Abstract
Chronic kidney disease (CKD) is a progressive loss in renal function over a period of months or years. End-stage renal disease (ESRD) or stage 5 CKD ensues when renal function deteriorates to under 15% of the normal range. ESRD requires either dialysis or, preferentially, a kidney organ allograft, which is severely limited due to organ shortage for transplantation. To combat this situation, one needs to either increase supply of organs or decrease their demand. Two strategies therefore exist: for those that have completely lost their kidney function (ESRD), we will need to supply new kidneys. Taking into account the kidneys' extremely complex structure, this may prove to be impossible in the near future. In contrast, for those patients that are in the slow progression route from CKD to ESRD but still have functional kidneys, we might be able to halt progression by introducing stem cell therapy to diseased kidneys to rejuvenate or regenerate individual cell types. Multiple cell compartments that fall into three categories are likely to be worthy targets for cell repair: vessels, stroma (interstitium) and nephron epithelia. Different stem/progenitor cells can be linked to regeneration of specific cell types; hematopoietic progenitors and hemangioblastic cell types have specific effects on the vascular niche (vasculogenesis and angiogenesis). Multipotent stromal cells (MSC), whether derived from the bone marrow or isolated from the kidney's non-tubular compartment, may, in turn, heal nephron epithelia via paracrine mechanisms. Nevertheless, as we now know that all of the above lack nephrogenic potential, we should continue our quest to derive genuine nephron (epithelial) progenitors from differentiated pluripotent stem cells, from fetal and adult kidneys and from directly reprogrammed somatic cells.
Key words: kidney regeneration, kidney stem cells, surface markers, reprogramming, tissue specific stem cells, renal progenitors
Introduction
Modern lifestyle and the shift of various diseases into a chronic course have led to a significant increase in the number of people suffering from severe disabling illnesses. To name a few examples, the number of hospitalizations with any mention of heart failure (HF) tripled from 1,274,000 in 1979 to 3,860,000 in 2004;1 the world prevalence of diabetes mellitus (DM) among adults is predicted to reach 439 million adults by 2030,2 and the Medicare cost of end-stage renal disease (ESRD) has risen from $12.2 in 2000 to $20.8 billion in 2007.3 HF, DM and ESRD are all manageable but incurable, chronic diseases.
ESRD patients, for example, are currently faced with two options: dialysis, which leads to severe morbidity, and kidney transplantation, which is limited due to lack of donor organs and also requires lifelong immunosuppressive treatment.4
In light of these worrisome figures, stem cell-based cell therapy, capable of regenerating damaged organs or tissues, is emerging as a new, promising therapeutic option for these various chronic maladies.
In order to provide definitive cure for chronic renal failure, which is essentially a decrease in the number of intact nephrons, the functional units of the kidney, the ideal approach is generation of new nephrons. Such de novo generation calls for the use of stem cells with a potential for renal differentiation. Therefore, tissue-specific stem cells, in particular, are emerging as a promising option for renewing damaged tissues. We will present the two sources for such cells, fetal and adult tissues, and discuss the advantages, limitations and future challenges related to this exciting, rapidly evolving field, highlighting the kidney.
Selecting the Optimal Cell for Tissue Regeneration
The ultimate goal of regenerative medicine is establishment of unlimited supply of cells for replenishment of a relevant cell type in the damaged organ (e.g., β cells for DM or cardiomyocytes for HF), preferably being autologous.
In recent years, several stem cell-based approaches for regeneration have emerged.
The first relies on differentiation of pluripotent cells [embryonic stem cells (ESCs) or induced pluripotent stem (iPS) cells] into the desired cell type. Pluripotent cells, by definition, can give rise to all cell types in the body, and in the case of iPS cells, offer the advantage of being an autologous source of cells. However, several problems surround the use of these cells, including the danger of maldifferentiation into unwanted tissues, the ethical and religious issues of deriving ESCs from early human embryos and lack of precise differentiation protocols into mature cells.5
A second approach is the use of various non-tissue-specific multipotent cells, most of which are bone marrow-derived, including hematopoietic stem cells (HSCs), endothelial progenitor cells (EPCs)/hemangioblasts and multipotent mesenchymal stromal cells (MSCs). Although once considered to posses substantial plasticity and regenerative potential, including kidney regeneration potential,6–8 the consensus held today is that these cells are multipotent only within the limits of their respective lineage (e.g., MSCs give rise to the mesodermal tissues, HSCs give rise to hematopoietic cells, etc.). However, they cannot cross lineage boundaries to differentiate into the cells of other damaged tissues, including the kidney.9–11 Despite this lack of nephrogenic differentiation potential, these cells have been repeatedly shown to enhance the intrinsic reparative capabilities of the kidney.12–15 EPCs/hemangioblasts, for example, demonstrate vasculogenic/angiogenic potential in various organs and specifically in the kidney16–20 and can therefore potentially restore the damaged microvasculature and reverse tissue hypoxia. These are two crucial factors in the chain of events leading to kidney fibrosis and ESRD, and this restoration may, in turn, heal nephron epithelia.21 In addition, MSCs have shown therapeutic potential in different models of kidney damage14,22,23 and are currently being tested in a clinical trial investigating the safety and efficacy of human MSCs administered to open-heart surgery patients who are at high risk of postoperative acute kidney injury (AKI).24
These beneficial effects are ascribed to various paracrine mechanisms, such as secretion of growth factors, inhibition of apoptosis and recruitment of local, tissue-specific stem cells.25
Of note, while most studies described above were performed on small animal models, a recent study found no clinical benefit in the administration of MSCs into sheep suffering from kidney failure.26
A third approach is the use of tissue-specific stem cells, which can be derived from both fetal and adult tissues.
Although details of their origin are not always known, tissue-specific stem cells in the adult organism usually share the expression of key transcription factors with stem cells of the embryonic rudiment from which they arise and are probably in a similar developmental state.27 Thus, by recapitulation of the processes of organogenesis, tissue-specific stem cells afford the advantage of being restricted to the relevant lineage and can therefore generate the entire plethora of cells necessary to replenish the injured organ without the risks of maldifferentiation.
While many adult tissues (especially rapidly-cycling tissues, such as the hematopoietic system, skin and intestine28–30) are considered to harbor stem cells, the slowly cycling kidney has only limited regenerative capacity.31 To date, there is no definite evidence for the existence in the adult kidney of stem cells,4 i.e., cells with a clonal capacity for self-renewal and differentiation into at least one type of mature progeny,31 which in the case of the kidney, is mature nephron epithelia.
In summary, tissue-specific stem cells, whether fetal or adult, represent an excellent source for cell replacement therapy. On the one hand, they are inherently committed only to their tissue of origin and, on the other hand, are guaranteed to possess a broad enough differentiation potential to produce the entire spectrum of mature progeny. Thus, they harbor the exact differentiation potential necessary to regenerate an organ (Table 1).
Table 1.
Three cell sources for kidney regeneration
| Tissue specific stem cell | ESCs | Extrarenal | ||
| Adult | Fetal | |||
| Potency | Multipotent | Multipotent | Pluripotent | Multipotent |
| Availability | Existence questionable, requires biopsy | Exist but rare, paucity of markers | Available | Readily available |
| Ethical problems | None | Problematic | Problematic | None |
| Immunogenicity | Autologous | Allogeneic | Allogeneic | Autologous |
| Expansion | Limited | Limited | Indefinitely self-renewing | Scalable |
| Renal differentiation | Inherent | Inherent | Possible, currently impractical | None |
| Maldifferentiation | Unlikely | Unlikely | Possible (e.g., teratoma) | Possible (e.g., mesoderm) |
Abbreviations: ESC, Embryonic stem cell.
The Uniqueness of the Kidney Dictates the Use of Multipotent Cells for Successful Regeneration
Apart from the need to generate large amounts of mature, functional cells, the kidney presents a unique challenge in terms of achieving successful regeneration.
In contrast to other organs, the kidney relies on the orchestrated action of more than 14 different cell types to fulfill its function.32 The hematopoietic system, for instance, is also composed of various cell types, but in contrast to the kidney, the cells function mostly as individual units, and therefore, the concept of achieving a functional “structure” is irrelevant. In addition, because hematopoietic cells constantly flow in the blood stream, the various niches (e.g., bone marrow, lymph nodes, etc.) are readily accessible. For example, in vivo deletion of Pax5 in mature B cells of mice was sufficient to cause them to dedifferentiate into uncommitted progenitors in the bone marrow and rescue T lymphopoiesis in the thymus of T-cell-deficient mice.33 However, even when considering solid organs, we can see that clinically meaningful regeneration can sometimes be achieved, even without establishing the original three-dimensional structure of the organ. If we take the pancreas and regenerative medicine for diabetic patients as an example, we can see that individual β cells are capable of sensing blood glucose levels and secreting insulin in response, such that above a critical mass of β cells, diabetes can be ameliorated, regardless of the location or spatial organization of the cells. For example, in a study by Zhou et al., exocrine pancreatic cells were reprogrammed into insulin-producing β cells and even though the reprogrammed cells did not organize into islet structures, they led to significant and long-lasting improvement in fasting blood glucose levels of hyperglycemic animals.34
In contrast, kidney function not only requires the combined action of various cell types (i.e., podocytes, parietal epithelial cells, principal cells, etc.) organized into specific segments (i.e., proximal tubule, loop of Henle, distal tubule, etc.) but also necessitates a special three-dimensional structure allowing interactions (i.e., the countercurrent mechanism) between the luminal ultra-filtrate, tubular epithelial cells and the interstitial space or peri-tubular vessels.4 The best strategy to tackle this high degree of complexity and cellular heterogeneity is probably establishment of multipotent stem/progenitor cells that could be administered into the diseased kidney, where in situ differentiation would take place, thereby replenishing the full spectrum of renal cells, leading to regeneration.4 Nonetheless, it cannot be excluded that progenitor cells with a more limited differentiation potential may also suffice as a therapeutic tool, since some pathologies are limited to specific cell types, such as podocyte loss seen in many glomerular diseases (e.g., focal segmental glomerulosclerosis4).
In order to fully appreciate the development, characteristics and function of the multipotent nephron stem cells, one must first understand the processes involved in kidney development, which is the only circumstance of de novo formation of nephrons in humans.
Kidney Organogenesis as a Model for Understanding Neo-Nephrogenesis
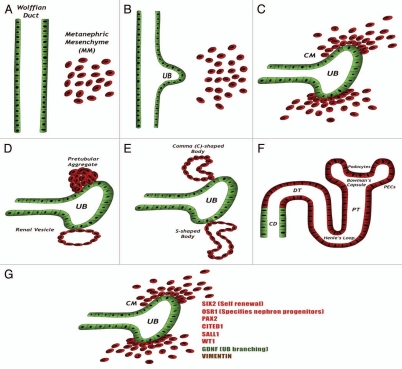
The metanephros, the mature mammalian kidney, is formed via reciprocal interactions between two intermediate mesoderm (IM)-derived precursor tissues, the metanephric mesenchyme (MM) and ureteric bud (UB), a derivative of the Wolffian duct.35,36 This complex process is summarized in Figure 1. Early in the process, a fraction of MM cells, called the cap mesenchyme (CM), located just adjacent to the UB tip, condense and maintain themselves at the tips of the branching UB while, at the same time, giving off cells that differentiate into mature nephrons.37 Recent studies13–16,38–41 have established that these CM cells are able to self-renew and differentiate into different types of nephron epithelia, thereby fitting within the criteria of renal stem cells. Prior to their induction, CM cells express a unique combination of transcription factors, including the Hox11 paralogs, Osr1, Pax2, Eya1, Wt1, Six2, Sall1 and Cited1,35 all considered early markers of kidney progenitor cells. Notably, it was shown that continued expression of Six2 is required for self-renewal of this stem cell pool as nephrogenesis continues.41 Osr1 has recently been shown to mark an even earlier lineage in the IM, capable of giving rise to all metanephric cell components, including the Six2+ epithelial nephron progenitors, renal vasculature and smooth muscle cells.40 Importantly, silencing of most of these genes coincides with termination of nephrogenesis (human, 34th gestational week; mice, 2 weeks postnatal).42,43 Thus, nephrons are formed only prenatally in humans and in the first 2 weeks after birth in rodents.44
Figure 1.
Kidney development: (A) The kidney is formed via reciprocal interactions between the Wolffian duct and the MM. (B) MM-derived signals induce the formation of the ureteric bud (UB) from the Wolffian duct. The UB then invades the MM and attracts MM cells. (C) MM cells condense around the tips of the branching UB, forming the condensed mesenchyme, or CM. In response to UB signals, CM cells are induced to undergo mesenchymal-to-epithelial transition (MET). (D–F) The induced cells acquire an epithelial phenotype concomitantly with shutting down of the major transcription factors described before. The cells sequentially form the pretubular aggregate, renal vesicle, C- and S-shaped bodies and finally the mature nephron. The cells derived from the CM form most of the nephron body (from glomerulus to distal tubule), whereas the UB-derived cells form the collecting duct. (G) The CM expresses a unique combination of genes, including the transcription factors SIX2 (which promotes self-renewal of CM cells), OSR1 (which specifies progenitors of the nephron), PAX2, CITED1, SALL1 and WT1, the secreted protein GDNF (which promotes branching morphogenesis of the UB) and the mesenchymal marker Vimentin. Abbreviations: CD, collecting duct; CM, cap mesenchyme; DT, distal tubule; PECs, parietal epithelial cells; PT, proximal tubule; UB, ureteric bud.
Therefore, the ultimate goal of renal regenerative medicine is isolation and/or creation of an unlimited supply of cells equivalent to the CM renal stem/progenitor cells, capable of regenerating epithelial cells within the nephron.
The strong correlation between the shutting down of renal developmental transcription factors and the post-natal loss of the stem cell pool, and, consequently, lack of regenerative potential of the adult kidney, implies that in order to establish CM-like cells, one should apply one of two strategies.
The first is the isolation of cells expressing these transcription factors from fetal kidneys. The second is to artificially reactivate these factors so as to drive adult kidney cells back into the multipotent state (cellular reprogramming).
Derivation of Tissue-Specific Stem Cells from Fetal Kidney
Developing fetal organs are the site of active organogenesis, and thus, the presence of stem/progenitor cells is guaranteed.27 Nonetheless, not much attention has been given to the advantages of fetal-derived stem cells for regenerative purposes, possibly due to low availability of fetal organs and the ethical and religious controversies surrounding the use of these cells. Although being an allogeneic source of cells, fetal-derived cells might, in time, develop into an “on the shelf ” product for kidney regeneration.
Indeed, several seminal studies32,38,41 have demonstrated that the embryonic kidney harbors multipotent nephron stem cells. The existence of such fetal stem cells was established in other organs as well (i.e., the pancreas,45 heart46 and intestine47). The major obstacle that currently limits the use of fetal organs as a source for tissue-specific stem cells is the need to develop methods to isolate the cells from within developing organs.
Upon isolation of the desired cells, another challenge arises, namely, the need to establish ex-vivo culture conditions to enable cell expansion into clinically relevant numbers. This need is underscored by the limited amount of cells that often characterizes stem cell populations in vivo.4
Several approaches have emerged for the purpose of isolating tissue-specific stem/progenitor cells from human fetal kidneys that can principally be applied to other organs as well:
Step-wise analysis of human tissues for identification of novel stem cell surface markers.
Hampering the identification of stem/progenitor cells in the developing kidney is that, as opposed to other organs (e.g., the hematopoietic system), specific surface markers have yet to be identified.4 How can we find such markers?
One approach to marker identification is the use of global gene expression analysis. M.H. Little and colleagues used laser dissection microscopy in order to isolate the MM and compare its gene expression profile to that of differentiated areas in the murine fetal kidney. This analysis identified CD24a and cadherin11 as MM surface markers,48 which implies that these markers can be used to isolate fetal kidney stem cells.
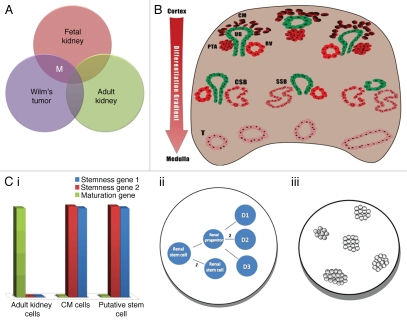
Work previously performed in our lab used a unique step-wise strategy that applied several criteria in order to identify potential markers for isolation of stem cells from human fetal kidneys (Fig. 2).
Figure 2.
Step-wise strategy for identification of renal stem cell surface markers: Putative surface markers for identification and isolation of renal stem cells were established through the application of three criteria: (A) Global gene expression analysis that compared fetal kidneys (FK), adult kidneys (AK) and Wilms tumor (WT) revealed several markers (M) that were overexpressed in FK and WT, which are progenitor-rich tissues but not in AK. (B) Illustration of histological section of FK. FK is characterized by a differentiation gradient, such that the undifferentiated structures [i.e., cap mesenchyme (CM)] are located in the renal cortex and the mature structures are located in the medulla. Thus, the expression of the markers revealed in (A) was examined in FK sections, and only markers that localized to cortical structures were considered for further evaluation. (C) The surface markers that fit the first two criteria were further subjected to in vitro assays to assess their stem cell capabilities: (i) Gene expression similar to CM cells; (ii) Self-renewal (1) and renal multi-potentiality (2); (iii) Colony formation capability.
Criterion 1: Upregulation in progenitor-rich tissues. Based on the assumption that a stem cell marker should be upregulated in stem cell-rich tissues and not in mature tissues, the first step in our quest was microarray-based analysis of three different types of renal tissue: fetal kidney, adult kidney and the pediatric malignancy Wilms tumor (WT).49
Similarly to fetal kidney and in contrast to adult kidney, WT, which originates from multipotent renal embryonic precursors that undergo a partial differentiation arrest, contains both undifferentiated elements (blastema) and differentiated elements (epi thelial and stromal).50 Serial transplantations of WT cells into mice lead to expansion of the progenitor blastema at the expense of differentiated elements, creating WT stem-like xenografts (WT-Xn).42 The latter contain mostly transformed progenitors, thereby combining the properties of both kidney progenitors and tumor cells.
Our first criterion for the stem cell marker was, therefore, upregulation in FK and WT-Xn relative to AK.
Upon identification of genes that are upregulated in both fetal kidney tissue (containing progenitors but also other fetal cell populations) and WT xenografts (containing progenitors but also other differentiated cell populations) but not in adult kidney tissue (Fig. 2A), we established a renal “stemness” signature.51 This signature included a large number of genes from different groups, including nephron “progenitor genes,” Wnt pathway-related genes, polycomb group genes and a limited number of surface markers (NCAM1, PSA-NCAM, FZD7, FZD2, DLK1, ACVRIIb and NTRK2).
In order to assess which of these markers can select for the stem cell population, we next defined several more criteria for the alleged stem cell markers, in addition to upregulation in stem cell rich tissues.52
Criterion 2: Exclusive expression in undifferentiated structures. The ideal marker should localize to and only to the primitive, undifferentiated structures within the developing organ that harbor the stem cell population, not to the differentiated ones. Pinpointing of these structures can be achieved by several methods, depending on the organ of interest.
One way is histological identification of the primitive structures. The kidney, for instance, develops in such a way that histological sections reveal a “differentiation gradient,” with the most primitive structures (CM and/or its early nephron derivatives) located at the outer parts of the organ (i.e., renal cortex) and the mature, differentiated ones (formed tubules) at the innermost parts (i.e., renal medulla)35 (Fig. 2B). In addition, different structures (e.g., CM, pretubular aggregates, C- and S-shaped bodies and differentiated tubules) are morphologically distinct, allowing identification upon histological inspection.
When assessing where each putative renal progenitor surface marker is expressed, we expect true markers to localize to the primitive cortical structures.
Notably, in organs that undergo mesenchymal-epithelial transition (MET) to produce differentiated cells, such as the kidney, mature structures are epithelial, while primitive structures are mesenchymal in phenotype.
Thus, another option for pinpointing the primitive structures is to look within the mesenchymal regions of the organ. The putative marker should localize to these regions.
Criterion 3: Demonstration of stem cell traits. The third and last criterion is that cells expressing the putative marker should demonstrate properties characteristic of tissue-specific stem cells. Testing this criterion requires a series of assays, including a gene expression profile compatible with the relevant stem cells (e.g., high levels of renal “progenitor” genes alongside low levels of genes characteristic of mature kidney cells), self-renewal, multipotentiality and colony formation capability. Only cells that possess these traits should be considered tissue-specific stem cells (Fig. 2C).
In this context, it is worth mentioning that currently there is no accepted in vitro assay to test the renal multipotentiality of alleged renal stem/progenitor cells.4 We have recently established an in vivo assay to test the potential of putative nephron progentiros to proliferate and organize into renal tubules upon transplantation onto the chorioallantoic membrane (CAM) of the chick embryo (presented in this issue of Organogenesis).
NCAM as a marker for kidney stem cells. We attempted to apply the aforementioned strategy in order to identify a marker for stem cells in the human fetal kidney.
Among the microarray-predicted markers, described previously, NCAM1 (CD56) was the only one that fit, at least partially, all of the criteria that were defined above (ref. 52).
Regarding the second criterion, NCAM localized to the primitive structures in the renal cortex but also to the kidney stroma (which does not contain stem cells of the nephron). Therefore, we tested its expression with respect to the epithelial marker EpCAM. The NCAM+EpCAM− fraction included the renal stem cells but also stromal cells, which are, indeed, mesenchymal in phenotype but cannot develop into nephrons. The NCAM+EpCAM+ fraction included nephron progenitors. Although probably more limited in differentiation potential than the former, the latter fraction contained only nephron progrnitors.
Finally, NCAM+ cells showed remarkable overexpression of several renal progenitor genes, including Six2, Cited1, Sall1, Wt1 and Pax2.52
In summary, using a step-wise strategy, we identified NCAM+EpCAM− and NCAM+EpCAM+ cells as putative nephron stem/progenitor cells, representing a framework that can be used to derive human renal stem/progenitors cells from fetal kidney, to be used in the future for regenerative purposes.52
An alternative to the aforementioned approach is to apply so-called universal human stem cell markers, such as CD133 and CD24 (not equivalent to the murine CD24a48), in order to isolate tissue-specific stem cells. Indeed, these markers have been reported to specify renal progenitors in human embryonic kidneys.53
Lower organisms as model systems for human stem cell biology.
While humans have no renal regenerative capacity after birth, several primitive organisms retain a post-natal reservoir of stem cells and therefore represent a good model for the human state during fetal development.
Thus, an additional option to identify candidate markers for stem cell isolation is to infer about stem cell characteristics in humans from processes of regeneration in lower organisms. For example, adult urodeles can regenerate their limbs by local formation of a mesenchymal growth zone or blastema;54 adult zebrafish have a remarkable capacity for cardiac regeneration;55 and adult teleost fish exhibit an enormous potential to produce new neurons in the adult central nervous system and to replace damaged neurons by newly generated ones.56
Although differences related to interspecies variability should always be considered, such primitive organisms can provide excellent model systems to study the mechanisms underlying neo-nephrogenesis and kidney regeneration.
For instance, partial nephrectomy of the skate kidney has been shown to be followed by regrowth of new nephrons, probably from renal-specific progenitors residing within the adult skate kidney.57 Very recently, Diep et al. used lineage-tracing analysis to identify nephron progenitors capable of kidney regeneration in the adult zebrafish. To determine how similar these zebrafish progenitors are to Six2+ murine CM cells, a microarray-based comparison was conducted. Interestingly, while at a global level, the genes upregulated in these two populations were not significantly similar, conservation of several factors implicated in renal development and/or stem cell self-renewal was noted. Notably, orthologs of the mammalian renal transcription factors Six2 (six2a) and Wt1 (wt1a), which are essential for CM maintenance, were upregulated in the zebrafish progenitor population as well, suggesting that this approach can indeed provide insight into mammalian and, hopefully, human stem cell research. Several other potentially important regulators were also identified in the comparison, including Meis2, Ezh2 and Tcf3, which participate in Wnt signaling and/or stem cell function. The authors concluded that, despite having distinct molecular identities, zebrafish progenitor cells and Six2+ CM cells share a core set of regulatory genes that may be important for conferring renal stem/progenitor cell potential.58
Genetic tagging for pinpointing the fetal stem cell population.
An alternative to cell isolation, according to surface markers, is to rely on distinctive expression of transcription factors known to characterize stem cells.
As previously described, intensive research has come up with several cardinal transcription factors defining kidney stem cells, including Six2, Osr1, Cited1 and Pax2.4
Therefore, if we could, in some way, isolate cells expressing the relevant “stemness genes” from within the heterogeneous pool of cells by the use of a reporter gene, we would have obtained the precursor of the nephron and could possibly use it for therapeutic applications.
The use of reporter genes to mark and isolate cell populations expressing major renal developmental genes has already been employed in animal models. McMahon and colleagues, for example, used transgenic mice to genetically label the fetal Six2+ population in vivo and prove its self-renewal capacity and multipotentiality.41 Similar approaches were applied to characterize the murine fetal Cited1+39 and Osr1+40 populations. Osafune et al. used knock-in Sall1-GFP mice to isolate Sall1high cells and demonstrate via in vitro assays that the renal stem cell pool is contained within this population.59
Obviously, in order to apply this method to the human state, an in vitro approach should be used consisting of initial establishment of human kidney cell culture and then a genetic manipulation to specifically mark the desired cells and permit their isolation.
Post-injury reactivation of fetal markers.
Another possible way to discover potential markers of stem/progenitor cells is to subject the organ or tissue to an insult and rely on the fact that, in some cases, stem cells are activated in response to injury. Thus, identification of upregulated markers in the post-injury adult organ can provide a clue about possible markers for tissue-specific stem cells. This phenomenon was described in several organs, such as the intestine, heart, brain and skeletal muscle;60–63 the latter three are considered slow-cycling organs. Therefore, even in this type of organs, injury provides a driving force for dedifferentiation and/or activation of tissue-specific stem cells, which might allow for identification of potential markers of progenitor/stem cells.
Interestingly, when mammalian kidneys are subjected to acute ischemia, proximal tubular cells upregulated NCAM in a pattern that recapitulates its expression in the developing kidney.64 This post-ischemic expression of NCAM, besides validating our own microarray-based results,49 highlights the possibility that the post-injury milieu reactivates developmental programs, thereby providing a “window” to an earlier developmental stage of the cells.
Derivation of Tissue-Specific Stem Cells from Adult Kidney
A second alternative for obtaining tissue-specific stem cells is to try and isolate them from the adult organism.
The great advantage of this type of cells is their potential to become an autologous source for cell therapy. However, while the developing human, as previously mentioned, is guaranteed to harbor such multipotent cells, the presence of such cells in the postnatal organism is very controversial.31 Moreover, what is the relationship between the fetal stem cells and their adult counterparts?
Regional specification in embryonic development proceeds as a hierarchy. Starting from the epiblast, any particular tissue rudiment or cell type is formed by a sequence of developmental decisions. At each step, the production of a particular combination of transcription factors is activated or repressed in response to a particular extracellular signal. It is believed that the stem cell state arises as the final developmental decision involved in creating a particular tissue type. This implies that tissue-specific stem cells will be in a similar state of developmental commitment to the embryonic rudiment that produced them. It is important to note that this theory still awaits final confirmation via, for instance, detailed comparisons of the fetal and adult populations of tissue-specific stem cells and/or precise lineage tracing analyses.27
Does the adult kidney harbor stem cells?
It is well established that the human kidney can survive various acute injuries involving severe reduction in the glomerular filtration rate (GFR) and recover back into normal function within a period of days. This recovery can, in theory, be attributed to one of two mechanisms:4 “true” regeneration, involving neo-nephrogenesis, or “simple” repair, occurring through repopulation of injured nephrons by the surviving tubular cells, and not involving stem cells.
As discussed previously, upon completion of nephrogenesis, the renal stem cell pool is entirely exhausted, and therefore, no cell population with nephrogenic potential similar to the CM exists in the adult.44 This has been elegantly demonstrated by Humphreys et al.65 in a lineage tracing study that not only showed lack of expression of the CM marker gene Six2 in healthy adult mice kidneys, but also excluded its reactivation during ischemic kidney damage. These findings suggest that the CM population is not re-established post-injury by recapitulation of the developmental genetic pathways.
These findings strongly imply that renal repair in the adult organism relies upon simple repair, which consists of replacement of necrotic tubular cells in surviving nephrons via the proliferation of the surviving tubular cells (a process possibly involving initial dedifferentiation). This is, for example, the recognized mechanism in the pancreas.66 Vogetseder et al. explored this mechanism during normal kidney homeostasis in the S3 segment of rat kidneys,67–69 and found that cycling and non-cycling cells were both differentiated cells, and that most tubular cells divide or enter the cell cycle in a period of 2 weeks, suggesting that a potential for proliferation exists in most, if not all cells of the S3 segment. It was shown that a large proportion of tubular cells are in the G1 phase, and that quiescent cells subjected to a mitotic stimulus re-enter the cell cycle, suggesting that tubular cells, many of which are in the G1 phase, are ready to respond to injury with a rapid proliferative response.
In summary, both during normal kidney turnover and after damage, the kidney is able to repair injured nephrons but not generate new nephrons.
Nonetheless, since Bussolati et al. first reported the presence in the adult human kidney of multipotent progenitors characterized by a CD133+ phenotype,70 several groups have isolated cells harboring progenitor potential via different methodologies.71–75 The question that arises is, “What have we been isolating?” Various explanations, may account for this discrepancy.4 First, similarly to other organs, the kidney contains “resident” progenitors. In contrast to intrinsic, tissue-specific stem cells, which are remnant of the fetal population that developed into the organ parenchyma, resident stem cells are cells that did not originate from within the organ, (i.e., the MM in the kidney) but rather localized to areas outside of the organ parenchyma, such as bone marrow-derived cells.4 These resident progenitors are probably devoid of the potential to differentiate into cells of the respective organ, but nonetheless might have beneficial effects, mostly via paracrine mechanisms.25 Second, most solid organs contain progenitors for the interstitial compartment, which is usually devoid of the organ-specific functions of parenchymal cells. The fetal kidney, for instance, contains a stromal progenitor population, marked by the expression of FoxD1, which is mutually exclusive to the Six2+ tubular progenitor compartment and, accordingly, cannot differentiate into tubuli. It does, however, posses stem cell traits, including clonogenicity, long-term expansion capacity, multidifferentiation potential, etc. Third, the cells presented as “progenitors” in various studies might in fact be terminally differentiated. This misinterpretation can be attributed to acquisition of some stem cell traits upon ex vivo culturing of cells. Differentiated epithelial cells, even when isolated from adult organisms, have already been documented to possess clonogenic and self-renewal capabilities.76 In addition, in vitro conditions sometimes result in a non-specific phenotypic switch of differentiated epithelial cells during epithelial-mesenchymal transition (EMT). Although these cells present enhanced proliferation and migration and appear in a progenitor state, they are in fact mostly fibroblast/mesenchymal-like cells, lacking functional relevance.77 A third reason for misinterpretation of mature cells as progenitors is the use of surface markers or functional parameters for isolation that overlap with those of differentiated cell types. Examples of such markers include “universal” stem cell markers such as CD133, CD24, Sca-1 and c-Kit, which have all been shown to be heavily expressed in differentiated epithelia, including renal epithelia.52,78–82 Examples of overlapping functional parameters are label retention and dye efflux capacity.31
Finally, the adult kidney might harbor one or more populations of progenitors with a more restricted potential than the fetal CM population, possibly activated in response to injury.
Reprogramming renal progenitors.
A third alternative for establishing nephron progenitor cells for kidney regeneration is to artificially create them from other cell sources.
A large number of studies in the field of cellular reprogramming have proven that, by manipulating the transcriptional profile of cells, one can induce a change of phenotype into a desired one. Such manipulations include the expression34,83–87 or repression33,88 of potent transcription factors, induction of epigenetic changes,89,90 manipulations of micro-RNA expression91,92 and somatic cell nuclear transfer.93–95
Such manipulations have been used for two main reprogramming types: one is reprogramming back into pluripotency,83,86,90,95 and the other is reprogramming between two types of non-pluripotent cells, either multipotent or fully differentiated.34,84,87
The major issue in reprogramming for kidney regeneration is determining the ideal endpoint or, in other words, what cell we are attempting to establish through this process.4 In order to achieve renal regeneration, several options can be proposed, including early states of kidney development (i.e., IM, MM and CM), uni/ oligopotent progenitors, capable of differentiating only into one or several types of nephron epithelia and mature kidney cells. However, in light of the uniqueness of the adult kidney, described in a previous section, the first two options seem more suitable.
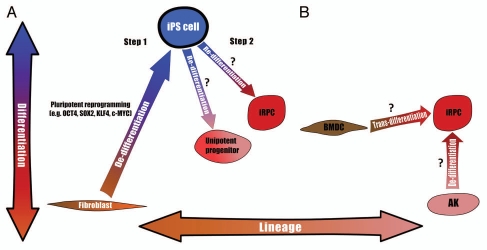
These two types of reprogramming dictate two possible strategies for obtaining the desired cells: one is “indirect” reprogramming, consisting of an initial dedifferentiation step into pluripotency and then a second step of differentiation into the desired cell type. The second strategy is single-step reprogramming directly into the desired phenotype. These two strtategies are described in detail below and summarized in Figure 3 and Table 2.
Figure 3.
Two strategies for reprogramming into renal progenitors: (A) Indirect reprogramming, mature cells (e.g., fibroblasts) are first (Step 1) dedifferentiated into a pluripotent state (i.e., iPS cell) via one of the many previously described protocols and then (Step 2) re-differentiated into multipotent induced renal progenitor cells (iRPC) or unipotent progenitors. The major limitation of this strategy is the absence of such re-differentiation protocols for obtaining renal progenitors. (B) Direct reprogramming, various cell sources can theoretically be reprogrammed into iRPCs, for instance by the ectopic expression of developmental transcription factors. Two examples are transdifferentiation from non-renal cells (e.g., bone marrow-derived cells; BMDC) and dedifferentiation from adult kidney (AK) cells. Two axes are presented: Differentiation axis, from pluripotency at the top down to fully differentiated cells at the bottom, such that cells at different vertical positions possess different differentiation grades; Lineage axis, such that cells at different horizontal positions are situated within different lineages.
Table 2.
Two strategies for reprogramming renal progenitors
| Indirect reprogramming | Direct reprogramming | |
| Cell of origin | Any (e.g., skin fibroblast) | Preferably kidney-derived |
| De-differentiated state | Pluripotent iPS cell | Multipotent iRP cell |
| Immunogenicity | Autologous | Autologous |
| Availability of cell of origin | Good (e.g., skin biopsy) | Good, but requires invasive biopsy |
| Establishment of target cell | Extracellular factors | Genetic manipulations |
| Maldifferentiation | Possible | Unlikely |
| Site of reprogramming | In vitro | In vitro or in situ |
| Major challenge | Differentiation protocol | Dedifferentiation protocol |
Abbreviations: iPS, Induced pluripotent stem; iRP, Induced renal progenitor.
Strategy 1: Indirect reprogramming. The first way in which cellular reprogramming can be used to obtain the desired cells includes two distinct steps. The first is pluripotent reprogramming through the use of any of the various protocols already described for generating iPS cells from human cells,89,96,97 and the second is re-differentiation into the desired cells.
Importantly, many types of cells can serve as the starting material to be reverted into pluripotency,86,96,98,99 affording an important advantage of a large and readily obtainable cell source for this initial step. Of note, several recent studies have shown that iPS cells derived from different sources are not equal in their potential to differentiate into a specific cell type,99–101 such that iPS cells derived from a certain tissue are skewed towards redifferentiating into the same tissue, probably due to incomplete erasure of the epigenetic signature during dedifferentiation into pluripotency or to epigenetic “memory.”100 Therefore, it is possible that kidney-derived iPS cells would prove to be better sources for obtaining renal tissue.
While much is already known about the first step of pluripotent reprogramming, the second step poses the major obstacle here, in light of the lack of well-defined differentiation protocols from pluripotent cells into renal tissue.
Despite many attempts to induce pluripotent cells in this manner, applying both growth factor combinations [bone morphogenetic protein (BMP)/Activin/Retinoic acid] and genetic approaches,102–108 most studies, even after successfully inducing renal lineage genes, failed to pinpoint the exact stage along the renal lineage that was obtained. Moreover, most studies did not analyze the induced cells in functional, in vivo models.
A second problem surrounding this strategy is the unlimited differentiation potential of pluripotent cells, which, if not controlled properly, could lead to the formation of unwanted tissues, such as non-renal mesodermal cells, cells from other germ layers and even tumors (e.g., teratoma). To overcome this danger and permit safe administration of the cells, it will be necessary to ensure that the entire population of cells has indeed differentiated fully and properly.
In summary, indirect reprogramming provides an unlimited supply of an autologous cell source for deriving renal tissue. However, for this strategy to become practical, precise differentiation protocols for establishment of kidney cells must be first developed.
Strategy 2: Direct reprogramming. An alternative to the two-step strategy described before is to directly convert the initial cell population into the final product that will be used for tissue regeneration.
The most important advantage of this approach is elimination of most of the risk of maldifferentiation that accompanies the use of cells derived from pluripotent cells. In the case of the kidney, instead of dedifferentiation all the way back into pluripotency, culminating in induced pluripotent cells, we can purpose to dedifferentiate mature cells only to the extent necessary to achieve renal multipotentiality, thereby ending up in induced renal progenitor cells (iRPCs).
A second advantage is the possibility of reprogramming cells in situ, thereby avoiding the confounding factors of the in vitro environment, the risks of malignant transformation during ex vivo expansion and the need to home the cells to the desired tissue.
The cellular niche is a complex microenvironment, which is, in essence, the sum of extracellular factors and neighboring cells.109 In vivo, this microenvironment is the main factor that determines the phenotype and function of the cells, dictating whether they will self-renew to maintain the stem cell phenotype, differentiate, proliferate or undergo apoptosis.109 Therefore, in order to successfully apply the in situ reprogramming approach, it is essential to provide the reprogrammed cells with a suitable niche, because otherwise, the newly established stem cell phenotype will be unstable. Although rare, there have already been reports of such in situ reprogramming.33,34 Thus, the possibility of reprogramming iRPCs in the adult human could be severely limited by the probable lack of a kidney stem cell niche. Nonetheless, precise recapitulation of this complex microenvironment is extremely challenging in vitro as well, representing one of the greatest hurdles in the field of regenerative medicine in general and kidney regeneration in particular. Indeed, to date, no one has been able to establish long-term culture of human CM cells while marinating their nephrogenic potential.
Another difference between the in situ and in vitro approaches is that, while in vitro reprogramming allows for genetic manipulations in human cells, the in situ approach is clearly limited, at least initially, to animal models.
When attempting to design a reprogramming strategy, for instance, for establishing iRPCs, three important issues must be addressed: the cell of origin, the reprogramming factors and the criteria for successful reprogramming.
Regarding the cell of origin, several candidates can be proposed, each affording its own advantages and disadvantages.
The first and most obvious candidates are adult kidney-derived cells, which, in theory, should be the most amenable to reprogramming into renal progenitors, as they are already situated within the renal lineage. It is widely accepted that a crucial factor in determining the probability of achieving successful reprogramming and the efficiency of the process is the developmental distance between the cell of origin and target cell. This distance is, in essence, the difference in epigenome between the two cell types.110 In the case of kidney cells as starting material, the epigenetic barriers presented will be minimal, and consequently, reprogramming should be the most efficient and/or require the least genetic manipulations. Derivation of kidney cells, however, will probably require renal biopsy, a relatively invasive procedure.
The second candidates are extrarenal cells, most notably bone marrow-derived cells (e.g., MSCs). Such cells are readily obtainable from the bone marrow, peripheral blood and even from adipose tissue111 and have already been intensively studied in the context of kidney repair (reviewed in ref. 112), including attempts to ectopically overexpress factors in order to improve their renal reparative potential.113 In addition, large scale expansion of MSCs into clinically relevant amounts, while at the same time avoiding in vitro malignant transformation has already been achieved and applied to treat human patients.114
The major limitation here, however, is the large developmental distance that will have to be crossed to achieve reprogramming.
As for the second issue of the reprogramming factors, it is essential to determine the exact combination of transcription factors necessary to drive adult cells back into a state of renal progenitors. Surely, establishment of such a protocol will be based upon firm understanding of the developmental processes and molecular pathways governing kidney development.4 To date, several cardinal transcription factors controlling renal stem cells have already been described (ref. 35), but much is yet to be learnt before the exact genetic code of this cell population can be applied to cellular reprogramming.
Finally, the product of reprogramming will have to go through strict tests to confirm establishment of the exact phenotype. In addition, taking into account that not all cells undergo complete reprogramming,115 a method for selecting the reprogrammed cells would have to be developed.
In summary, the two strategies presented above will hopefully enable the establishment of a large-scale source of CM-like cells, which will allow for neo-nephrogenesis in the adult human. To determine which of the two is most successful in terms of kidney regeneration, in vitro and, eventually, in vivo tests will have to be carried out to assess both efficacy and safety and, ultimately, to pave the way for a successful treatment for chronic kidney disease.
References
- 1.Fang J, Mensah GA, Croft JB, Keenan NL. Heart failure-related hospitalization in the US 1979 to 2004. J Am Coll Cardiol. 2008;52:428–434. doi: 10.1016/j.jacc.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 2.Farag YM, Gaballa MR. Diabesity: an overview of a rising epidemic. Nephrol Dial Transplant. 2010;26:28–35. doi: 10.1093/ndt/gfq576. [DOI] [PubMed] [Google Scholar]
- 3.Trivedi H. Cost implications of caring for chronic kidney disease: are interventions cost-effective? Adv Chronic Kidney Dis. 2010;17:265–270. doi: 10.1053/j.ackd.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Pleniceanu O, Harari-Steinberg O, Dekel B. Concise review: Kidney stem/progenitor cells: differentiate, sort out or reprogram? Stem Cells. 2010;28:1649–1660. doi: 10.1002/stem.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amabile G, Meissner A. Induced pluripotent stem cells: current progress and potential for regenerative medicine. Trends Mol Med. 2009;15:59–68. doi: 10.1016/j.molmed.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 7.Gupta S, Verfaillie C, Chmielewski D, Kim Y, Rosenberg ME. A role for extrarenal cells in the regeneration following acute renal failure. Kidney Int. 2002;62:1285–1290. doi: 10.1111/j.1523-1755.2002.kid569.x. [DOI] [PubMed] [Google Scholar]
- 8.Fang TC, Alison MR, Cook HT, Jeffery R, Wright NA, Poulsom R. Proliferation of bone marrow-derived cells contributes to regeneration after folic acid-induced acute tubular injury. J Am Soc Nephrol. 2005;16:1723–1732. doi: 10.1681/ASN.2004121089. [DOI] [PubMed] [Google Scholar]
- 9.Krause D, Cantley LG. Bone marrow plasticity revisited: Protection or differentiation in the kidney tubule? J Clin Invest. 2005;115:1705–1708. doi: 10.1172/JCI25540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, et al. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest. 2005;115:1743–1755. doi: 10.1172/JCI22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 12.Sugimoto H, Mundel TM, Sund M, Xie L, Cosgrove D, Kalluri R. Bone marrow-derived stem cells repair basement membrane collagen defects and reverse genetic kidney disease. Proc Natl Acad Sci USA. 2006;103:7321–7326. doi: 10.1073/pnas.0601436103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.C Chade AR, Zhu XY, Krier JD, Jordan KL, Textor SC, Grande JP, et al. Endothelial progenitor cells homing and renal repair in experimental renovascular disease. Stem Cells. 28:1039–1047. doi: 10.1002/stem.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:31–42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 15.Li B, Cohen A, Hudson TE, Motlagh D, Amrani DL, Duffield JS. Mobilized human hematopoietic stem/progenitor cells promote kidney repair after ischemia/reperfusion injury. Circulation. 121:2211–2220. doi: 10.1161/CIRCULATIONAHA.109.928796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sangidorj O, Yang SH, Jang HR, Lee JP, Cha RH, Kim SM, et al. Bone marrow-derived endothelial progenitor cells confer renal protection in a murine chronic renal failure model. Am J Physiol Renal Physiol. 2010;299:325–335. doi: 10.1152/ajprenal.00019.2010. [DOI] [PubMed] [Google Scholar]
- 17.Dekel B, Shezen E, Even-Tov-Friedman S, Katchman H, Margalit R, Nagler A, et al. Transplantation of human hematopoietic stem cells into ischemic and growing kidneys suggests a role in vasculogenesis but not tubulogenesis. Stem Cells. 2006;24:1185–1193. doi: 10.1634/stemcells.2005-0265. [DOI] [PubMed] [Google Scholar]
- 18.Dekel B, Hochman E, Sanchez MJ, Maharshak N, Amariglio N, Green AR, et al. Kidney, blood and endothelium: developmental expression of stem cell leukemia during nephrogenesis. Kidney Int. 2004;65:1162–1169. doi: 10.1111/j.1523-1755.2004.00489.x. [DOI] [PubMed] [Google Scholar]
- 19.Dekel B, Metsuyanim S, Garcia AM, Quintero C, Sanchez MJ, Izraeli S. Organ-injury-induced reactivation of hemangioblastic precursor cells. Leukemia. 2008;22:103–113. doi: 10.1038/sj.leu.2404941. [DOI] [PubMed] [Google Scholar]
- 20.Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol. 2007;18:2486–2496. doi: 10.1681/ASN.2007020140. [DOI] [PubMed] [Google Scholar]
- 21.Fine LG. First heal thyself: rescue of dysfunctional endothelial progenitor cells restores function to the injured kidney. Am J Pathol. 176:1586–1587. doi: 10.2353/ajpath.2010.091282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magnasco A, Corselli M, Bertelli R, Ibatici A, Peresi M, Gaggero G, et al. Mesenchymal stem cells protective effect in adriamycin model of nephropathy. Cell Transplant. 2008;17:1157–1167. doi: 10.3727/096368908787236567. [DOI] [PubMed] [Google Scholar]
- 23.Choi S, Park M, Kim J, Hwang S, Park S, Lee Y. The role of mesenchymal stem cells in the functional improvement of chronic renal failure. Stem Cells Dev. 2009;18:521–529. doi: 10.1089/scd.2008.0097. [DOI] [PubMed] [Google Scholar]
- 24.Togel FE, Westenfelder C. Mesenchymal stem cells: A new therapeutic tool for AKI. Nat Rev Nephrol. 6:179–183. doi: 10.1038/nrneph.2009.229. [DOI] [PubMed] [Google Scholar]
- 25.Humphreys BD, Bonventre JV. Mesenchymal stem cells in acute kidney injury. Annu Rev Med. 2008;59:311–325. doi: 10.1146/annurev.med.59.061506.154239. [DOI] [PubMed] [Google Scholar]
- 26.Behr L, Hekmati M, Lucchini A, Houcinet K, Faussat AM, Borenstein N, et al. Evaluation of the effect of autologous mesenchymal stem cell injection in a large-animal model of bilateral kidney ischaemia reperfusion injury. Cell Prolif. 2009;42:284–297. doi: 10.1111/j.1365-2184.2009.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slack JM. Origin of stem cells in organogenesis. Science. 2008;322:1498–1501. doi: 10.1126/science.1162782. [DOI] [PubMed] [Google Scholar]
- 28.Kondo M, Wagers AJ, Manz MG, et al. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- 29.Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22:1856–1864. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alison MR, Islam S. Attributes of adult stem cells. J Pathol. 2009;217:144–160. doi: 10.1002/path.2498. [DOI] [PubMed] [Google Scholar]
- 32.Herzlinger D, Koseki C, Mikawa T, et al. Metanephric mesenchyme contains multipotent stem cells whose fate is restricted after induction. Development. 1992;114:565–572. doi: 10.1242/dev.114.3.565. [DOI] [PubMed] [Google Scholar]
- 33.Cobaleda C, Jochum W, Busslinger M. Conversion of mature B cells into T cells by dedifferentiation to uncommitted progenitors. Nature. 2007;449:473–477. doi: 10.1038/nature06159. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Q, Brown J, Kanarek A, et al. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reidy KJ, Rosenblum ND. Cell and molecular biology of kidney development. Semin Nephrol. 2009;29:321–337. doi: 10.1016/j.semnephrol.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dressler GR. Advances in early kidney specification, development and patterning. Development. 2009;136:3863–3874. doi: 10.1242/dev.034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schedl A. Renal abnormalities and their developmental origin. Nat Rev Genet. 2007;8:791–802. doi: 10.1038/nrg2205. [DOI] [PubMed] [Google Scholar]
- 38.Self M, Lagutin OV, Bowling B, Hendrix J, Cai Y, Dressler GR, et al. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J. 2006;25:5214–5228. doi: 10.1038/sj.emboj.7601381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boyle S, Misfeldt A, Chandler KJ, Deal KK, Southard-Smith EM, Mortlock DP, et al. Fate mapping using Cited1-CreERT2 mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Dev Biol. 2008;313:234–245. doi: 10.1016/j.ydbio.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mugford JW, Sipilä P, McMahon JA, McMahon AP. Osr1 expression demarcates a multi-potent population of intermediate mesoderm that undergoes progressive restriction to an Osr1-dependent nephron progenitor compartment within the mammalian kidney. Dev Biol. 2008;324:88–98. doi: 10.1016/j.ydbio.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, et al. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 2008;3:169–181. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Metsuyanim S, Pode-Shakked N, Schmidt-Ott KM, Keshet G, Rechavi G, Blumental D, et al. Accumulation of malignant renal stem cells is associated with epigenetic changes in normal renal progenitor genes. Stem Cells. 2008;26:1808–1817. doi: 10.1634/stemcells.2007-0322. [DOI] [PubMed] [Google Scholar]
- 43.Rosenblum ND. Developmental biology of the human kidney. Semin Fetal Neonatal Med. 2008;13:125–132. doi: 10.1016/j.siny.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Hartman HA, Lai HL, Patterson LT. Cessation of renal morphogenesis in mice. Dev Biol. 2007;310:379–387. doi: 10.1016/j.ydbio.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fishman MP, Melton DA. Pancreatic lineage analysis using a retroviral vector in embryonic mice demonstrates a common progenitor for endocrine and exocrine cells. Int J Dev Biol. 2002;46:201–207. [PubMed] [Google Scholar]
- 46.Vincent SD, Buckingham ME. How to make a heart: the origin and regulation of cardiac progenitor cells. Curr Top Dev Biol. 90:1–41. doi: 10.1016/S0070-2153(10)90001-X. [DOI] [PubMed] [Google Scholar]
- 47.Heath JK. Transcriptional networks and signaling pathways that govern vertebrate intestinal development. Curr Top Dev Biol. 90:159–192. doi: 10.1016/S0070-2153(10)90004-5. [DOI] [PubMed] [Google Scholar]
- 48.Challen GA, Martinez G, Davis MJ, Taylor DF, Crowe M, Teasdale RD, et al. Identifying the molecular phenotype of renal progenitor cells. J Am Soc Nephrol. 2004;15:2344–2357. doi: 10.1097/01.ASN.0000136779.17837.8F. [DOI] [PubMed] [Google Scholar]
- 49.Dekel B, Metsuyanim S, Schmidt-Ott KM, Fridman E, Jacob-Hirsch J, Simon A, et al. Multiple imprinted and stemness genes provide a link between normal and tumor progenitor cells of the developing human kidney. Cancer Res. 2006;66:6040–6049. doi: 10.1158/0008-5472.CAN-05-4528. [DOI] [PubMed] [Google Scholar]
- 50.Sebire NJ, Vujanic GM. Paediatric renal tumours: recent developments, new entities and pathological features. Histopathology. 2009;54:516–528. doi: 10.1111/j.1365-2559.2008.03110.x. [DOI] [PubMed] [Google Scholar]
- 51.Pode-Shakked N, Metsuyanim S, Rom-Gross E, Mor Y, Fridman E, Goldstein I, et al. Developmental tumouri-genesis: NCAM as a putative marker for the malignant renal stem/progenitor cell population. J Cell Mol Med. 2009;13:1792–1808. doi: 10.1111/j.1582-4934.2008.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Metsuyanim S, Harari-Steinberg O, Buzhor E, Omer D, Pode-Shakked N, Ben-Hur H, et al. Expression of stem cell markers in the human fetal kidney. PLoS One. 2009;4:6709. doi: 10.1371/journal.pone.0006709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lazzeri E, Crescioli C, Ronconi E, Mazzinghi B, Sagrinati C, Netti GS, et al. Regenerative potential of embryonic renal multipotent progenitors in acute renal failure. J Am Soc Nephrol. 2007;18:3128–3138. doi: 10.1681/ASN.2007020210. [DOI] [PubMed] [Google Scholar]
- 54.Brockes JP. Amphibian limb regeneration: Rebuilding a complex structure. Science. 1997;276:81–87. doi: 10.1126/science.276.5309.81. [DOI] [PubMed] [Google Scholar]
- 55.Raya A, Koth CM, Büscher D, Kawakami Y, Itoh T, Raya RM, et al. Activation of Notch signaling pathway precedes heart regeneration in zebrafish. Proc Natl Acad Sci USA. 2003;100:11889–11895. doi: 10.1073/pnas.1834204100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zupanc GK. Adult neurogenesis and neuronal regeneration in the central nervous system of teleost fish. Brain Behav Evol. 2001;58:250–275. doi: 10.1159/000057569. [DOI] [PubMed] [Google Scholar]
- 57.Elger M, Hentschel H, Litteral J, Wellner M, Kirsch T, Luft FC, et al. Nephrogenesis is induced by partial nephrectomy in the elasmobranch Leucoraja erinacea. J Am Soc Nephrol. 2003;14:1506–1518. doi: 10.1097/01.asn.0000067645.49562.09. [DOI] [PubMed] [Google Scholar]
- 58.Diep CQ, Ma D, Deo RC, Holm TM, Naylor RW, Arora N, et al. Identification of adult nephron progenitors capable of kidney regeneration in zebrafish. Nature. 470:95–100. doi: 10.1038/nature09669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Osafune K, Takasato M, Kispert A, Asashima M, Nishinakamura R. Identification of multipotent progenitors in the embryonic mouse kidney by a novel colony-forming assay. Development. 2006;133:151–161. doi: 10.1242/dev.02174. [DOI] [PubMed] [Google Scholar]
- 60.Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4:49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y, Li TS, Lee ST, Wawrowsky KA, Cheng K, Galang G, et al. Dedifferentiation and proliferation of mammalian cardiomyocytes. PLoS One. 5:12559. doi: 10.1371/journal.pone.0012559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miles DK, Kernie SG. Activation of neural stem and progenitor cells after brain injury. Prog Brain Res. 2006;157:187–197. doi: 10.1016/s0079-6123(06)57012-8. [DOI] [PubMed] [Google Scholar]
- 63.Mu X, Peng H, Pan H, Huard J, Li Y. Study of muscle cell dedifferentiation after skeletal muscle injury of mice with a Cre-Lox system. PLoS One. 6:16699. doi: 10.1371/journal.pone.0016699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abbate M, Brown D, Bonventre JV. Expression of NCAM recapitulates tubulogenic development in kidneys recovering from acute ischemia. Am J Physiol. 1999;277:454–463. doi: 10.1152/ajprenal.1999.277.3.F454. [DOI] [PubMed] [Google Scholar]
- 65.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 66.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 67.Vogetseder A, Karadeniz A, Kaissling B, Le Hir M. Tubular cell proliferation in the healthy rat kidney. Histochem Cell Biol. 2005;124:97–104. doi: 10.1007/s00418-005-0023-y. [DOI] [PubMed] [Google Scholar]
- 68.Vogetseder A, Palan T, Bacic D, Kaissling B, Le Hir M. Proximal tubular epithelial cells are generated by division of differentiated cells in the healthy kidney. Am J Physiol Cell Physiol. 2007;292:807–813. doi: 10.1152/ajpcell.00301.2006. [DOI] [PubMed] [Google Scholar]
- 69.Vogetseder A, Picard N, Gaspert A, Walch M, Kaissling B, Le Hir M. Proliferation capacity of the renal proximal tubule involves the bulk of differentiated epithelial cells. Am J Physiol Cell Physiol. 2008;294:22–28. doi: 10.1152/ajpcell.00227.2007. [DOI] [PubMed] [Google Scholar]
- 70.Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D, et al. Isolation of renal progenitor cells from adult human kidney. Am J Pathol. 2005;166:545–555. doi: 10.1016/S0002-9440(10)62276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dekel B, Zangi L, Shezen E, Reich-Zeliger S, Eventov-Friedman S, Katchman H, et al. Isolation and characterization of nontubular sca-1+ lin− multipotent stem/progenitor cells from adult mouse kidney. J Am Soc Nephrol. 2006;17:3300–3314. doi: 10.1681/ASN.2005020195. [DOI] [PubMed] [Google Scholar]
- 72.Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q. The renal papilla is a niche for adult kidney stem cells. J Clin Invest. 2004;114:795–804. doi: 10.1172/JCI20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kitamura S, Yamasaki Y, Kinomura M, Sugaya T, Sugiyama H, Maeshima Y, et al. Establishment and characterization of renal progenitor like cells from S3 segment of nephron in rat adult kidney. Faseb J. 2005;19:1789–1797. doi: 10.1096/fj.05-3942com. [DOI] [PubMed] [Google Scholar]
- 74.Gupta S, Verfaillie C, Chmielewski D, Kren S, Eidman K, Connaire J, et al. Isolation and characterization of kidney-derived stem cells. J Am Soc Nephrol. 2006;17:3028–3040. doi: 10.1681/ASN.2006030275. [DOI] [PubMed] [Google Scholar]
- 75.Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, et al. Isolation and characterization of multipotent progenitor cells from the Bowman's capsule of adult human kidneys. J Am Soc Nephrol. 2006;17:2443–2456. doi: 10.1681/ASN.2006010089. [DOI] [PubMed] [Google Scholar]
- 76.Cicero SA, Johnson D, Reyntjens S, Frase S, Connell S, Chow LM, et al. Cells previously identified as retinal stem cells are pigmented ciliary epithelial cells. Proc Natl Acad Sci USA. 2009;106:6685–6690. doi: 10.1073/pnas.0901596106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 78.Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, Milde T, et al. CD133 expression is not restricted to stem cells and both CD133+ and CD133− metastatic colon cancer cells initiate tumors. J Clin Invest. 2008;118:2111–2120. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weigmann A, Corbeil D, Hellwig A, Huttner WB. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc Natl Acad Sci USA. 1997;94:12425–12430. doi: 10.1073/pnas.94.23.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van de Rijn M, Heimfeld S, Spangrude GJ, Weissman IL. Mouse hematopoietic stem-cell antigen Sca-1 is a member of the Ly-6 antigen family. Proc Natl Acad Sci USA. 1989;86:4634–4638. doi: 10.1073/pnas.86.12.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Droz D, Rousseau-Merck MF, Jaubert F, Diebold N, Nezelof C, Adafer E, et al. Cell differentiation in Wilms tumor (nephroblastoma): an immunohistochemical study. Hum Pathol. 1990;21:536–544. doi: 10.1016/0046-8177(90)90011-s. [DOI] [PubMed] [Google Scholar]
- 82.Natali PG, Nicotra MR, Sures I, Santoro E, Bigotti A, Ullrich A. Expression of c-kit receptor in normal and transformed human nonlymphoid tissues. Cancer Res. 1992;52:6139–6143. [PubMed] [Google Scholar]
- 83.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 84.Laiosa CV, Stadtfeld M, Xie H, de Andres-Aguayo L, Graf T. Reprogramming of committed T cell progenitors to macrophages and dendritic cells by C/EBP alpha and PU.1 transcription factors. Immunity. 2006;25:731–744. doi: 10.1016/j.immuni.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 85.Choi J, Costa ML, Mermelstein CS, Chagas C, Holtzer S, Holtzer H. MyoD converts primary dermal fibroblasts, chondroblasts, smooth muscle and retinal pigmented epithelial cells into striated mononucleated myoblasts and multinucleated myotubes. Proc Natl Acad Sci USA. 1990;87:7988–7992. doi: 10.1073/pnas.87.20.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.KiKim JB, Greber B, Araúzo-Bravo MJ, Meyer J, Park KI, Zaehres H, et al. Direct reprogramming of human neural stem cells by OCT4. Nature. 2009;461:649–643. doi: 10.1038/nature08436. [DOI] [PubMed] [Google Scholar]
- 87.Efe JA, Hilcove S, Kim J, Zhou H, Ouyang K, Wang G, et al. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat Cell Biol. 13:215–222. doi: 10.1038/ncb2164. [DOI] [PubMed] [Google Scholar]
- 88.Galloway JL, Wingert RA, Thisse C, Thisse B, Zon LI. Loss of gata1 but not gata2 converts erythropoiesis to myelopoiesis in zebrafish embryos. Dev Cell. 2005;8:109–116. doi: 10.1016/j.devcel.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 89.Huangfu D, Maehr R, Guo W, Eijkelenboom A, Snitow M, Chen AE, et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008;26:795–797. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huangfu D, Osafune K, Maehr R, Guo W, Eijkelenboom A, Chen S, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26:1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 91.Lin SL, Chang DC, Chang-Lin S, Lin CH, Wu DT, Chen DT, et al. Mir-302 reprograms human skin cancer cells into a pluripotent ES-cell-like state. Rna. 2008;14:2115–2124. doi: 10.1261/rna.1162708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin SL, Chang DC, Lin CH, Ying SY, Leu D, Wu DT. Regulation of somatic cell reprogramming through inducible mir-302 expression. Nucleic Acids Res. 39:1054–1065. doi: 10.1093/nar/gkq850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 94.Gurdon JB, Melton DA. Nuclear reprogramming in cells. Science. 2008;322:1811–1815. doi: 10.1126/science.1160810. [DOI] [PubMed] [Google Scholar]
- 95.Hochedlinger K, Jaenisch R. Monoclonal mice generated by nuclear transfer from mature B and T donor cells. Nature. 2002;415:1035–1038. doi: 10.1038/nature718. [DOI] [PubMed] [Google Scholar]
- 96.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 97.Chang CW, Lai YS, Pawlik KM, Liu K, Sun CW, Li C, et al. Polycistronic lentiviral vector for “hit and run” reprogramming of adult skin fibroblasts to induced pluripotent stem cells. Stem Cells. 2009;27:1042–1049. doi: 10.1002/stem.39. [DOI] [PubMed] [Google Scholar]
- 98.Staerk J, Dawlaty MM, Gao Q, Maetzel D, Hanna J, Sommer CA, et al. Reprogramming of human peripheral blood cells to induced pluripotent stem cells. Cell Stem Cell. 7:20–24. doi: 10.1016/j.stem.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hu Q, Friedrich AM, Johnson LV, Clegg DO. Memory in induced pluripotent stem cells: reprogrammed human retinal-pigmented epithelial cells show tendency for spontaneous redifferentiation. Stem Cells. 28:1981–1991. doi: 10.1002/stem.531. [DOI] [PubMed] [Google Scholar]
- 100.Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, Tan KY, et al. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol. 28:848–855. doi: 10.1038/nbt.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Quattrocelli M, Palazzolo G, Floris G, Schöffski P, Anastasia L, Orlacchio A, et al. Intrinsic cell memory reinforces myogenic commitment of pericyte-derived iPSCs. J Pathol. 223:593–603. doi: 10.1002/path.2845. [DOI] [PubMed] [Google Scholar]
- 102.Kramer J, Steinhoff J, Klinger M, Fricke L, Rohwedel J. Cells differentiated from mouse embryonic stem cells via embryoid bodies express renal marker molecules. Differentiation. 2006;74:91–104. doi: 10.1111/j.1432-0436.2006.00062.x. [DOI] [PubMed] [Google Scholar]
- 103.Kim D, Dressler GR. Nephrogenic factors promote differentiation of mouse embryonic stem cells into renal epithelia. J Am Soc Nephrol. 2005;16:3527–3534. doi: 10.1681/ASN.2005050544. [DOI] [PubMed] [Google Scholar]
- 104.Vigneau C, Polgar K, Striker G, Elliott J, Hyink D, Weber O, et al. Mouse embryonic stem cell-derived embryoid bodies generate progenitors that integrate long term into renal proximal tubules in vivo. J Am Soc Nephrol. 2007;18:1709–1720. doi: 10.1681/ASN.2006101078. [DOI] [PubMed] [Google Scholar]
- 105.Bruce SJ, Rea RW, Steptoe AL, Busslinger M, Bertram JF, Perkins AC. In vitro differentiation of murine embryonic stem cells toward a renal lineage. Differentiation. 2007;75:337–349. doi: 10.1111/j.1432-0436.2006.00149.x. [DOI] [PubMed] [Google Scholar]
- 106.Kobayashi T, Tanaka H, Kuwana H, Inoshita S, Teraoka H, Sasaki S, et al. Wnt4-transformed mouse embryonic stem cells differentiate into renal tubular cells. Biochem Biophys Res Commun. 2005;336:585–595. doi: 10.1016/j.bbrc.2005.08.136. [DOI] [PubMed] [Google Scholar]
- 107.Batchelder CA, Lee CC, Matsell DG, Yoder MC, Tarantal AF. Renal ontogeny in the rhesus monkey (Macaca mulatta) and directed differentiation of human embryonic stem cells towards kidney precursors. Differentiation. 2009;78:45–56. doi: 10.1016/j.diff.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nakane A, Kojima Y, Hayashi Y, Kohri K, Masui S, Nishinakamura R. Pax2 overexpression in embryoid bodies induces upregulation of integrin alpha8 and aquaporin-1. In Vitro Cell Dev Biol Anim. 2009;45:62–68. doi: 10.1007/s11626-008-9151-8. [DOI] [PubMed] [Google Scholar]
- 109.Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol. 2008;9:11–21. doi: 10.1038/nrm2319. [DOI] [PubMed] [Google Scholar]
- 110.Zhou Q, Melton DA. Extreme makeover: converting one cell into another. Cell Stem Cell. 2008;3:382–388. doi: 10.1016/j.stem.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 111.Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217:318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yeagy BA, Cherqui S. Kidney repair and stem cells: a complex and controversial process. Pediatr Nephrol. doi: 10.1007/s00467-011-1789-x. [DOI] [PubMed] [Google Scholar]
- 113.Hagiwara M, Shen B, Chao L, Chao J. Kallikrein-modified mesenchymal stem cell implantation provides enhanced protection against acute ischemic kidney injury by inhibiting apoptosis and inflammation. Hum Gene Ther. 2008;19:807–819. doi: 10.1089/hum.2008.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Caimi PF, Reese J, Lee Z, Lazarus HM. Emerging therapeutic approaches for multipotent mesenchymal stromal cells. Curr Opin Hematol. 17:505–513. doi: 10.1097/MOH.0b013e32833e5b18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hanna J, Saha K, Pando B, van Zon J, Lengner CJ, Creyghton MP, et al. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature. 2009;462:595–601. doi: 10.1038/nature08592. [DOI] [PMC free article] [PubMed] [Google Scholar]