A computer program for the GCS model may be obtained from TB Kinraide (USDA) and the authors. When the ionic composition of the bathing medium is known, the values for ψ0o can be computed with a simplified method (Equation 11 of Kinrade4).
The GCS model, incorporating the parameter values used by us, appears to be quite robust,1,5–8 but we are least confident the parameter for intrinsic surface charge density (σ0). We have employed a value of 0.3074 µmol negatively charged sites/m2 (σ0 = −30 mC/m2), but this appears to be somewhat variable with species, tissue and preparation of vesicles and protoplasts.3 The uncertainties mean that the values for ψ0o, and the ion activities at the PM exterior surface computed from ψ0o with the Nernst equation, may be only proportional to the actual values. The feature of proportionality is very robust,3 and uncertainties regarding the model fail to reduce seriously the great superiority of expressing plant-ion interactions (including uptake, toxicity and the alleviation of toxicity) in terms of surface activities rather than bulk-phase activities,3–5,7,9–13 and the results are presented in the present article. As for the value of σ0 used in the GCS model, much evidence preponderantly supports the suitability of σ0 = −30 mCm−2 while noting the occurrence of variability among membranes. This variability is small relative to the difference in estimates of σ0 by many methods.3 A proposed, fully parameterized GCS model appears to be applicable for the calculation of ψ0o and be suitable for the interpretation of many plant responses to the ionic environment.
Electrostatic Models
Increases in major cations concentrations or decreases in pH in root bathing media reduced the negativity of ψ0o with negligible effects upon the bulk-phase-to-bulk-phase transmembrane potential difference (Em). This reduction caused decreases in the activities of metallic cations at the PM exterior surface and increases in the activities of anions, such as H2AsO4−. The reduced surface negativity increased the surface-to-surface transmembrane potential difference (Em,surf), thus increasing the electrical driving force for cation transport and decreasing the driving force for anion transport across the PM. Ion uptake and toxicity depended on the balance of the dual effects of ψ0o (altered ion PM exterior surface activity and surface-to-surface transmembrane electrical gradients). In light of this knowledge, the electrostatic uptake model (Equations 1 to 4 of Wang et al.15) and the electrostatic toxicity model (Equations 5 to 7 of Wang et al.15) were developed to model the uptake and toxicity of both metallic and metalloid ions. Regression analysis of measured uptake and toxicity of ions with the electrostatic models demonstrated the dual effects of ψ0o. For example, Parker et al. investigated Cu2+ rhizotoxicity to wheat roots (Triticum aestivum L. cv. Yecora Rojo) in response to CuCl2 (0 ∼ 1.6 µM), variable CaCl2 (0 ∼ 5.0 mM), MgCl2 (0 ∼ 4.9 mM) and pH (4.5 ∼ 6.5) in a factorial array. It is clear from the authors' figures that increasing Ca2+ or Mg2+ concentrations or reducing pH alleviated Cu2+ stress (the study considered theoretical aspects of FIAM). In our reanalysis with the electrostatic toxicity model, it is evident from Figure 1 that RRL correlated more strongly with the calculated RRL based on the electrostatic toxicity model (R2 = 0.921) than with Cu2+ surface activity (R2 = 0.878) or bulk-phase activity (R2 = 0.745). Similarly, almost all the measured RRLs were within 95% confidence limits of the predicted one based on the electrostatic toxicity model (Fig.-1D), indicating that alleviation of Cu2+ by cations and pH was attributed to the dual effects of ψ0o.
Figure 1.
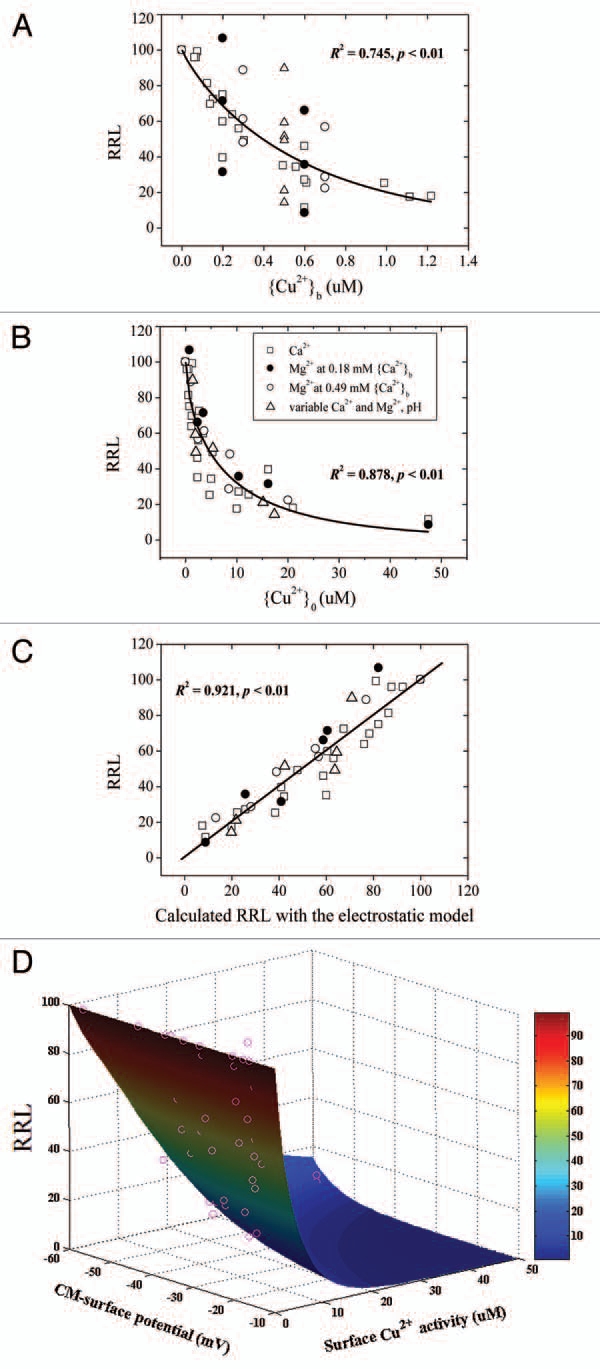
Relative root elongation (RRL) of wheat (cv. Yecora Rojo) seedlings exposed to toxic levels of Cu2+ affected by Ca2+, Mg2+ and pH. Data were taken from Parker et al. The RRL is plotted as functions of (A) Cu2+ activities in the bulk medium, (B) Cu2+ ion activities at the CMSe, (C) calculated Cu2+ toxicity based on the electrostatic toxicity model or (D) the ψ0o (x-axis) and Cu2+ surface activity (y-axis).
Prediction of Ion Uptake and Toxicity with the FIAM, SIAM and Electrostatic Models
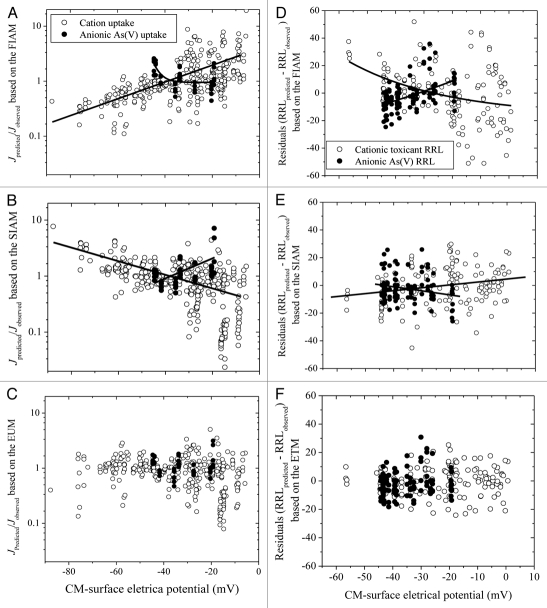
The ion uptake and toxicity predicted by the FIAM, Surface Ion Activity Model (SIAM) and the Electrostatic Uptake Models (EUM) or Electrostatic Toxicity Model (ETM) are compared in Figure 2. For cationic uptake or toxicity, the ratio (predicted Juptake/observed Juptake) based on the FIAM increases from 0.1 to 10 (Fig. 2A) and the residuals (RRLpredicted − RRLobserved) become more negative (Fig.-2D) with decreasing negativity of ψ0o. That is, the FIAM underestimates the bioavailability and toxicity of metallic ions at a higher negativity of ψ0o (possibly corresponding to a situation with lower ion strength or higher pH, which is exactly what was found for the toxicity of copper to aquatic organisms) and overestimates at least one. For the SIAM, the ratio declines and gradually deviates from 1 (Fig. 2B) and the residuals become more positive (Fig. 2E) with decreasing the negativity of ψ0o, indicating that SIAM underestimates uptake and toxicity of metallic ions. The SIAM neglects the gradually enhanced uptake and toxicity as ψ0o becomes less negative. For anion uptake, such as H2AsO4−, the situation is reversed. For the EUM or ETM, the ratio and residuals are not significantly different from 1 and 0 (p > 0.05), respectively (Fig. 2 and bottom).
Figure 2.
Predicted Juptake/observed Juptake (left column) and residual values (RRLpredicted − RRLobserved) (right column) are plotted against the PM-surface electrical potential (ψ0o). The predicted uptake or RRL was based on the free ion activity model (FIAM, top), the surface ion activity model (SIAM, middle) or the electrostatic uptake model (EUM) and electrostatic toxicity model (ETM) (bottom). Curves are plotted where p < 0.05.
General Evaluation of the Electrostatic Approach to Ion Uptake and Toxicity
The BLM is an attempt to generalize the concepts of toxicity and alleviation on the basis of toxic metal ion binding to cell-surface ligands as a key step leading to toxicity. The alleviation of toxicity by major cations is caused by the competition of these ions with toxic ions for binding to the same ligands. These assumptions may not be true in the present study. For example, {Ca2+}0° increased initially and reached a peak value with the increase of {Ca2+}b from 0.25 to 1.0 mM in the bulk medium, and then slightly declined with further increases in {Ca2+}b (Fig. 3A). In contrast, the CMSe activities of other cations, especially Mg2+ and H+, declined markedly (Fig. 3B) despite their constant concentrations in the BM. Interestingly, the sum of the PM exterior surface activities of Ca2+ and Mg2+ was also decreased with increasing {Ca2+}b (Fig. 3B). It is indicated that the competition effect from surface Ca2+, if existent, only took place at low {Ca2+}b concentrations (e.g., lower than 1.0 mM; Fig. 3A) and that the overall competition effects from Ca2+, Mg2+ and H+ may be weakened with adding of Ca2+ in the BM. However, the surface activity of cationic toxicants at the PM exterior surface declined at all times, caused by a reduction in the negativity of ψ0o that accompanied an increase in CaCl2. Therefore, ψ0o effects could give the false appearance of competition in cases where competition is weak or does not occur at all.
Figure 3.
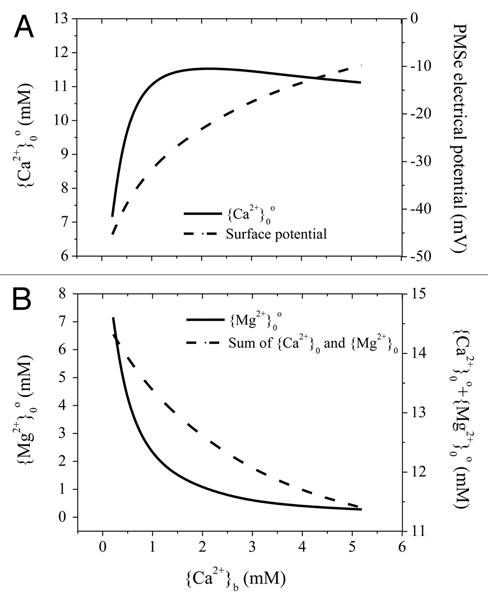
Electrical potentials (ψ0o) and activities of Ca2+ and Mg2+ at the PM exterior surface in response to Ca2+ addition to the bulk-phase medium (BM). The subscript b refers to variables in the BM. The subscript 0 refers to variables at the out CM-surface. The values of ψ0o were calculated for each datum point with the GCS model, and then surface activities of Ca2+ and Mg2+ were computed with the Nernst equation. The BM contained 0.25 mM MgCl2 and variable CaCl2 at pH 6.0.
The BLM also fails to interpret the enhancement of uptake and toxicity of anions by the treatments that reduce the toxicity of toxic cations.5,10 These latter phenomena, therefore, require a special interpretation, but we have now shown that electrostatic mechanisms provide a unified interpretation of both phenomena—the cation alleviation of cation uptake and toxicity and the enhancement of anion uptake and toxicity. The alleviation of cation toxicity by ameliorative cations may entail site-specific competitions, but that effect may be not existent or minor.4,5 In nearly all cases, the dual effects of ψ0o (electrostatic enhancement or depletion of cationic or anionic toxicants; increases or decreases in the electrical driving force for cationic of anionic toxicant transport across the PM) is sufficient to explain most ion-toxicant interactions. Most previous studies did not take an electrostatic approach, but their results are better explained by the dual effects.4,5
References
- 1.Kinraide TB, Yermiyahu U, Rytwo G. Computation of surface electrical potentials of plant cell membranes. Correspondence to published zeta potentials from diverse plant sources. Plant Physiol. 1998;118:505–512. doi: 10.1104/pp.118.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barber J. Membrane surface charges and potentials in relation to photosynthesis. Biochim Biophys Acta. 1980;594:253–308. doi: 10.1016/0304-4173(80)90003-8. [DOI] [PubMed] [Google Scholar]
- 3.Kinraide TB, Wang P. The surface charge density of plant cell membranes (σ): An attempt to resolve conflicting values for intrinsic σ. J Exp Bot. 2010;61:2507–2518. doi: 10.1093/jxb/erq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinraide TB. Plasma membrane surface potential (PM) as a determinant of ion bioavailability: A critical analysis of new and published toxicological studies and a simplified method for the computation of plant PM. Environ Toxicol Chem. 2006;25:3188–3198. doi: 10.1897/06-103r.1. [DOI] [PubMed] [Google Scholar]
- 5.Wang P, Zhou DM, Kinraide TB, Luo XS, Li LZ, Li DD, et al. Cell membrane surface potential (ψo) plays a dominant role in the phytotoxicity of copper and arsenate. Plant Physiol. 2008;148:2134–2143. doi: 10.1104/pp.108.127464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yermiyahu U, Rytwo G, Brauer DK, Kinraide TB. Binding and electrostatic attraction of lanthanum (La3+) and aluminum (Al3+) to wheat root plasma membranes. J Membr Biol. 1997;159:239–252. doi: 10.1007/s002329900287. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Q, Smith FA, Sekimoto H, Reid RJ. Effect of membrane surface charge on nickel uptake by purified mung bean root protoplasts. Planta. 2001;213:788–793. doi: 10.1007/s004250100555. [DOI] [PubMed] [Google Scholar]
- 8.Kinraide TB, Yermiyahu U. A scale of metal ion binding strengths correlating with ionic charge, Pauling electronegativity, toxicity and other physiological effects. J Inorg Biochem. 2007;101:1201–1213. doi: 10.1016/j.jinorgbio.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Kinraide TB. Three mechanisms for the calcium alleviation of mineral toxicities. Plant Physiol. 1998;118:513–520. doi: 10.1104/pp.118.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinraide TB. The controlling influence of cell-surface electrical potential on the uptake and toxicity of selenate (SeO42-) Physiol Plantarum. 2003;117:64–71. [Google Scholar]
- 11.Yermiyahu U, Kinraide TB, Huang PM, Gobran GR. Binding and electrostatic attraction of trace elements to plant root surfaces. In: Huang PM, Gobran GM, editors. Biogeochemistry of Trace Elements in the Rhizosphere. Amsterdam: Elsevier; 2005. pp. 365–389. [Google Scholar]
- 12.Kopittke PM, Blamey FPC, Asher CJ, Menzies NW. Trace metal phytotoxicity in solution culture: A review. J Exp Bot. 2010;61:945–954. doi: 10.1093/jxb/erp385. [DOI] [PubMed] [Google Scholar]
- 13.Wang P, Zhou DM, Peijnenburg WJGM, Li LZ. Evaluating mechanisms for plant-ion (Ca2+, Cu2+, Cd2+ or Ni2+) interactions and their effectiveness on rhizotoxicity. Plant Soil. 2010;344:277–288. [Google Scholar]
- 14.Parker DR, Pedler JF, Thomason DN, Li HY. Alleviation of copper rhizotoxicity by calcium and magnesium at defined free metal-ion activities. Soil Sci Soc Am J. 1998;62:965–972. [Google Scholar]
- 15.Wang P, Kinraide TB, Zhou DM, Kopittke PM, Peijnenburg WJGM. Plasma membrane surface potential: dual effects upon ion uptake and toxicity. Plant Physiol. 2011;155:808–820. doi: 10.1104/pp.110.165985. [DOI] [PMC free article] [PubMed] [Google Scholar]