Abstract
Background
Kidneys from brain-dead donors are cold preserved until transplanted. However, prolonged cold storage can contribute to allograft failure. Studies suggest that donor preconditioning with dopaminergics may reduce cold-ischemic transplant injury, but whether heme oxygenase-1 (HO1) induction is an underlying mechanism is not known.
Objective
To test whether preconditioning with fenoldopam (FD) induce HO1 and protect kidneys against cold storage injury and HO1 plays a role in protection.
Method
We used human renal proximal tubular epithelial (RPTE) cells, rat kidney transplants and HO1(−/−) null mice kidneys.
Results
FD-preconditioning of cells for 4 h significantly protected against cell death from 24 h cold-hypoxia and was associated with a dose-dependent increase in HO1 expression. In a syngeneic rat kidney transplant model, FD-preconditioning for 18 h markedly increased kidney HO1 expression and protected kidneys against 24 h cold ischemic transplant injury. To test the role of HO1, RPTE cells were treated with HO1 siRNA, followed by FD-preconditioning. siRNA inhibited the HO1 mRNA expression and reversed the FD protection. Suspension of kidneys of HO1(−/−) null and wild type (WT) mice preconditioned with FD or saline were subjected to 24 and 48 h cold storage. N-Acetyl-glucosaminidase, a specific tubular injury marker, was significantly lower in FD-preconditioned WT kidneys, but not in HO1 null kidneys suggesting a role for HO1 in FD’s preconditioning.
Conclusion
Our data suggest HO1 induction as an underlying mechanism for FD-preconditioning, and support the idea of testing FD-preconditioning in the clinical setting. Studies are required to determine the optimum FD-preconditioning protocol.
Keywords: Transplant, Kidney, Preconditioning, Hemoxegense-1, HO1, cold storage, Oxidative stress, Cold Preservation, Acute Kidney Injury
Introduction
In clinical practice, kidneys retrieved from brain-dead donors are cold preserved until transplanted. Cold storage provides the time necessary to perform tissue matching and to transport kidneys to suitable recipients. However, prolonged cold storage, e.g., over 20 h, can lead to allograft failures (1, 2). The delayed graft function (DGF) and the accompanying ARF shortens the graft survival, increases recipients’ morbidity and mortality, and adds to the health-care cost (1, 2). For several logistical reasons, the CIT is unlikely to lower from the current level (3). Therefore, the focus is on developing effective strategies to block the cold ischemic injury. An analysis of a kidney transplant database suggested that administration of catecholamines including dopamine to donors might be associated with reduced graft loss (4). Further, in experimental studies, administration of such compounds was also shown to reduce cold storage associated injuries of cells and transplanted kidneys (5, 6). However, the mechanism underlying such protection is not clearly understood. Since dopaminergic compounds are known to induce HO1 in cell culture studies and deliberate induction of HO1 in in-vivo studies has been shown to protect kidney against acute injuries including that of transplants, we speculated that the preconditioning effect of dopaminergic compounds against cold storage could be arising from its ability to induce HO1 (7–14). Therefore, using a dopaminergic compound fenoldopam, we first tested its ability to induce HO1 and protect kidney and kidney cells against cold ischemic injury. Then, using HO1 siRNA in cell culture and using kidneys of HO1 deficient mice, we tested whether HO1 was critical for FD’s preconditioning protection. Our results confirm the preconditioning properties of dopaminergic compounds, and demonstrate for the first time that HO1 induction is critical for protection.
Results
Effect of FD-preconditioning on cold-hypoxic cell death
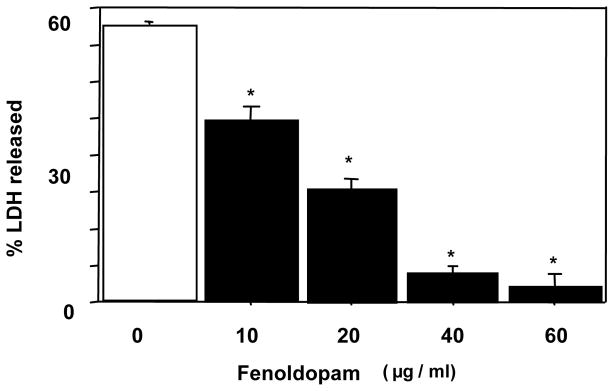
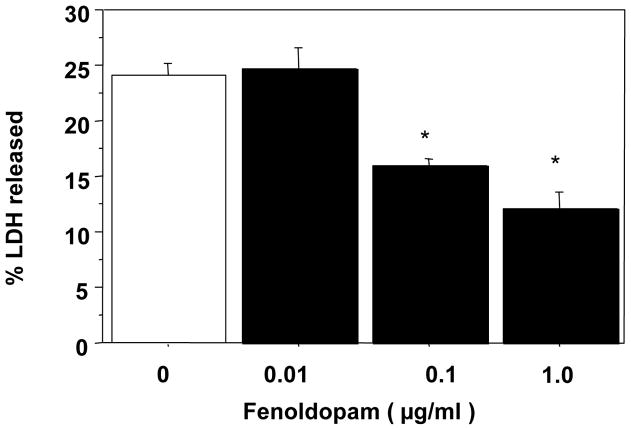
The human RPTE cells grown to near confluence in 24-well plates were preconditioned with FD (10–60 μg/ml) or FD vehicle, normal saline, in DMEM at 37°C for 18 h. This was followed by hypoxic (<1%) and hypothermic (4°C) storage of cells in UW solution for 48 h. The cells preconditioned with FD had a dose-dependent reduction in LDH release and, compared with the saline treated group, the mean LDH release was significantly lower in the FD-preconditioned groups including the lowest dose used of 10 μg/ml (Fig 1a), suggesting an FD-mediated cytoprotection against cold storage injury. The experiment was repeated using doses lower than10 μg/ml, i.e., 0.01–1 μg/ml as well as a preconditioning duration shorter than 18 h, i.e., for 4 h. The cells were then subjected to 24 h cold-hypoxia, followed by LDH measurement. The saline-preconditioned control group had a LDH release (mean ±SEM) of 24±4 % (Fig 1b), which was significantly lower in the FD-preconditioning groups starting with as low as 0.1μg/ml (Fig 1b).
Fig. 1.
a: Effect of FD-preconditioning on 48-h cold hypoxic storage-induced LDH release in human RPTE cells (mean±SEM; n=4 in duplicates; * P <0.05 vs. 0 μg/ml FD by ANOVA). For preconditioning, FD was used at 10–60 μg/ml for 18 h.
b: Effect of a shorter and low dose FD-preconditioning on 24-h cold hypoxic storage-induced LDH release in human RPTE cells (n=3 in duplicates; * P <0.05 vs. 0 μg/ml FD by ANOVA). For preconditioning, FD was used at 0.10–1.0 μg/ml for 4h.
c: Effect of a shorter and low dose FD-preconditioning on 24-h cold hypoxic storage and rewarming-induced apoptosis in human RPTE cells (n=4 in duplicates; * P <0.05 vs. 0 μg/ml FD by ANOVA). For preconditioning, FD was used at 0.10–10 μg/ml for 4h.
Effect of FD on HO1 induction
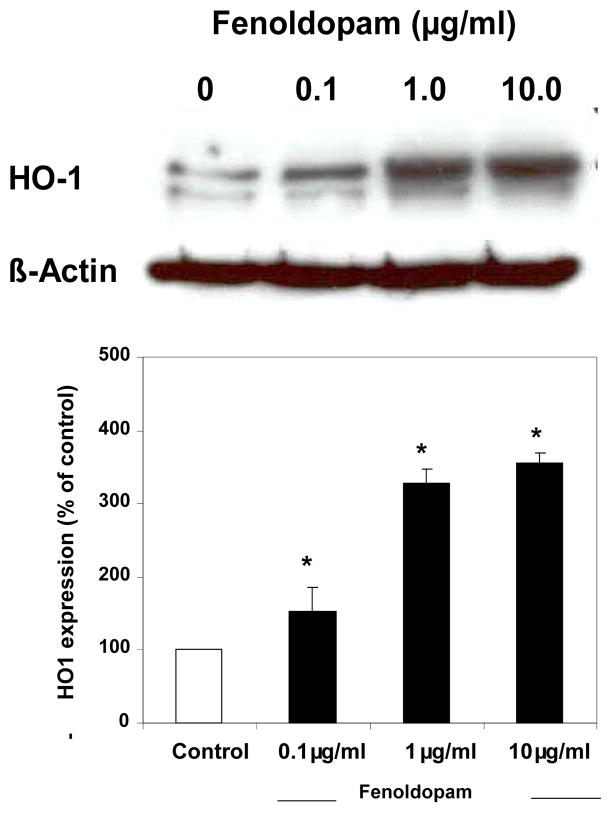
Cells were subjected to preconditioning for 4 h with FD at 0.1–10 μg/ml. This was followed by a 24-h cold-hypoxic storage in UW solution. Cell lysates were subjected to immunoblot analysis for HO1. As displayed in Fig. 2, FD-preconditioning was associated with a dose-related induction of HO1 protein expression that was evident as more than a 50% increase in HO1 expression with the lowest dose of FD tested, i.e., 0.1 μg/ml, compared to saline control; a 300% increase was noted with 10 μg/ml of FD.
Fig. 2.
Immunoblot analysis of HO1 expression in human RPTE cells following a 4-h lower-dose FD-preconditioning followed by 24-h cold hypoxic storage. The top panel shows the Western blot, and the lower panel displays the densitometric values for the blot (n=3 in duplicates; * P <0.05 vs. 0 μg/ml FD by ANOVA).
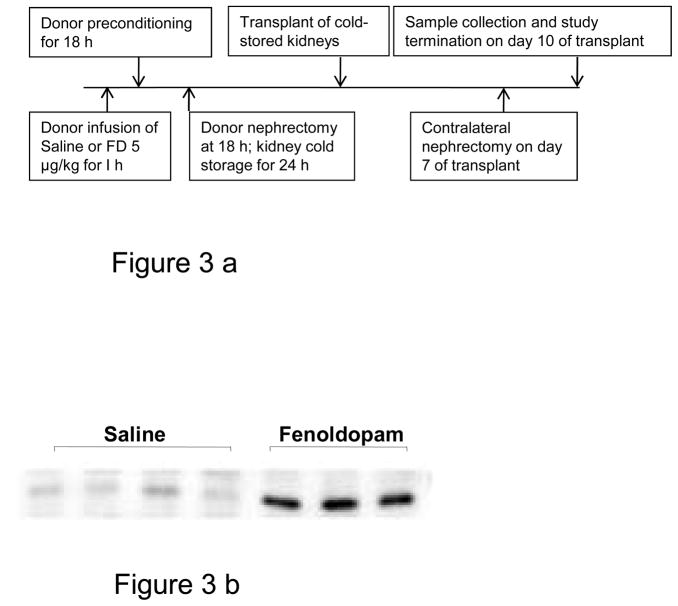
As a part of the transplantation study, protocol described below and displayed on Fig. 3a, the donor rats were infused with either FD (5 μg/kg/min for 60 min) or the saline vehicle. The homogenates of right kidneys removed 18 h after FD or saline were subjected to HO1 immunoblot analysis. As displayed in Fig. 3b, the kidneys of rats infused with FD had a striking increase in the induction of HO1 expression compared to saline-treated controls.
Fig. 3.
a: A flow chart of the protocol used in the transplant study.
b: Immunoblot analysis of the FD-induced HO1 expression in whole kidney homogenate. The rats were injected with saline or FD, and the kidneys were retrieved after 18 h (n = 4 saline and 3 FD; the blot was standardized by loading equal amounts of protein).
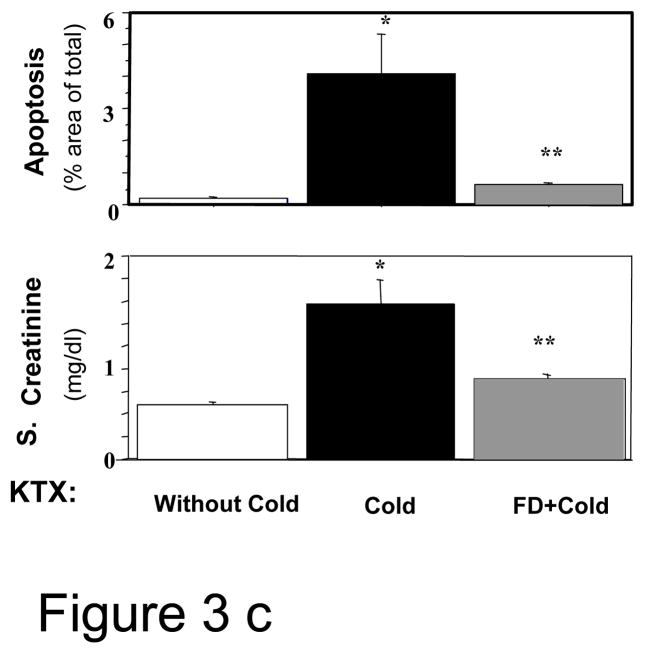
c: The serum creatinine levels and the extent of renal apoptosis in the syngeneic kidney transplant (KTX) study; n=5–6; * P <0.05 of KTX between kidneys with and without cold storage and ** P<0.05 of KTX between cold stored kidneys with and without FD by ANOVA).
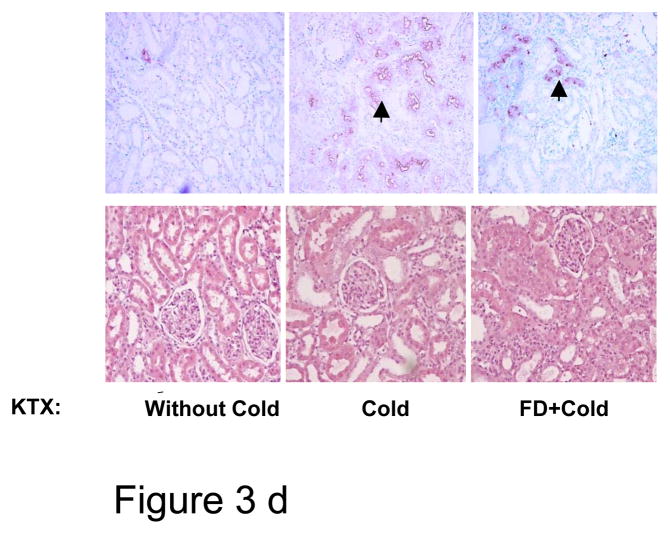
d: Top panel: Immunohistochemical staining for apoptosis of kidneys removed at day 10 of transplantation (KTX); arrow-heads indicate apoptotic staining. The extent of apoptosis quantified by computer program is given in Fig. 3b. Bottom panel shows hematoxilyn and eosin staining of these kidneys and by day 10 of KTX no discernible necrosis is seen.
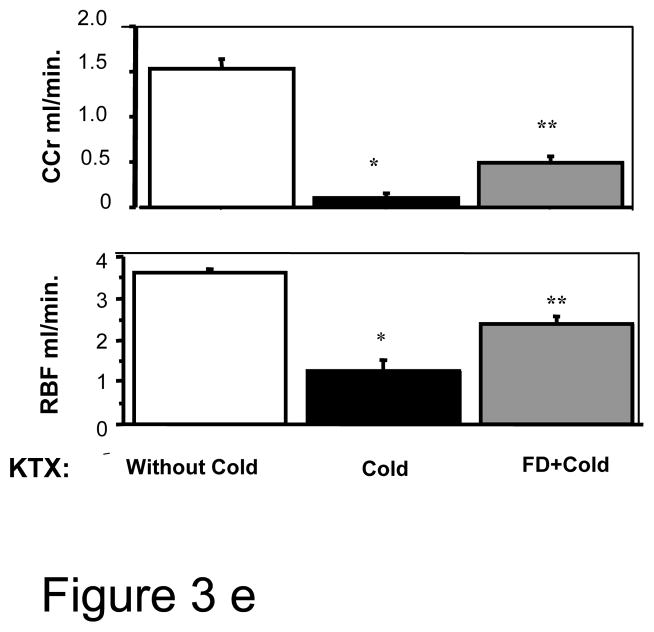
e: Renal clearance measurements: GFR measured by creatinine clearance of timed urine collections and renal blood flow by flow probe were higher in the FD group than the saline control KTX that did not receive FD preconditioning. (n=5–6; * P <0.05 of KTX between kidneys with and without cold storage and ** P<0.05 of KTX between cold stored kidneys with and without FD by ANOVA).
Effect of FD on cold-ischemia induced transplant kidney injury
As displayed in Fig. 3a, the kidneys from donor rats were removed 18 h after the 1-h of FD (n=6) or saline (n=5) infusion. The kidneys were flushed with cold UW solution through renal arteries using a perfusion chamber. The right kidneys, as mentioned above, were used for HO1 immunoblot analysis, and the left kidneys were subjected to 24 h cold storage pending transplantation. Prior to transplantation, the recipient rats first underwent left nephrectomies. Then, in the same surgery, the cold stored left kidneys from the donor rats, flushed clear of UW solution with normal saline, were transplanted to the recipients’ left renal fosse. The right nephrectomies of the recipient rats were delayed for 7 days to provide interim renal support, which allowed time for the transplanted kidneys to begin recovering from DGF. In preliminary studies, we found that when right nephrectomies were performed at the time of transplantation, the recipients suffered high morbidity and mortality; a 24-h cold storage of kidneys is often associated with DGF. On day 10 of transplant (i.e., 3 days after the right native kidney nephrectomy, which leaves the transplanted kidneys as the sole supplier of kidney function), the transplanted kidneys were subjected to renal clearance and sampling for histology. A 3-day gap between right nephrectomy and final transplanted kidney study was allowed to reach a steady-state especially the serum creatinine. The details on transplant surgery and the technique used were published, and also provided as on-line data (15).
The function of the transplanted kidney, measured 10 days after transplant, was significantly better in the FD group than in the saline-treated group. FD infusion was associated with a significantly lower serum creatinine than the normal saline-infused group (0.89±0.50 vs. 1.71±0.29 mg/dl, p <0.05), but both were higher than control rats transplanted without cold (0.60±0.04 mg/dl, p <0.05) (Fig 3c). The serum creatinine level in a 250–300 g rat is 0-3-0.5 mg/dl(16). Tubular necrosis was minimal in these kidneys, 10 days post-injury (Fig 3d, lower panel). However, apoptosis was significantly lower in the FD group than normal saline group (0.06±0.01% vs. 0.42±0.21%, P< 0.05) (Fig. 3d, upper panel).
During renal hemodynamic measurements, consistent with the serum creatinine finding, GFR based on creatinine clearances (Ccr) of the transplanted kidneys were also significantly higher in the FD group vs. normal saline group (0.49±0.08 vs. 0.10±0.05 ml/min; P<0.05), but both were lower than the GFR in the control kidneys transplanted without cold storage(1.53±0.10; p <0.05) (Fig. 3e). The Ccr in a normal rat is 1.25–1.75 ml/min(17). RBF was significantly higher in FD group compared with saline group (2.40 ±0.18 vs. 1.28±0.25 ml/min, P<0.05), but lower than non-cold control group (3.63±0.08 ml/min, P<0.05) (Fig. 3e). The RBF in a normal rat is 4–6 ml/min/kidney(18). RVR also provided results consistent with RBF suggesting less vasoconstriction in the FD-treated kidneys (data not provided). Furthermore, as noted above, the FD-infusion group had markedly higher HO1 expression than the saline control group (Fig. 3b).
Effect of HO1 siRNA in suppressing hemin- and FD-induced HO1 expression in RPTE cells
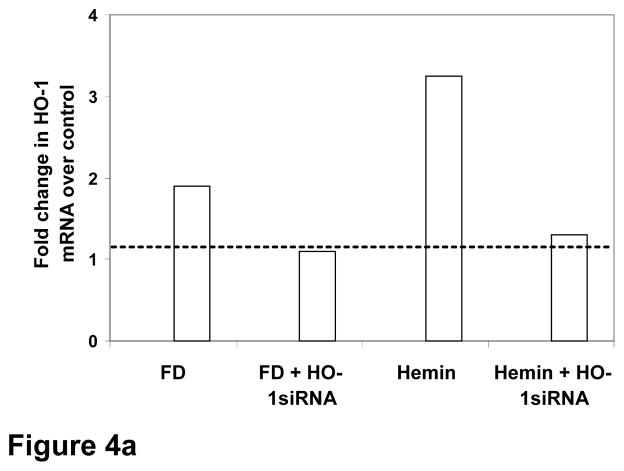
We first verified the transfecting efficiency of HO1 siRNA in RPTE cells as described in the method section by using 5′-fluorescein-labeled HO1 siRNAs (Orbigen.com) in Oligofectamine™ (Invitrogen.com). The fluorescence uptake of cells after incubating them with fluorescence-tagged HO1 siRNA in oligofectamine showed an approximately 80% of transfecting efficacy of our method. To test the effect of HO1 siRNA on FD-induced HO1 expression, the following groups of RPTE cells in DMEM were used 1) oligofectamine, 2) oligofectamine and FD, 3) HO1 siRNA, oligofectamine and FD, 4) oligofectamine and hemin (a potent inducer of HO1 as positive control) 5) HO1 siRNA, oligofectamine and hemin. To facilitate siRNA uptake, all groups were incubated for 24 h at 37° C. This was followed by addition of FD 10 μg/ml or equal volume of saline in the appropriate groups followed by incubation at 37° C for 24 h. The cell lysates were used for real time PCR. Hemin, a known stimulant of HO1, caused over a three-fold increase in HO1 mRNA (Fig. 4a), which was suppressed in cells preloaded with HO1 siRNA to a level found in control cells treated with vehicle. Similarly, HO1 siRNA also suppressed the FD-induced two-fold increase in HO1 mRNA to a level found in control cells treated with vehicle. Thus the custom-synthesized HO1 siRNA and the oligofectamine transfecting protocol had achieved an 80–90% knock down of HO1 gene in our experiment.
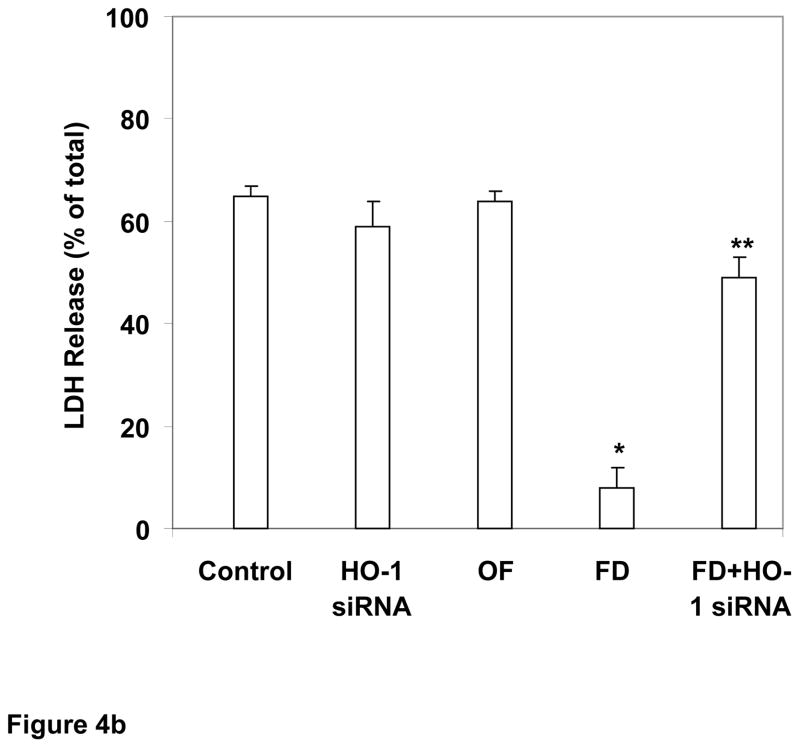
Fig. 4. Role of HO1 in FD-induced protection of human renal tubular cells against cold hypoxic injury: studies using HO1 siRNA.
a: Real-time PCR data: the inhibitory effect of HO1 siRNA on hemin- and FD-induced HO1 mRNA is displayed (average fold change over control based on result from 3 experiments in triplicate is shown).
b: HO1 siRNA reverses FD’s cytoprotection against cold hypoxic cell injury. Control cells, preconditioned with saline instead of FD, sustained marked injury from 48-h of cold hypoxic storage. The transfecting agent oligofectamine (OF) or HO1 siRNA alone had no significant effect on cold storage injury. FD preconditioning was associated with significant protection against cold storage injury, which was significantly reversed by HO1 siRNA and OF treatment (n=4 in duplicates; *P<0.05 vs. the rest and **P<0.05 vs. the rest, by ANOVA).
Effect of HO1 siRNA in reversing FD-afforded cytoprotection against cold hypoxic injury
In an experiment similar to above, preconditioned cells were subjected to cold hypoxic storage to determine whether HO1-siRNA would reverse the FD’s protection. Following groups of cells were included: 1) saline, 2) oligofectamine, 2) oligofectamine and HO1 siRNA, 3) oligofectamine and FD and 4) oligofectamine, HO1 siRNA and FD. Cells were preconditioned with FD for 24 h at 37° C. After replacing DMEM with UW solution, cells were stored at 4° C and <1% O2 for 48 h. Cell injury was measured by LDH method. Cells preconditioned with FD and cold stored for 48 h had significantly lower LDH release compared to cold stored cells preconditioned with saline (Fig. 4b: FD: 9±5% vs. Saline 65±4%, mean±SEM; P<0.05 by ANOVA); oligofectamine or siRNA alone had no significant effect on cold hypoxic cell injury. However, FD preconditioning of cells treated with HO1 siRNA had significantly lower protection against cold storage than cell preconditioned with FD in the absence of HO1 siRNA (50±5% vs. 9±5%; P<0.05 by ANOVA), indicating a role for HO1 gene expression in FD-mediated cytoprotection.
Effect of FD-preconditioning of HO1 null and WT mice on cold storage-induced kidney injury
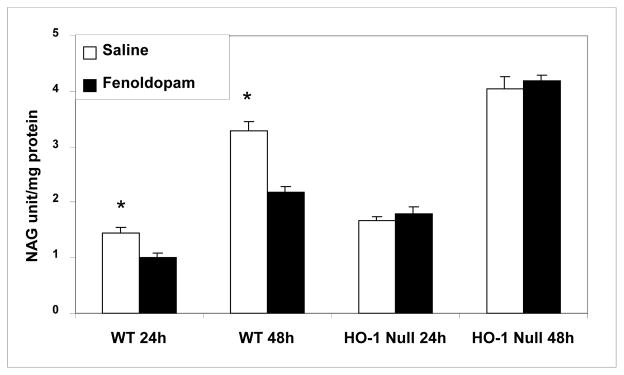
In this study, we extended the above siRNA cell culture data to HO1(−/−) null mice. The mice were 10 weeks old, weighing 25–30 g and their genotypes were confirmed by tail snip PCR. The null and WT mice were preconditioned for 24 h with fenoldopam (5 μg/kg/min for 60 minutes) or equal volume of saline administered through tail veins. The four groups were the null mice infused with saline (n=6) or FD (n=6), and WT mice infused with saline (n=4) or FD (n=4). A fine suspension of kidneys perfused completely clear of red cells as described in the Method Section were subjected to cold storage studies. The NAG, a specific marker of renal tubular injury, was sequentially assayed in the supernatant at 24 and 48 h of and expressed as units per mg of protein. The NAG levels were significantly lower in the WT mice preconditioned with FD, but not in the saline treated group (Fig. 5). For example, the NAG levels in the 48 h cold stored kidney suspension of FD-preconditioned WT mice was 2.20±0.10 unit/mg protein vs. 3.30±0.20 unit/mg protein in the saline-preconditioned mice (P<0.05). More importantly, the FD-preconditioning did not lower the NAG levels in the cold-stored-kidney-suspension of HO1 deficient mice indicating the prerequisite for HO1 gene to marshal the FD-mediated protection.
Fig. 5.
Role of HO1 in FD-induced kidney protection against cold ischemic injury: studies using HO1 null mice HO1 null (n=4) and WT type mice (n=6) were injected with FD or saline, and their kidneys were subjected to cold storage in fine suspension. The extent of NAG release (a marker for tubular injury) into supernatant was determined at 24 and 48 h of cold storage (* P<0.05 vs. FD by ANOVA).
Discussion
Our study demonstrates that preconditioning of tubular cells or donor kidneys with FD is associated with HO1 induction, and that such preconditioning is associated with attenuation of cold-hypoxic injury. Moreover, based on HO1 siRNA and HO1 null mice experiments, we demonstrate for the first time that HO1 induction is critical for the FD’s preconditioning effect.
FD is a strong renal vasodilator, but this is unlikely a main mechanism for its preconditioning as FD’s cytoprotection was also seen in our culture studies. Consistent with a role for HO1 in the present study is the direct finding that FD induces HO1 in human kidney cells in a potent and dose-dependent manner. Earlier reports from other investigators using neuronal and endothelial cells in culture had noted HO1 induction with dopamine (19, 20). In our study, using two compelling approaches, i.e., one, transiently suppressing the HO1 gene in human proximal tubular cells with siRNA and, two, using mice with genetically deleted HO1 gene, we were able to demonstrate that HO1 is essential for the FD’s preconditioning effect.
In our in-vitro study, a short FD-preconditioning of cells seems to continue to engender HO-1 expression in spite of the cold and hypoxic condition of storage (figure 2) and confer protection. If this is corroborated in a transplant model (in our transplant study, we had employed an 18 h preconditioning period) that would suggest that preconditioning kidneys a few hours prior to cold storage would result in protection against cold ischemic injury. Future studies are required in the transplant model to address this key question. Additionally, although increase in HO-1 expression with FD-preconditioning can only be expected to last for about 48 hours(14), renal protection in our study was demonstrable 10 days after transplant. This suggests that suppressing early cold ischemic injury may limit subsequent injury process—possibly suppressing the downstream cascade of events as proposed in the “Response-to-Injury Hypothesis”(21).
Although we have focused on HO1 for FD’s protection, several other redox sensitive genes activated by the Nrf2 pathway could also be important in FD’s cytoprotection. A recent study by Gottmann et al showed that protection afforded by dopamine-preconditioning in a kidney transplant model was associated with up-regulation of transforming growth factor beta (TGF-β) along with suppression of cellular infiltrates (6). Interestingly, HO1 has also been shown to modulate cytokines and cellular infiltrates (22). Thus, it is possible that the up-regulation of TGF-β noted by Gottmann et could be a downstream salutary effect of the HO1 induction. Indeed, the anti-inflammatory effect of dopaminergic compounds could also arise from the by-product of HO1 enzymatic reaction, namely CO (23, 24). In kidney tubular cell studies, CO has been directly shown to protect cells against cold storage injury (13). Moreover, several studies have demonstrated a role for iron toxicity in cold ischemic injury of organs (14, 15, 25). Thus, the increase in iron-sequestrating ferritin, well documented phenomenon occurring with HO1 induction, could be an additional mechanism by which FD confer protection against cold ischemic injury.
In summary, our experimental study demonstrates that preconditioning of donor rats with FD induces HO1 and suppresses kidney transplant injury from cold-ischemia and reperfusion injury. We further demonstrate that the preconditioning effect of FD could be explained at least in part on HO1 induction. Our present finding of protection with FD-preconditioning in cold ischemic injury is consistent with the known tissue protective properties of HO1, especially in settings where iron toxicity exists. Collectively, therefore, our data provide certain scientific rational for testing FD-preconditioning in the clinical settings of transplantation. Studies are, however, needed to determine the optimum timing for FD-preconditioning.
Methods
Cell culture model
Acute tubular necrosis (ATN) is the most frequent form of injury sustained by kidneys subjected to cold storage, and it occurs frequently at the proximal tubular level. Cells used for this study were renal proximal tubular epithelial (RPTE) cells of human origin. The techniques of growing cells are standard and have been reported in detail (25–27).
Cell death measurements
LDH as a measure of cell death was assayed by a kinetic method(28) as described by us previously (25–27). For apoptotic cell death, an apoptotic quantification kit from Biotium Inc., Hayward, CA was used as per manufacturer’s suggestion. Briefly, the apoptotic cells take up green fluorescein-labeled Annexin-V. The cells were sorted within one hour of staining by flow cytometry. The data is presented as percentage of total cells.
HO1 protein detection
Cell lysate or kidney homogenate was subjected to standard immunoblot technique using commercially available antibodies and an enhanced chemiluminescence detection system (ECL plus Western Blotting system from Amersham, Buckinghamshire HP7 9NA UK) as described by us previously (29). The HO1 (H4535) and β-actin (A1978) antibodies were obtained from Sigma-Aldrich, St. Louis, MO.
Kidney transplantation
We reported in detail a stable syngeneic kidney transplantation model using in-bred male Wistar-Furth rats (Charles River Laboratories, Inc., Wilmington, MA)(21, 25) (see also on-line data).
Renal function measurements
Briefly, catheterization of femoral artery was used for BP measurement and blood sampling, internal jugular vein for IV fluids and bladder for urine collection. RBF was measured by direct ultrasonic flow probe, and RVR, derived from BP, RBF and Hct. Measurements were made under euvolemia while maintaining BP and temperature, methods described in detail by us previously(15). The serum creatinine was measured using the autoanalyzer (Beckman Coulter, Fullerton, CA).
Assessment of necrosis and apoptosis in the transplanted kidneys
We have described the method in detail (29). Tubular sections with necrosis were point-counted with a line grid using Image-Pro® image analysis software program (Media Cybernetics, Carlsbad, CA) (29). For in-situ detection of apoptosis, kidney sections were stained with an apoptosis detection kit (ApopTag®, Serological Corporation Inc., Norcross, GA). The area of apoptosis was measured with ImagePro® program and a mean score for each kidney was used for statistical analysis(29).
HO1 gene silencing
Using a successfully employed HO1 siRNA-sequence targeting human HO1 isoform (see on-line data), RPTE cells in DMEM at 69–70% confluence were mixed with siRNA (synthesized by Dharmacon Research, Inc., Lafayette, CO) in Oligofectamine vehicle (Invitrogen, Carlsbad, CA) to a final concentration of 20 nM. The cells were incubated for 24 h at 37° C. The cells were then washed in DMEM and subjected to preconditioning at 37° C with FD followed by cold storage studies. Genetically altered mice were obtained from Dr. Dore’s laboratory at Johns Hopkins University, Baltimore, MD.
Real-time PCR
Real-Time PCR was performed using iQ SYBR Green Supermix. See on-line data for more details.
Cold storage studies of kidney suspension from HO1 null and WT mice
Immediately after euthanization of HO1 null and WT mice subjected to experiment, kidneys were removed and perfused with saline in-situ through the cardiac approach. The left kidney is brought to a fine suspension on ice using a surgical blade over 10 minutes. The suspension, cycled three times through normal saline-wash and centrifugation, was placed in UW solution. While under continuous low-speed stir, samples were aliquoted equally for cold storage experiments. The results from these experiments were reported as units of n-acetyl glucosaminidase (NAG) per mg protein. NAG is released with renal tubular cell injury, and in acute kidney injury, its increase is regarded as an early and reliable biomarker of tubular injury(30). NAG is measured by a spectrophotometeric enzymatic assay kits from Sigma Inc., USA.
Statistical Analysis
Statistical analyses were conducted using the StatView Program from Abacus Concepts, Inc., Berkeley, CA. The results are presented as mean±SEM. For in-vitro studies, 3–4 experiments, performed at least in duplicates, were conducted. The differences among multiple groups were determined by ANOVA followed by Fisher’s post-hoc test. A P<0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This research was supported by NIDDK NIH grant # RO-1 DK-56835-01 to AKS.
Abbreviations
- FD
Fenoldopam
- HO1
Heme oxygenase1
- WT
Wild type
- RPTE
Human renal proximal tubular epithelial
- CIT
Cold ischemia time
- UW
University of Wisconsin
- DGF
Delayed graft function
- DMEM
Dulbecco’s Modified Eagle’s Medium
Footnotes
AKS: Participated in research design, performance of the research, data analysis and writing of the paper. MY and HH: Participated in the performance of the research and data analysis. SD: Contributed new reagents and analytic tools and writing the paper. DES: Participated in research design, performance of the research, and data analysis.
None of the authors has any potential conflict of interest related to this work to disclose.
References
- 1.Ojo AO, Wolfe RA, Held PJ, Port FK, Schmouder RL. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation. 1997;63 (7):968. doi: 10.1097/00007890-199704150-00011. [DOI] [PubMed] [Google Scholar]
- 2.Salahudeen AK, Haider N, May W. Cold ischemia and the reduced long-term survival of cadaveric renal allografts. Kidney Int. 2004;65 (2):713. doi: 10.1111/j.1523-1755.2004.00416.x. [DOI] [PubMed] [Google Scholar]
- 3.Salahudeen AK, May W. Reduction in cold ischemia time of renal allografts in the United States over the last decade. Transplant Proc. 2008;40 (5):1285. doi: 10.1016/j.transproceed.2008.03.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schnuelle P, Lorenz D, Mueller A, Trede M, Van Der Woude FJ. Donor catecholamine use reduces acute allograft rejection and improves graft survival after cadaveric renal transplantation. Kidney Int. 1999;56 (2):738. doi: 10.1046/j.1523-1755.1999.00567.x. [DOI] [PubMed] [Google Scholar]
- 5.Yard B, Beck G, Schnuelle P, et al. Prevention of cold-preservation injury of cultured endothelial cells by catecholamines and related compounds. Am J Transplant. 2004;4 (1):22. doi: 10.1046/j.1600-6143.2003.00268.x. [DOI] [PubMed] [Google Scholar]
- 6.Gottmann U, Brinkkoetter PT, Bechtler M, et al. Effect of pre-treatment with catecholamines on cold preservation and ischemia/reperfusion-injury in rats. Kidney Int. 2006;70 (2):321. doi: 10.1038/sj.ki.5001501. [DOI] [PubMed] [Google Scholar]
- 7.Tsuchihashi S, Fondevila C, Kupiec-Weglinski JW. Heme oxygenase system in ischemia and reperfusion injury. Ann Transplant. 2004;9 (1):84. [PubMed] [Google Scholar]
- 8.Immenschuh S, Schroder H. Heme oxygenase-1 and cardiovascular disease. Histol Histopathol. 2006;21 (6):679. doi: 10.14670/HH-21.679. [DOI] [PubMed] [Google Scholar]
- 9.Nath KA, Haggard JJ, Croatt AJ, Grande JP, Poss KD, Alam J. The indispensability of heme oxygenase-1 in protecting against acute heme protein-induced toxicity in vivo [see comments] Am J Pathol. 2000;156 (5):1527. doi: 10.1016/S0002-9440(10)65024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otterbein LE, Kolls JK, Mantell LL, Cook JL, Alam J, Choi AM. Exogenous administration of heme oxygenase-1 by gene transfer provides protection against hyperoxia-induced lung injury. J Clin Invest. 1999;103 (7):1047. doi: 10.1172/JCI5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nath KA. Heme oxygenase-1: a redoubtable response that limits reperfusion injury in the transplanted adipose liver [In Process Citation] J Clin Invest. 1999;104 (11):1485. doi: 10.1172/JCI8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soares MP, Lin Y, Anrather J, et al. Expression of heme oxygenase-1 can determine cardiac xenograft survival. Nat Med. 1998;4 (9):1073. doi: 10.1038/2063. [DOI] [PubMed] [Google Scholar]
- 13.Stec DE, Bishop C, Rimoldi JM, Poreddy SR, Vera T, Salahudeen AK. Carbon monoxide (CO) protects renal tubular epithelial cells against cold-rewarm apoptosis. Ren Fail. 2007;29 (5):543. doi: 10.1080/08860220701391878. [DOI] [PubMed] [Google Scholar]
- 14.Salahudeen AA, Jenkins JK, Huang H, Ndebele K, Salahudeen AK. Overexpression of heme oxygenase protects renal tubular cells against cold storage injury: studies using hemin induction and HO-1 gene transfer. Transplantation. 2001;72 (9):1498. doi: 10.1097/00007890-200111150-00005. [DOI] [PubMed] [Google Scholar]
- 15.Huang H, He Z, Roberts LJ, Salahudeen AK. Deferoxamine reduces cold-ischemic renal injury in a syngeneic kidney transplant model. Am J Transplant. 2003;3 (12):1531. doi: 10.1046/j.1600-6135.2003.00264.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang C, Salahudeen AK. Cyclosporine nephrotoxicity: attenuation by an antioxidant-inhibitor of lipid peroxidation in vitro and in vivo. Transplantation. 1994;58 (8):940. doi: 10.1097/00007890-199410270-00014. [DOI] [PubMed] [Google Scholar]
- 17.Wang C, Salahudeen AK. Lipid peroxidation accompanies cyclosporine nephrotoxicity: effects of vitamin E. Kidney Int. 1995;47 (3):927. doi: 10.1038/ki.1995.138. [DOI] [PubMed] [Google Scholar]
- 18.Nath KA, Salahudeen AK. Induction of renal growth and injury in the intact rat kidney by dietary deficiency of antioxidants. J Clin Invest. 1990;86 (4):1179. doi: 10.1172/JCI114824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt J, Mertz K, Morgan JI. Regulation of heme oxygenase-1 expression by dopamine in cultured C6 glioma and primary astrocytes. Brain Res Mol Brain Res. 1999;73 (1–2):50. doi: 10.1016/s0169-328x(99)00231-4. [DOI] [PubMed] [Google Scholar]
- 20.Berger SP, Hunger M, Yard BA, Schnuelle P, Van Der Woude FJ. Dopamine induces the expression of heme oxygenase-1 by human endothelial cells in vitro. Kidney Int. 2000;58 (6):2314. doi: 10.1046/j.1523-1755.2000.00415.x. [DOI] [PubMed] [Google Scholar]
- 21.Salahudeen A, Wang C, McDaniel O, Lagoo-Denadyalan S, Bigler S, Barber H. Antioxidant lazaroid U-74006F improves renal function and reduces the expression of cytokines, inducible nitric oxide synthase, and MHC antigens in a syngeneic renal transplant model. Partial support for the response-to-injury hypothesis. Transplantation. 1996;62 (11):1628. doi: 10.1097/00007890-199612150-00017. [DOI] [PubMed] [Google Scholar]
- 22.Nath KA, Vercellotti GM, Grande JP, et al. Heme protein-induced chronic renal inflammation: suppressive effect of induced heme oxygenase-1. Kidney Int. 2001;59 (1):106. doi: 10.1046/j.1523-1755.2001.00471.x. [DOI] [PubMed] [Google Scholar]
- 23.Kohmoto J, Nakao A, Stolz DB, et al. Carbon monoxide protects rat lung transplants from ischemia-reperfusion injury via a mechanism involving p38 MAPK pathway. Am J Transplant. 2007;7 (10):2279. doi: 10.1111/j.1600-6143.2007.01940.x. [DOI] [PubMed] [Google Scholar]
- 24.Otterbein LE. Carbon monoxide: innovative anti-inflammatory properties of an age-old gas molecule. Antioxid Redox Signal. 2002;4 (2):309. doi: 10.1089/152308602753666361. [DOI] [PubMed] [Google Scholar]
- 25.Huang H, Salahudeen AK. Cold induces catalytic iron release of cytochrome P-450 origin: a critical step in cold storage-induced renal injury. Am J Transplant. 2002;2 (7):631. doi: 10.1034/j.1600-6143.2002.20708.x. [DOI] [PubMed] [Google Scholar]
- 26.Salahudeen AK, Huang H, Patel P, Jenkins JK. Mechanism and prevention of cold storage-induced human renal tubular cell injury. Transplantation. 2000;70 (10):1424. doi: 10.1097/00007890-200011270-00005. [DOI] [PubMed] [Google Scholar]
- 27.Salahudeen AK, Joshi M, Jenkins JK. Apoptosis versus necrosis during cold storage and rewarming of human renal proximal tubular cells. Transplantation. 2001;72 (5):798. doi: 10.1097/00007890-200109150-00010. [DOI] [PubMed] [Google Scholar]
- 28.Wahlefeld AW. UV Method with L-Lactate and NAD. 3. Deerfield Beach: Verlag Chemie; 1983. [Google Scholar]
- 29.Salahudeen AK, Haider N, Jenkins J, et al. Antiapoptotic properties of erythropoiesis-stimulating proteins in models of cisplatin-induced acute kidney injury. Am J Physiol Renal Physiol. 2008;294 (6):F1354. doi: 10.1152/ajprenal.00131.2008. [DOI] [PubMed] [Google Scholar]
- 30.Han WK, Waikar SS, Johnson A, et al. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2008;73 (7):863. doi: 10.1038/sj.ki.5002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.