Abstract
The glomerular basement membrane (GBM) is a crucial component of the kidney's filtration barrier that separates the vasculature from the urinary space. During glomerulogenesis, the GBM is formed from fusion of two distinct basement membranes, one synthesized by the glomerular epithelial cell (podocyte) and the other by the glomerular endothelial cell. The main components of the GBM are laminin-521 (α5β2γ1), collagen α3α4α5(IV), nidogen and the heparan sulfate proteoglycan, agrin. By studying mice lacking specific GBM components, we have shown that during glomerulogenesis, laminin is the only one that is required for GBM integrity and in turn, the GBM is required for completion of glomerulogenesis and glomerular vascularization. In addition, our results from laminin β2-null mice suggest that laminin-521, and thus the GBM, contribute to the establishment and maintenance of the glomerular filtration barrier to plasma albumin. In contrast, mutations that affect GBM collagen IV or agrin do not impair glomerular development or cause immediate leakage of plasma proteins. However, collagen IV mutation, which causes Alport syndrome and ESRD in humans, leads to gradual damage to the GBM that eventually leads to albuminuria and renal failure. These results highlight the importance of the GBM for establishing and maintaining a perfectly functioning, highly selective glomerular filter.
Key words: laminin, collagen IV, nephrotic syndrome, alport syndrome, podocyte, mesangial cell, glomerulogenesis
Introduction
Human kidneys generate an enormous primary filtrate of about 180 liters from the approximately 900 L of blood that pass through them on a daily basis. Due to resorption of water and solutes by the tubular epithelial cells of the kidney, this primary filtrate is concentrated to a urinary output of only 1–2 L. The glomerular filtration barrier, which lies between the vasculature and the urinary space, retards the passage of plasma proteins, primarily of albumin and immunoglobulins, while ensuring the efficient flow of water and small solutes that comprise the primary filtrate. Since the realization that the glomerular capillary wall consists of two cells, podocyte and endothelium, separated by an extracellular matrix called the glomerular basement membrane (GBM), there have been vigorous debates about which of these three layers serves as the major barrier to plasma proteins.1 In this review, I will summarize our current understanding of the cellular and extracellular components of the glomerular capillary wall and focus on the composition of the GBM as it relates to glomerulogenesis, permselectivity and kidney disease.
The Glomerular Filtration Barrier
The glomerular filtration barrier (Fig. 1) is a three-layered structure that lies between the vasculature and Bowman's space. Within the glomerulus, it is the only separation between the bloodstream and the urine. The GBM is a specialized extracellular matrix situated between the podocytes and endothelial cells, which, during glomerulogenesis, synthesize and secrete components of the GBM. Furthermore, both cell types are important for maintaining the GBM's structure and function after glomerular maturation.2–4 Although the focus of this review is the GBM, podocytes and endothelial cells will also be discussed, as all three layers are necessary for establishing and maintaining an intact filtration barrier.
Figure 1.
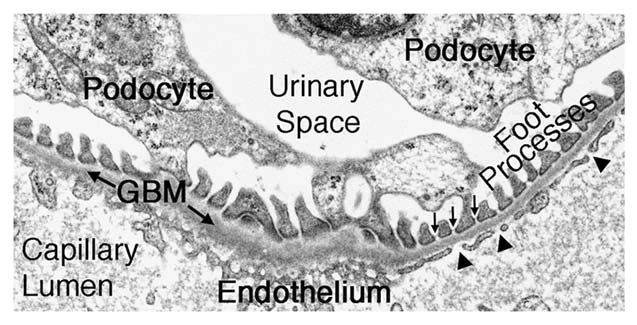
The ultrastructure of the glomerular filtration barrier. The capillary lumen is lined by an endothelium with fenestrations (arrowheads). The endothelium is adjacent to the ribbon-like glomerular basement membrane (GBM). Podocyte foot processes, which are bridged by slit diaphragms (arrows), abut the opposite aspect of the GBM and are surrounded by the primary filtrate present in the urinary space.
Podocytes
Podocytes, also called glomerular visceral epithelial cells, are morphologically complex, nascent nephron epithelium-derived cells that reside within the urinary space (also called Bowman's space) and are therefore bathed in the primary urine. Podocytes enwrap the outer aspect of the glomerular capillaries by extending narrow foot processes that interdigitate with those of adjacent podocytes. Juxtaposed foot processes are directly linked to one another by the glomerular slit diaphragms (Fig. 1); these derive from gradual modification and apical-to-basal migration of the tight junctions that originally joined the previously columnar, immature podocytes.5
Like the tubular epithelial cells of the nephron, podocytes are descendants of the metanephric mesenchyme, a population of intermediate mesoderm-derived cells that are determined to form nephrons. At the beginning of development of the definitive kidney, the ureteric bud grows towards and into the metanephric mesenchyme and induces it to condense and undergo a dramatic mesenchyme-to-epithelium transition to form what is referred to as the renal vesicle. Activation of numerous important signaling pathways results in morphological changes to the renal vesicle and segmentation of the resulting “S-shaped” figure into the podocytes, the parietal epithelial cells that form Bowman's capsule,and the epithelial cells that comprise the tubular segments of the nephron.6
Glomerular Endothelial Cells
Glomerular capillaries are lined by endothelial cells that are different than most, because they bear many fenestrations (Fig. 1). Fenestrations are seemingly patent “holes” in the endothelial cells that allow the passage of fluid across the cell layer. Ultrastructural analyses have shown that some of these fenestrations have wagon wheel-like diaphragms similar to those found in peritubular capillaries and in islet endothelial cells in the pancreas, but many of the fenestrations in glomerular endothelial cells do not have diaphragms.7 This lack of diaphragms likely allows more efficient flow of plasma across this proximal layer of the glomerular filtration barrier.
Despite the presence of what usually appear by electron microscopy to be unobstructed fenestrae in the glomerular endothelium, special methods of fixation and staining indicate the presence of filamentous plugs in the fenestrations. These plugs are thought to be composed of a form of glycocalyx (a sugary coat) assembled by the glomerular endothelial cells at their cell surfaces. These fenestral plugs have been hypothesized to serve as an impedance to the passage of plasma proteins and therefore an important component of the glomerular filtration barrier to albumin.8–10
Glomerular Basement Membrane (GBM)
The GBM is, in some ways, a typical basement membrane: it has an electron-dense lamina densa when viewed by transmission electron microscopy, and it is formed by protein-protein interactions among the same classes of macromolecules found in all other basement membranes. However, the GBM also has some atypical features. First, it is unusually thick compared to most other basement membranes; as mentioned above, the GBM forms during glomerulogenesis by the fusion of two distinct basement membranes, the endothelial and the visceral epithelial (or podocyte) basement membranes.3 Second, the GBM has an unusual composition compared to most other basement membranes, and this presumably imparts its unique functional properties. The GBM contains laminin, type IV collagen, nidogen and heparan sulfate proteoglycan (HSPG), components found in all basement membranes, but for some of these types of matrix proteins, the specific isoforms present in the GBM are very different than those found elsewhere in basement membranes. Even the contiguous Bowman's capsule and tubular basement membranes, for example, possess different complements of isoforms compared to the GBM.
The GBM occupies a prime position within the glomerular filtration barrier (Fig. 1). In addition, it is very dense by electron microscopy. Together with structural and functional data regarding the GBM and other basement membranes, published by many different labs over a period of decades, we hypothesize that the GBM functions by (1) providing adhesive and signaling substrates to the adjacent glomerular cells (podocytes, endothelial cells and mesangial cells); (2) concentrating and presenting diffusible factors, such as vascular endothelial growth factor; and (3) contributing directly to the glomerular filtration barrier to albumin. These functions, if correct, likely depend upon the composition of the GBM, which will now be discussed.
Laminin
Laminins are a family of large, evolutionarily related glycosylated proteins that assemble with each other to form at least 15 different αβγ heterotrimeric macromolecules. These laminin trimers are synthesized and secreted by diverse cell types into the extracellular space. Laminin trimers in the most recent laminin nomenclature scheme are named based solely on their chain composition; e.g., a trimer containing the laminin α5, β2 and γ1 chains is called laminin-521, or LM-521.11 Most laminin heterotrimers are cross-shaped macromolecules (LM-521 is shown in Fig. 2), with the one “long arm” formed by association of the α, β and γ chains via coiled-coil interactions and disulfide bonding. The three free “short arms” contain NH2-terminal globular domains (called LN domains) that mediate laminin trimer-trimer interactions in the extracellular space. These interactions lead to laminin polymerization, which initiates basement membrane formation. The ∼100 kDa laminin globular (LG) domain at the distal end of the long arm (found exclusively at the COOH termini of α chains) mediates interactions with integrin and nonintegrin receptors on the surfaces of cells. A number of studies have shown that laminin polymerization in the extracellular space is required for initiation of basement membrane formation. Moreover, laminin polymerization via short arm-short arm interactions is facilitated by LG domain interactions with cellular receptors, likely due in part to the increase in effective laminin concentration that results at cell surfaces. Intracellular signaling events induced by laminin binding are also involved in promoting laminin polymerization and basement membrane formation.12
Figure 2.
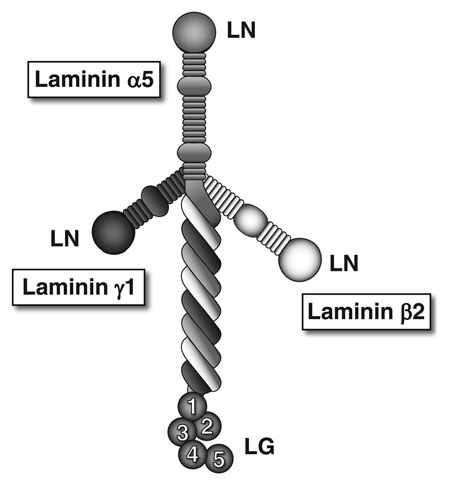
The laminin-521 heterotrimer. This full-sized cruciform laminin is composed of the α5, β2 and γ1 chains. Globular laminin amino-terminal (LN) domains interact with each other to facilitate laminin polymerization in the extracellular matrix. The α chain laminin globular (LG) domain is recognized by receptors on cells, such as integrins, dystroglycan and the Lutheran blood group glycoprotein.
LM-521 is the major laminin found in the mature GBM, and it is secreted by both podocytes and endothelial cells.2 During the early stages of glomerulogenesis, when glomerular endothelial cells are migrating towards the differentiating podocytes to begin to form the glomerular capillaries, and the GBM is first recognizable as a distinct basement membrane, LM-111 and LM-511 are the major laminin components.13 But as capillary maturation begins, LM-521 is deposited by both podocytes and endothelial cells, and LM-111 and -511 are eventually eliminated.13,14 The molecular mechanisms that drive the developmental transitions in laminin expression and deposition have yet to be discovered, but as discussed below, these transitions are crucial to ensure the establishment of a proper glomerular filtration barrier.
Type IV Collagen
Type IV collagen, like all collagens, is secreted as a trimer (also called protomer) composed of three α chains, the sequences of which include collagenous regions that consist of repeated Gly-X-Y amino acid triplets. The presence of glycine at every third residue is absolutely necessary to allow the chains to assemble into a stable triple helix; this is because glycine is the only amino acid with a side chain (a hydrogen) that is able to fit at the center of the helix. But unlike the rigid fibril-forming types of collagen that are associated with bone and tendon, for example, the six distinct collagen IV α chains contain numerous interruptions of the Gly-X-Y repeats that are scattered throughout the collagenous domain. These interruptions provide flexibility to the collagen IV trimers and therefore to the collagen IV network, which is formed by covalent and non-covalent interactions among trimers in the extracellular matrix.15
Besides the central collagenous region containing the Gly-X-Y interruptions, all six collagen IV α chains bear a small non-collagenous NH2-terminal domain called 7S and a larger COOH-terminal noncollagenous domain called NC1. Both of these domains are crucial for linking trimers to each other to promote collagen IV network formation. Moreover, the NC1 domain plays an important role in directing the composition of collagen IV heterotrimers; information present within the NC1 domain sequence ensures that only three types of collagen IV trimers form: (α1)2α2, α3α4α5 and (α5)2α6.16
The collagen IV network of the mature GBM consists primarily of α3α4α5 heterotrimers. Grafting experiments in vivo have shown that podocytes but not endothelial cells synthesize the α3α4α5 network,4 so it is likely the small amount of collagen α1 and α2(IV) chains that are present in the mature GBM derive mainly from glomerular endothelial cells. Similar to the situation for laminins discussed above, there are developmental transitions in collagen IV gene expression and collagen trimer deposition during glomerulogenesis. At the earliest stages, only the α1 and α2(IV) chains are detected, but as the glomerular capillaries begin to mature, there is a gradual increase in deposition of the α3, α4 and α5 chains.14 This developmental transition in collagen IV network production has been shown to occur during human, dog and mouse kidney development.14,17,18
Nidogens
There are two nidogen proteins. Each is a dumbbell-shaped basement membrane component that binds tightly to the laminin γ1 chain short arm as well as to type IV collagen.19 Therefore, nidogen was thought initially to be crucial for basement membrane formation as a potential link between the separate laminin and type IV collagen networks. However, analysis of single and double nidogen-1 and -2 knockout mice proved that basement membranes can form in the absence of either or both nidogen proteins, although some basement membranes subjected to high levels of stress during embryogenesis exhibit breaks and discontinuities in the absence of both nidogens.20 As far as the GBM is concerned, both nidogens-1 and -2 are present, but little is known about their differential expression during development.
Heparan Sulfate Proteoglycans (HSPGs)
HSPGs consist of a protein core with covalently linked glycosaminoglycan side chains that can dramatically increase the molecular weight of the molecule. The side chains are frequently modified by sulfation, which confers a significant anionic charge to the proteoglycan and thus to the basement membrane (or other extracellular matrix) in which the proteoglycan is located. Although perlecan is probably the most common HSPG found in most basement membranes, the mature GBM contains very little perlecan but, instead, contains primarily agrin.21 Agrin plays an important role in the neuromuscular system, where a special isoform secreted by motor neurons is crucial for development and organization of the neuromuscular junction.22 Although agrin is the major GBM HSPG at maturity, during glomerular development there appears to be a codistribution of agrin and perlecan, and then perlecan disappears from the GBM and becomes confined to the mesangial matrix.23
The GBM's Contribution to Glomerulogenesis
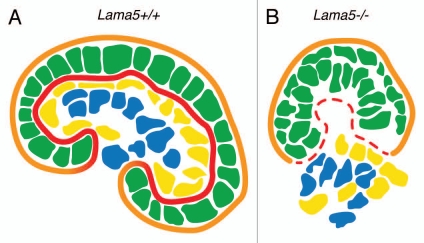
Evidence that the GBM plays an important role in glomerulogenesis comes from our studies of laminin α5 mutant and transgenic mice. As alluded to above, during glomerulogenesis there are developmental transitions in GBM components, including transitions from LM-111 to LM-511 and then to LM-521, which is the mature isoform.13 The appearance of LM-511 in the nascent GBM is accompanied by the abrupt removal/disappearance of LM-111 by an unknown mechanism. When laminin α5 is knocked out, LM-111 is still removed, and because there is no polymerizing laminin trimer present, the integrity of the GBM is compromised, and it breaks down.24 Without a GBM, none of the three glomerular cell types are able to maintain their positions within the developing glomerulus, leading to a disorganized glomerulus (Fig. 3) and failed glomerular vascularization.24 Subsequent studies of specific segments of the laminin α5 COOH-terminal LG domain reveal a previously unknown role for laminin α5 in mediating adhesion of mesangial cells to the GBM; this adhesion is required for maintaining the capillary looping that is a major anatomical characteristic of the glomerular tuft.25
Figure 3.
Schematic diagram showing that GBM breakdown due to Lama5 mutation results in failed glomerulogenesis. (A) A maturing wild-type (Lama5+/+) glomerulus with an intact podocyte epithelium (green) and endothelium (yellow) flanking the GBM (red). Mesangial cells (blue) are centrally located within the glomerulus, and parietal epithelial cells (not shown) sit on the Bowman's capsule basement membrane (orange) and line Bowman's space. (B) Failed glomerulogenesis in a Lama5−/− kidney. Discontinuities in the GBM (red) lead to stratification of the podocyte layer (green) and disorganized endothelial and mesangial cells (yellow and blue, respectively).
The GBM's Contribution to Permselectivity
Since the discovery that nephrin (NPHS1), a primary component of the glomerular slit diaphragm, is mutated in congenital nephrotic syndrome of the Finnish type,26 podocytes have been the major focus of research aimed at defining the nature of the glomerular filtration barrier and understanding the etiology of glomerular disease. There is now known to be a large cohort of genes expressed in podocytes, the mutation of which in humans and/or mice causes defects in glomerular permselectivity, nephrotic range proteinuria and in some cases, focal segmental glomerulosclerosis.27 This certainly lends support to the concept that podocytes are crucial for establishing and maintaining the glomerular filtration barrier. However, the GBM should not be ignored or forgotten.28 Of the nine proteins that are known to be present in the mature GBM, mutations that affect four of them cause glomerular disease in humans (as well as in mice), and mutation of a fifth causes nephrotic syndrome in mice.29 These will now be discussed.
The first GBM protein shown to be affected in a human disease is collagen α5(IV), which is encoded by COL4A5.30 Mutations in COL4A5 cause the X-linked form of Alport syndrome, a hereditary glomerulonephritis leading to kidney failure.31 As discussed above, the α5 chain is a component of the α3α4α5(IV) trimer that is present in the mature GBM. In the absence of α5(IV), these trimers, and thus the network they would normally form within the GBM, are totally absent. Likewise, homozygous or compound heterozygous mutations that affect the autosomal COL4A3 or COL4A4 genes also prevent production of the α3α4α5(IV) trimer and cause the more rare, autosomal recessive form of Alport syndrome.
In the absence of the usual collagen α3α4α5(IV) network that is present in the GBM, there is substitution or compensation by the (α1)2α2(IV) network.32 Interestingly, in some Col4a3−/− Alport mice, especially those on the C57BL/6J strain background, there is also compensation in the GBM by the (α5)2α6(IV) network, which may slow progression of the disease.33 Although this network functions adequately for several years in most Alport syndrome patients, eventually there is a characteristic splitting and thickening of the GBM, visible by electron microscopy (Fig. 4), that sometimes imparts a “basket weave” appearance. This pathology is associated first with hematuria and later with proteinuria. Because proteinuria is a relatively late feature of Alport syndrome, it seems that the identity of the collagen IV network is not an important determinant of glomerular permselectivity. It is important to note here that dominant mutations in human COL4A1, which encodes the collagen α1(IV) chain, also cause several forms of kidney disease, and there are a number of interesting extrarenal manifestations.34,35
Figure 4.
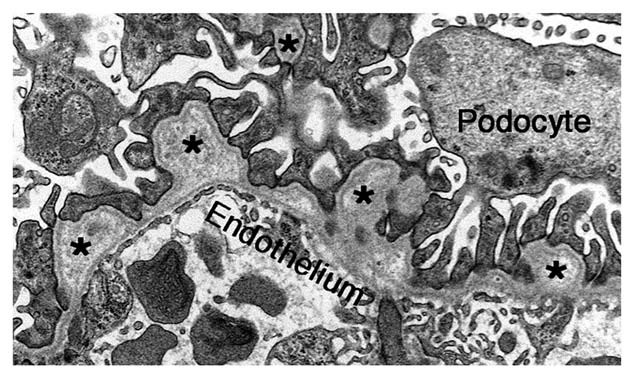
The ultrastructure of Alport mouse GBM. In the absence of the collagen α3α45α5(IV) network, the GBM becomes split and thickened, with moderately electron-lucent outpocketings (asterisks). However, the endothelium and podocyte foot processes are mostly intact at this early stage of the disease process.
In contrast to the delayed onset proteinuria that develops in patients with Alport syndrome, those with Pierson syndrome, which is caused by truncating or severe missense mutations in the laminin β2 gene LAMB2, exhibit congenital nephrotic syndrome and diffuse mesangial sclerosis together with extrarenal manifestation such as small pupils (microcoria) and neurological abnormalities.36,37 Lamb2−/− mice exhibit a phenotype that is consistent with that observed in Pierson syndrome patients, including nephrotic syndrome,38 severe neuromuscular abnormalities39 and retinal defects.40 Interestingly, unlike the GBM breakdown that occurs in mice lacking laminin α5,24 GBM integrity is maintained in the absence of laminin β2. This is likely because there is retention of laminin β1 in the mature Lamb2−/− GBM38 as part of LM-511 and ectopically deposited LM-111, LM-211 and perhaps LM-311.41 We concluded initially that laminin β1 compensates structurally for the missing laminin β2 but cannot compensate functionally.38 However, currently we are testing the alternative hypothesis that the remaining laminin β1 in the GBM is quantitatively insufficient compared to the level of laminin β2 that is normally present, and that artificially increasing the level of β1 will increase the concentration of laminin trimers in the GBM and thereby greatly improve the filtration barrier, perhaps by increasing the density of the GBM.
Patients with what seem to be “less severe” missense LAMB2 mutations also have nephrotic syndrome, but it is accompanied by significantly milder extrarenal defects.42,43 These data suggest that laminin β2 and the laminin-521 trimer of which it is a component contribute to the glomerular filter by imparting appropriate barrier properties to the GBM. Indeed, our studies of Lamb2−/− mice showed clearly that albuminuria is detectable before foot process effacement and loss of slit diaphragms. Furthermore, foot process and slit diaphragm formation normally occurrs in the absence of LM-521. The straightforward interpretation is that albumin leaks across the filtration barrier because the GBM is defective, indicating that the GBM must normally serve as an important component of the filtration barrier. Loss of foot processes is likely a secondary response to proteinuria,41 but this remains to be investigated.
We recently investigated the mechanism whereby a particular pathogenic LAMB2 missense mutation (R246Q) found in a Turkish family42 causes congenital nephrotic syndrome. The mutation was engineered into the rat laminin β2 cDNA and expressed in the podocytes of transgenic mice in vivo via the mouse nephrin promoter.44 Transgenes from three different founder lines were expressed at different relative levels, determined to be high, medium and low based on mutant protein accumulation in the GBM. These three transgenes were bred individually onto the Lamb2−/− background, so that all laminin β2 in the GBM derived from the mutant transgene. These mice developed proteinuria, the level of which was inversely proportional to the level of laminin β2 in the GBM and in all cases much lower than that observed in Lamb2−/− mice without the transgene. Interestingly, the low expressor had levels of laminin β2 mRNA in podocytes that were higher than that normally observed for endogenous mouse laminin β2, suggesting that the nephrin promoter was more than adequate for driving the production of mutant laminin β2 mRNA, in agreement with our previous studies in reference 41 and 45. Because studies of the R246Q mutant in transfected 293 cells suggest that the mutation somehow inhibits laminin secretion, we concluded that this mutation causes misfolding and degradation of most of the laminin β2 polypeptide, but that the increased transcription imparted by the nephrin promoter allows the podocytes to overcome the secretion defect and secrete enough LM-521 into the GBM to make a partly functional albumin barrier.44
Concerning the other components of the GBM, lack of either nidogen-1 or nidogen-2 results in viable mice20 and no significant defects in glomerular filtration. Similarly, podocyte-specific knockout of agrin, which leads to its absence from the GBM, has no discernible effect on the filtration barrier, although its loss causes a dramatic reduction in the GBM's concentration of negatively charged sites.23 Finally, although no mutations affecting human laminin α5 or laminin γ1 have been discovered, likely due to early prenatal lethality that should occur due to widespread expression of both chains during embryogenesis, podocyte-specific mutation of Lama5 in mice results in proteinuria that, in many cases, progresses to nephrotic syndrome and renal failure.29
Questions and Answers
Dr. Dwight Towler, Ira M. Lang Professor of Medicine and Developmental Biology, Washington University School of Medicine: I am curious about the potential for acquired dysfunctions in the laminin β chains. On that particular Arg246 of laminin β2, is it susceptible to modification by advanced glycation end products, for example? Do you know whether or not one or more N-carboxymethyl arginine derivatives, advanced glycosylation end products described in collagen in the setting of diabetes, could also modify and nullify the function of that β chain?
Dr. Jeffrey Miner: I don't think anyone has looked at specific residues of laminins in the setting of hyperglycemia.
Dr. Towler: The structure-function implications from the family with Pierson syndrome phenotypes and your elegant data point to that possibility. It is clearly an important region of laminin β2 with respect to glomerular basement membrane function and proteinuria; I was wondering whether or not some of the acquired albuminuria states and GBM deficiencies also relate to biochemical modifications of that same β2 arginine residue.
Dr. Miner: No, not that I know of.
Dr. Towler: I know this presentation was “focused on the filter,” but I was curious about your hypomorphic mutation of the α5 chain C terminus. Did you happen to note any structural defects that occurred in the peritubular basement membranes, where laminin-511 might be important?
Dr. Miner: We did not, we focused on the filter. That was the only place where we found any obvious abnormalities. The tubular basement membranes also have other laminins. It is not pure LM-511.
Dr. Towler: So do you think that the mesangial cell interaction with the COOH terminus of laminin α5 is a fairly specific interaction in the glomerular basement membrane?
Dr. Miner: Yes
Dr. Feng Chen, Assistant Professor of Medicine and Cell Biology and Physiology, Washington University School of Medicine: I have two questions. First, GBM is made by endothelial cells and podocytes, and I am wondering if anyone has looked carefully for any qualitative or quantitative differences between the contributions from these two cell types. Second, in the laminin β2 knockouts, there is some β1 retention to partially compensate for the problem. Do you know if normally the β1 is from the podocyte or from the endothelial cells?
Dr. Miner: Dale Abrahamson has done a lot of work to address those issues, at least the first one. He has shown that the laminin is made by both, as he can detect LM-521 within the endoplasmic reticulum in both endothelial cells and in podocytes.2 My personal opinion is that podocytes make more, and that is based on our finding that we can rescue the laminin β2 knockout by only rescuing the podocytes and not affecting the endothelial cells, which don't express from the nephrin promoter that we used.45 Regarding collagen, it is a different story. Dr. Abrahamson showed that only podocytes make the collagen α3/4/5 network,4 and that makes perfect sense because one can always detect a low level of the α1/2 network on the subendothelial aspect of the basement membrane. So if one thinks about rescuing Alport syndrome, either by gene therapy or bone marrow stem cells, there would need to be an effect on the podocyte side. Your second question had to do with residual laminin β1 and where that comes from. That is something we don't know and is something a student in my laboratory is working on. My guess is that it is coming from both cells. Sometimes we can detect a little bit of β1 even in a normal GBM, but I think the levels of transcription are very low, because these proteins are so long lived in the basement membrane; turnover is very slow, so there never needs to be a lot of mRNA to make a basement membrane. I think it would be difficult for us to discover by in situ hybridization, for example, which cell is making β1. However, it is something we are interested in figuring out.
Dr. Raphael Kopan, Professor of Developmental Biology and of Medicine, Washington University School of Medicine: Have you looked to see if you have phenotypes in animals that are heterozygous for the β1 chain, the β2 chain or trans-heterozygous for β1 and β2? Your hypothesis predicts that it is a quantitative rather than a qualitative deficiency.
Dr. Miner: In every laminin mutant that I know of there has never been a defect shown in a heterozygote. Even though it is quantitatively less transcription, the proteins are so long lived that it doesn't seem to take much mRNA to make a normal basement membrane protein complement.
Dr. Kopan: What if you reduce the β1 on the β2 knockout background?
Dr. Miner: We haven't done that. We have β1 knockout animals; we should do that experiment.
Dr. Helen Liapis, Professor of Pathology and Immunology, Washington University School of Medicine: You mentioned podocyte foot process effacement as being caused by a variety of conditions, and your last diagram also points out that defects in a lot of molecules may lead to the same result. However, there are nephrotic syndromes that are not associated with foot process effacement. Can you try to explain that?
Dr. Miner: Well I know of endothelial cell injury in preeclampsia, and that has been pretty well worked out by Karumanchi and others.46,47 Our model, I think, is also proteinuria without foot process effacement. What are the other instances?
Dr. Liapis: Well, my experience is in humans. For instance, you can have patients with crescentic glomerulonephritis and lots of protein loss, but they do not have foot process effacement. We just had a pediatric case the other day, and I was scratching my head. Why does this kid have proteinuria, if the podocytes look normal and the foot processes are intact? Is it possible that you can have acquired laminin dysfunction?
Dr. Miner: I doubt it. It is possible that something is directly impacting the structure of the GBM, but I can't imagine why that might happen other than what Dr. Towler brought up. Thinking about the intact nephron hypothesis, it is possible that the biopsy did not get the right glomeruli, and there might be leaking glomeruli with foot process effacement in other parts of the kidney.
Dr. Keith Hruska, Professor of Pediatrics, Medicine and Cell Biology and Physiology, Washington University School of Medicine: For your Col4a3 model of Alport syndrome, you have reported the switch to the collagen α5/5/6(IV) network associated with improved renal function.33 You mentioned the potential for rescuing the laminin β2 knockout with a small molecule stimulator of β1 expression. Has anybody been able to use this approach to activate a switch to induce podocytes to express ectopic α6 in the GBM?
Dr. Miner: You mean in mice? No. In theory, it should work in humans as well, but no one has ever seen that same ectopic α5/5/6 collagen IV network in human Alport patients. It seems to be a mouse-specific phenomenon and has only been shown to occur on the C57BL/6J background. The senior author of that paper thinks that there is actually a SNP, or a difference in the promoter of α5/6, that allows high levels of α6 to be made. And of course, most human Alport patients have α5 mutations, so even if you could activate α6 expression in podocytes, it would not help without the α5. So that approach couldn't work for most patients.
Acknowledgements
My relevant research is supported by NIH grants R01DK078314, R01GM060432 and R01DK081156.
Abbreviations
- GBM
glomerular basement membrane
- LM
laminin
- HSPG
heparan sulfate proteoglycan
Note
Edited transcripts of research conferences sponsored by Organogenesis and the Washington University George M. O'Brien Center for Kidney Disease Research (P30 DK079333) are published in Organogenesis. These conferences cover organogenesis in all multicellular organisms, including research into tissue engineering, artificial organs and organ substitutes and are participated in by faculty at Washington University School of Medicine, St. Louis, MO USA.
References
- 1.Farquhar MG. Editorial: The primary glomerular filtration barrier—basement membrane or epithelial slits? Kidney Int. 1975;8:197–211. doi: 10.1038/ki.1975.103. [DOI] [PubMed] [Google Scholar]
- 2.St John PL, Abrahamson DR. Glomerular endothelial cells and podocytes jointly synthesize laminin-1 and -11 chains. Kidney Int. 2001;60:1037–1046. doi: 10.1046/j.1523-1755.2001.0600031037.x. [DOI] [PubMed] [Google Scholar]
- 3.Abrahamson DR. Origin of the glomerular basement membrane visualized after in vivo labeling of laminin in newborn rat kidneys. J Cell Biol. 1985;100:1988–2000. doi: 10.1083/jcb.100.6.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abrahamson DR, Hudson BG, Stroganova L, Borza DB, St John PL. Cellular origins of type IV collagen networks in developing glomeruli. J Am Soc Nephrol. 2009;20:1471–1479. doi: 10.1681/ASN.2008101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schnabel E, Anderson JM, Farquhar MG. The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J Cell Biol. 1990;111:1255–1263. doi: 10.1083/jcb.111.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dressler GR. The cellular basis of kidney development. Annu Rev Cell Dev Biol. 2006;22:509–529. doi: 10.1146/annurev.cellbio.22.010305.104340. [DOI] [PubMed] [Google Scholar]
- 7.Satchell SC, Braet F. Glomerular endothelial cell fenestrations: An integral component of the glomerular filtration barrier. Am J Physiol Renal Physiol. 2009;296:947–956. doi: 10.1152/ajprenal.90601.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deen WM, Lazzara MJ, Myers BD. Structural determinants of glomerular permeability. Am J Physiol Renal Physiol. 2001;281:579–596. doi: 10.1152/ajprenal.2001.281.4.F579. [DOI] [PubMed] [Google Scholar]
- 9.Haraldsson B, Nystrom J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev. 2008;88:451–487. doi: 10.1152/physrev.00055.2006. [DOI] [PubMed] [Google Scholar]
- 10.Haraldsson B, Jeansson M. Glomerular filtration barrier. Curr Opin Nephrol Hypertens. 2009;18:331–335. doi: 10.1097/MNH.0b013e32832c9dba. [DOI] [PubMed] [Google Scholar]
- 11.Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, et al. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–332. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Colognato H, Winkelmann DA, Yurchenco PD. Laminin polymerization induces a receptor-cytoskeleton network. J Cell Biol. 1999;145:619–631. doi: 10.1083/jcb.145.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miner JH, Patton BL, Lentz SI, Gilbert DJ, Snider WD, Jenkins NA, et al. The laminin alpha chains: expression, developmental transitions and chromosomal locations of alpha1-5, identification of heterotrimeric laminins 8–11 and cloning of a novel alpha3 isoform. J Cell Biol. 1997;137:685–701. doi: 10.1083/jcb.137.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miner JH, Sanes JR. Collagen IV α3, α4 and α5 chains in rodent basal laminae: Sequence, distribution, association with laminins and developmental switches. J Cell Biol. 1994;127:879–891. doi: 10.1083/jcb.127.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hudson BG. The molecular basis of Goodpasture and Alport syndromes: beacons for the discovery of the collagen IV family. J Am Soc Nephrol. 2004;15:2514–2527. doi: 10.1097/01.ASN.0000141462.00630.76. [DOI] [PubMed] [Google Scholar]
- 16.Khoshnoodi J, Cartailler JP, Alvares K, Veis A, Hudson BG. Molecular recognition in the assembly of collagens: Terminal noncollagenous domains are key recognition modules in the formation of triple helical protomers. J Biol Chem. 2006;281:38117–38121. doi: 10.1074/jbc.R600025200. [DOI] [PubMed] [Google Scholar]
- 17.Harvey SJ, Zheng K, Sado Y, Naito I, Ninomiya Y, Jacobs RM, et al. Role of distinct type IV collagen networks in glomerular development and function. Kidney Int. 1998;54:1857–1866. doi: 10.1046/j.1523-1755.1998.00188.x. [DOI] [PubMed] [Google Scholar]
- 18.Kalluri R, Shield CF, III, Todd P, Hudson BG, Neilson EG. Isoform switching of type IV collagen is developmentally arrested in X-linked Alport syndrome leading to increased susceptibility of renal basement membranes to endoproteolysis. J Clin Invest. 1997;99:2470–2478. doi: 10.1172/JCI119431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salmivirta K, Talts JF, Olsson M, Sasaki T, Timpl R, Ekblom P. Binding of mouse nidogen-2 to basement membrane components and cells and its expression in embryonic and adult tissues suggest complementary functions of the two nidogens. Exp Cell Res. 2002;279:188–201. doi: 10.1006/excr.2002.5611. [DOI] [PubMed] [Google Scholar]
- 20.Bader BL, Smyth N, Nedbal S, Miosge N, Baranowsky A, Mokkapati S, et al. Compound genetic ablation of nidogen 1 and 2 causes basement membrane defects and perinatal lethality in mice. Mol Cell Biol. 2005;25:6846–6856. doi: 10.1128/MCB.25.15.6846-6856.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groffen AJ, Ruegg MA, Dijkman H, van de Velden TJ, Buskens CA, van den Born J, et al. Agrin is a major heparan sulfate proteoglycan in the human glomerular basement membrane. J Histochem Cytochem. 1998;46:19–27. doi: 10.1177/002215549804600104. [DOI] [PubMed] [Google Scholar]
- 22.Kummer TT, Misgeld T, Sanes JR. Assembly of the postsynaptic membrane at the neuromuscular junction: Paradigm lost. Curr Opin Neurobiol. 2006;16:74–82. doi: 10.1016/j.conb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Harvey SJ, Jarad G, Cunningham J, Rops AL, van der Vlag J, Berden JH, et al. Disruption of glomerular basement membrane charge through podocyte-specific mutation of agrin does not alter glomerular permselectivity. Am J Pathol. 2007;171:139–152. doi: 10.2353/ajpath.2007.061116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miner JH, Li C. Defective glomerulogenesis in the absence of laminin α5 demonstrates a developmental role for the kidney glomerular basement membrane. Dev Biol. 2000;217:278–289. doi: 10.1006/dbio.1999.9546. [DOI] [PubMed] [Google Scholar]
- 25.Kikkawa Y, Virtanen I, Miner JH. Mesangial cells organize the glomerular capillaries by adhering to the G domain of laminin alpha5 in the glomerular basement membrane. J Cell Biol. 2003;161:187–196. doi: 10.1083/jcb.200211121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, et al. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- 27.Machuca E, Benoit G, Antignac C. Genetics of nephrotic syndrome: connecting molecular genetics to podocyte physiology. Hum Mol Genet. 2009;18:185–194. doi: 10.1093/hmg/ddp328. [DOI] [PubMed] [Google Scholar]
- 28.Farquhar MG. The glomerular basement membrane: not gone, just forgotten. J Clin Invest. 2006;116:2090–2093. doi: 10.1172/JCI29488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldberg S, Adair-Kirk TL, Senior RM, Miner JH. Maintenance of glomerular filtration barrier integrity requires laminin alpha5. J Am Soc Nephrol. 2010;21:579–586. doi: 10.1681/ASN.2009091004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barker DF, Hostikka SL, Zhou J, Chow LT, Oliphant AR, Gerken SC, et al. Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science. 1990;248:1224–1227. doi: 10.1126/science.2349482. [DOI] [PubMed] [Google Scholar]
- 31.Kashtan CE. Alport syndromes: Phenotypic heterogeneity of progressive hereditary nephritis. Pediatr Nephrol. 2000;14:502–512. doi: 10.1007/s004670050804. [DOI] [PubMed] [Google Scholar]
- 32.Kashtan CE, Kim Y. Distribution of the α1 and α2 chains of collagen IV and of collagens V and VI in Alport syndrome. Kidney Int. 1992;42:115–126. doi: 10.1038/ki.1992.269. [DOI] [PubMed] [Google Scholar]
- 33.Kang JS, Wang XP, Miner JH, Morello R, Sado Y, Abrahamson DR, et al. Loss of alpha3/alpha4(IV) collagen from the glomerular basement membrane induces a strain-dependent isoform switch to alpha5alpha6(IV) collagen associated with longer renal survival in Col4a3−/− Alport mice. J Am Soc Nephrol. 2006;17:1962–1969. doi: 10.1681/ASN.2006020165. [DOI] [PubMed] [Google Scholar]
- 34.Gould DB, Phalan FC, van Mil SE, Sundberg JP, Vahedi K, Massin P, et al. Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N Engl J Med. 2006;354:1489–1496. doi: 10.1056/NEJMoa053727. [DOI] [PubMed] [Google Scholar]
- 35.Plaisier E, Gribouval O, Alamowitch S, Mougenot B, Prost C, Verpont MC, et al. COL4A1 mutations and hereditary angiopathy, nephropathy, aneurysms and muscle cramps. N Engl J Med. 2007;357:2687–2695. doi: 10.1056/NEJMoa071906. [DOI] [PubMed] [Google Scholar]
- 36.Zenker M, Aigner T, Wendler O, Tralau T, Muntefering H, Fenski R, et al. Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet. 2004;13:2625–2632. doi: 10.1093/hmg/ddh284. [DOI] [PubMed] [Google Scholar]
- 37.Zenker M, Tralau T, Lennert T, Pitz S, Mark K, Madlon H, et al. Congenital nephrosis, mesangial sclerosis and distinct eye abnormalities with microcoria: an autosomal recessive syndrome. Am J Med Genet A. 2004;130:138–145. doi: 10.1002/ajmg.a.30310. [DOI] [PubMed] [Google Scholar]
- 38.Noakes PG, Miner JH, Gautam M, Cunningham JM, Sanes JR, Merlie JP. The renal glomerulus of mice lacking s-laminin/laminin β2: Nephrosis despite molecular compensation by laminin β1. Nat Genet. 1995;10:400–406. doi: 10.1038/ng0895-400. [DOI] [PubMed] [Google Scholar]
- 39.Noakes PG, Gautam M, Mudd J, Sanes JR, Merlie JP. Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin β2. Nature. 1995;374:258–262. doi: 10.1038/374258a0. [DOI] [PubMed] [Google Scholar]
- 40.Libby RT, Lavallee CR, Balkema GW, Brunken WJ, Hunter DD. Disruption of laminin beta2 chain production causes alterations in morphology and function in the CNS. J Neurosci. 1999;19:9399–9411. doi: 10.1523/JNEUROSCI.19-21-09399.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jarad G, Cunningham J, Shaw AS, Miner JH. Proteinuria precedes podocyte abnormalities in Lamb2−/− mice, implicating the glomerular basement membrane as an albumin barrier. J Clin Invest. 2006;116:2272–2279. doi: 10.1172/JCI28414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hasselbacher K, Wiggins RC, Matejas V, Hinkes BG, Mucha B, Hoskins BE, et al. Recessive missense mutations in LAMB2 expand the clinical spectrum of LAMB2-associated disorders. Kidney Int. 2006;70:1008–1012. doi: 10.1038/sj.ki.5001679. [DOI] [PubMed] [Google Scholar]
- 43.Matejas V, Hinkes B, Alkandari F, Al-Gazali L, Annexstad E, Aytac MB, et al. Mutations in the human laminin beta2 (LAMB2) gene and the associated phenotypic spectrum. Hum Mutat. 2010;31:992–1002. doi: 10.1002/humu.21304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Y, Kikkawa Y, Miner JH. A missense LAMB2 mutation causes congenital nephrotic syndrome by impairing laminin secretion. J Amer Soc Nephrol. 2011:22. doi: 10.1681/ASN.2010060632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miner JH, Go G, Cunningham J, Patton BL, Jarad G. Transgenic isolation of skeletal muscle and kidney defects in laminin beta2 mutant mice: Implications for Pierson syndrome. Development. 2006;133:967–975. doi: 10.1242/dev.02270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]