Abstract
The past decade has seen a substantial increase in the number and quality of clinical trials of new therapies for vasculitis, including randomized, controlled, multicenter trials that have successfully incorporated measures of disease activity and toxicity. However, because current treatment regimens for severe disease effectively induce initial remission and reduce mortality, future trials will focus on any of several goals including: (a) treatment of mild—moderate disease; (b) prevention of chronic damage; (c) reduction in treatment toxicity; or (d) more subtle differences in remission induction or maintenance. Thus, new trials will require outcome measure instruments that are more precise and are better able to detect effective treatments for different disease states and measure chronic manifestations of disease. The OMERACT Vasculitis Working Group comprises international clinical investigators with expertise in vasculitis who, since 2002, have worked collaboratively to advance the refinement of outcome measures in vasculitis, create new measures to address domains of illness not covered by current research approaches, and harmonize outcome assessment in vasculitis. The focus of the OMERACT group to date has been on outcome measures in small-vessel vasculitis with an overall goal of creating a core set of outcome measures for vasculitis, each of which fulfills the OMERACT filter of truth, discrimination, feasibility, and identifying additional domains requiring further research. This process has been informed by several ongoing projects providing data on outcomes of disease activity, disease-related damage, multidimensional health-related quality of life, and patient-reported ratings of the burden of vasculitis.
Key Indexing Terms: VASCULITIS, OUTCOMES, ACTIVITY, DAMAGE
The vasculitides are a group of multisystem diseases involving inflammatory vascular pathology as well as other types of tissue inflammation. Vasculitis is often an organ- or life-threatening disease. Treatments for vasculitis include high doses of glucocorticoids in combination with immunosup-pressive medications. The cumulative burden of disease for patients with vasculitis can be substantial, due to acute injury, persistent disease, recurrent flares, and drug toxicity.
The past decade has seen a substantial increase in the number and quality of clinical trials of new therapies for vasculitis, including randomized, controlled, multicenter trials1–6. These trials have successfully incorporated measures of disease activity and toxicity. However, because current treatment regimens for severe disease effectively induce initial remission and reduce mortality, future trials will focus on any of several goals including (a) treatment of mild—moderate disease; (b) prevention of chronic damage; (c) reduction in treatment toxicity; or (d) more subtle differences in remission induction or maintenance. Thus, new trials will require outcome measure instruments that are more precise, better able to detect effective treatments for different disease states, and measure chronic manifestations of disease. Additionally, there are domains of illness in vasculitis not addressed in currently available outcome tools.
The OMERACT Vasculitis Working Group comprises international clinical investigators with expertise in vasculitis who, since 2002, have worked collaboratively to advance the refinement of outcome measures in vasculitis and create new measures to address domains of illness not covered by current research approaches. The group met during OMERACT 7 in 2004, during which the spectrum of outcome measures was reviewed and broad-ranging discussions identified several key areas of success, challenge, and controversy. These discussions led the group to propose an ambitious research agenda and were the basis for the ongoing international collaboration to advance and harmonize outcome assessment in vasculitis7.
The OMERACT 8 meeting in 2006 focused on specific domains that could be considered for inclusion in a core set, such as disease activity, disease-associated damage, and identification of new domains to explore, such as patient-reported outcomes. To collect data directly relevant to this research agenda, new projects were planned and developed8.
The OMERACT 9 meeting was preceded by evaluation of data from these new projects relating to domains of illness studied in vasculitis, including disease activity and damage assessment, as well as newer domains of importance identified at OMERACT 8, such as multidimensional health-related quality of life and patient-reported ratings of the burden of vasculitis. Although there is ongoing work in creating validated outcome measures for large-vessel vasculitis (e.g., giant cell arteritis and Takayasu’s arteritis), the focus of the OMERACT group has been on outcome measures in small-vessel vasculitis. This focus is the result of a combination of the higher prevalence of small-vessel vasculitides and the availability of a large set of outcome data from randomized clinical trials for antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) [Wegener’s granulomatosis (WG) and microscopic polyangiitis]. However, many of the constructs and domains being studied in small-vessel vasculitis, as well as specific data elements, are also applicable to the study of large-vessel disease.
The overall goal of the OMERACT Vasculitis Working Group is to create a core set of outcome measures for vasculitis, each of which fulfills the OMERACT filter of truth, discrimination, and feasibility9, and to identify additional domains requiring further research; these additional domains may eventually contribute to, or complement, the core set. This article will (a) summarize the current status of the group’s work in 4 key domains under consideration for inclusion into the core set; (b) report on the feedback from the OMERACT community of outcomes researchers, clinicians, patients, regulatory officials, biopharmaceutical industry executives, and government research administrators; and (c) outline both the current thinking about the levels of validation for the key domains of interest (Figure 1) and the updated research agenda (Table 1).
Figure 1.
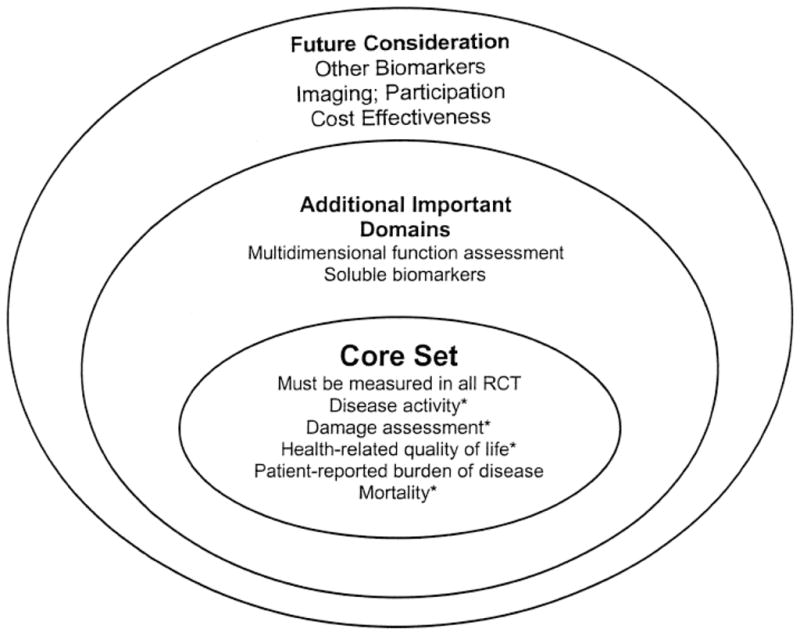
Domains of small-vessel vasculitis for study in clinical trials. *At least one instrument available for the domain has met the OMERACT filter9. RCT: randomized controlled trials.
Table 1.
Research agenda for the OMERACT Vasculitis Working Group.
| Domain | Issue | Potential Solution |
|---|---|---|
| Health-Related Quality of Life (HRQOL) | Usefulness of the SF-36 as an outcome measure for AAV | Analysis of SF-36 from randomized controlled trials and observational cohorts |
| Role of organ-specific instruments | Pilot such instruments in ongoing studies and compare to generic tool such as SF-36; evaluate addition of organ-specific tools to SF-36 | |
| Individual domain vs composite scores | Evaluate scoring approaches in ongoing studies | |
| Potential use of HRQOL data for health economics assessment | Evaluate EuroQol-5D in ongoing studies | |
| Patient-reported outcomes (PRO) | Item generation and selection | Patient focus groups |
| PROMIS NIH Toolbox for certain items, such as fatigue, sleep | ||
| Item reduction, item weighting, and overall revision of the instrument | Delphi exercise with patients with vasculitis | |
| Need for vasculitis type-specific instruments/modules | Pilot general vasculitis instrument and vasculitis type-specific instrument | |
| Validation of new PRO instrument | Use in randomized controlled trials | |
| Comparison of PRO and HRQOL | Compare PRO and HRQOL data within same patient cohort | |
| Damage | Item selection | Compile items from data in ongoing studies |
| Item weighting | Weighting informed expert rankings and trial data | |
| Need for vasculitis type-specific instrument | Comparison of VDI to AVID in ongoing studies; data from CDA | |
| Disease activity | Weighting of items | Determine data-driven weights using global assessment scores, medium-term outcomes (3–12 months), physician treatment decisions, relapse risk, death |
| Discrepancies between instruments | Harmonization, standardization of tools, new single instrument | |
| Definition of disease states (e.g., remission, relapse, low- disease activity, high-disease activity) | Longterm cohort studies | |
| Inclusion of other forms of vasculitis | Disease-specific evaluations; generic vasculitis instruments plus vasculitis type-specific modules |
SF-36: Medical Outcomes Survey Short Form 36 Questionnaire; PROMIS: Patient-Reported Outcomes Measurement System; NIH: US National Institutes of Health; VDI: Vasculitis Damage Index; AVID: ANCA Vasculitis Index of Damage; CDA: Combined Damage Assessment.
DISEASE ACTIVITY ASSESSMENT IN VASCULITIS
Disease activity is recognized as a central domain within the core set of outcome measures for clinical trials in systemic vasculitis. Development of disease activity assessment is more advanced than that of other domains in vasculitis. Measuring the degree of vasculitis disease activity has been achieved using structured clinical evaluation tools that catalogue abnormalities in multiple organ systems and derive numeric scores. The 3 main activity measurement instruments are the Birmingham Vasculitis Activity Score (BVAS)10,11 and its derivatives, the BVAS for WG (BVAS/WG)12, and the BVAS 2003 (reviewed in Flossmann, et al13). For all 3 versions of the BVAS, demonstration of validity, sensitivity to change, and reproducibility has been made10–13 or is under way14, and all have met, to various degrees, the OMERACT filter of truth, discrimination, and feasibility9.
While BVAS and BVAS 2003 were designed as tools applicable in a wide variety of forms of vasculitis, BVAS/WG specifically focuses on WG. Other between-instrument differences are in the number and weighting of rated, “pre-specified” disease manifestations. Moreover, while all versions offer an option to include open-ended/free-text “other” disease manifestations, only BVAS/WG allows for scoring of such manually added items. However, the results of a comparative study on WG and microscopic polyangiitis suggested that BVAS, BVAS 2003, and BVAS/WG ratings are highly correlated with each other and with a physician’s global assessment of disease activity15.
A recent study used data on BVAS/WG collected in a clinical trial of WG to examine various strategies for item selection and weighting to find the most appropriate methods for disease activity scoring16. This study concluded that the tool could be improved by omitting items that were rarely or never used, and by creating new items corresponding to disease manifestations frequently added in the free-text “other” section. A computational method generated a broader range of item weights compared to the expert opinion-based approach used in BVAS, BVAS 2003, and BVAS/WG.
The OMERACT Vasculitis Working Group recognizes that a number of challenges remain to be addressed in disease activity assessment of vasculitis. Although the various tools are conceptually quite similar, harmonization of disease activity assessment is a longterm aim, including using a single instrument designed based on data-driven item selection and weighting. Further, while all 3 instruments allow for separation of inactive and active disease, no cutoff values have been defined to differentiate between varying states of low, medium, or high disease activity. Finally, while the performance of these disease activity instruments has been evaluated extensively for WG and microscopic polyangiitis, there are only limited data available for other forms of vasculitis. Whether or not disease activity assessment tools should be disease-specific or remain universally applicable to all forms of vasculitis is still a matter of debate. Large datasets from completed and ongoing clinical trials and longitudinal studies will allow investigators to address these areas of research, each of which was endorsed by OMERACT attendees as important additions to the research agenda.
DAMAGE ASSESSMENT IN VASCULITIS
While the patient perspective on the burden of disease is important, significant damage from vasculitis can be present that may not have immediate subjective consequences for the patient, and yet is important to document. The goals of treatment are at least in part to avoid the accumulation of such damage. Thus, a physician’s perspective on damage is an additional key domain of interest in vasculitis. Damage denotes the consequences of vasculitis or its treatment that do not respond to immunosuppression17. For the patient, damage represents the chronic burden of disease. For the clinician, damage represents the manifestations of vasculitis that do not merit prolonged medical treatment.
The concept of damage is also an important outcome measure for clinical trials18. Because most vasculitis flares can be treated effectively in the short term, achievement of remission by itself may not be an adequate endpoint. Damage indicates the cost of achieving remission, aggregating the chronic morbidity that occurs after the disease flare has been treated. If 2 drugs both induce remission within 6 months of initiating therapy, the drug that also prevents the development of damage is superior. Damage thus represents a unique domain that is not captured by other domains under study for vasculitis, including disease activity, quality of life, and other patient-reported outcomes.
The Vasculitis Damage Index (VDI) was developed to record the diverse forms of damage that can occur in association with all forms of systemic vasculitis19. The VDI serves to ensure that patients are assessed systematically for damage in a way that is both reproducible and analyzable, yielding a summary “damage score.” The VDI performs well, but the OMERACT Vasculitis Working Group identified areas for reexamination, focusing on 3 issues related to damage assessment in AAV: content, weighting, and disease specificity7.
Content
Through expert consensus, the Combined Damage Assessment index (CDA) was developed and comprises 130 candidate items of damage8. The CDA is being applied to patients with WG or microscopic polyangiitis, and analysis of these data will help refine the index by identifying which items of damage should be included in future versions of the CDA.
Weighting
The VDI weights all forms of damage equally and thus each item contributes equally to the damage index score. To address the need for weighting, 50 experts in vasculitis were asked to rank items on the CDA using an 11-point Likert scale (from 0 to 10). These ranks will be used to create a candidate-weighting schema20. Future studies will determine if the use of weights improves the correlation between the damage index score and other outcomes that correlate with damage, such as mortality and quality of life.
Disease specificity
Because the VDI was designed to be broadly applicable to all forms of vasculitis, the instrument may not accurately record forms of damage specific to WG and microscopic polyangiitis. To test this hypothesis, the ANCA-associated Vasculitis Index of Damage (AVID) was developed21. AVID and the VDI are being applied simultaneously to 200 patients enrolled in a clinical trial, and these data should allow for testing of the hypothesis that, compared to the VDI, a disease-specific instrument has greater discriminant power for determining the overall prevalence of damage as well as changes in damage over time.
Feedback at OMERACT 9 reinforced the supposition that the concept of damage was indeed an important domain that should be considered for inclusion in the core set once an instrument has passed the OMERACT filter. Future work will include ongoing drafting and validation of a damage index.
MEASURING HEALTH-RELATED QUALITY OF LIFE IN VASCULITIS
The patient perspective has become increasingly recognized in OMERACT as an important aspect to be identified in assessing disease outcomes. One such patient-reported outcome (PRO), health-related quality of life (HRQOL), has been identified by the OMERACT Vasculitis Working Group as a key domain of interest to measure in vasculitis that should be considered for core set inclusion. HRQOL is the component of a patient’s quality of life that is thought to be attributable to their health status, rather than their education or socioeconomic status22. AAV has been transformed from a disease of almost certain mortality to one of chronic morbidity, thus making HRQOL an important outcome to assess. HRQOL of patients with AAV is likely adversely affected by disease activity, disease-related damage, and medication side effects. Measures of HRQOL may be influenced by many factors, and thus may serve as an overall measure of treatment efficacy. Importantly, HRQOL is only moderately associated with levels of disease activity or damage in longitudinal studies in other systemic inflammatory diseases23,24 and, therefore, should be considered for separate assessment.
The OMERACT Vasculitis Working Group has set goals of: (a) optimally defining which measures adequately assess HRQOL in AAV; (b) determining the best method to report HRQOL scores to adequately reflect patients’ experiences with vasculitis; and (c) assessing the factors, including therapies, that influence HRQOL in vasculitis. HRQOL in vasculitis has typically been measured to date using the generic Medical Outcomes Survey Short-Form 36 Questionnaire (SF-36)2–4. However, it is not clear that generic tools such as the SF-36 are able to measure important aspects of HRQOL in AAV with adequate precision and sensitivity. For example, patients with AAV may have manifestations such as fatigue and sino-nasal symptoms that influence HRQOL but are incompletely identified by a generic instrument such as the SF-36. Generic measures have been shown to be less responsive than disease-specific instruments in several clinical trials25–28. Thus, additional symptom- or disease-specific instruments may be necessary to improve our understanding of the effect of AAV and its therapies on HRQOL29. Further, because AAV is a complicated multisystem disease, the reporting of individual domains of HRQOL are best reported separately rather than as composite scores. The addition of health utility scores such as the EuroQol 5D may provide important information for overall summaries of health that can be incorporated into health economic assessments30,31.
The OMERACT 9 attendees agreed that HRQOL was an important domain meriting further research that could enable its inclusion as a core set domain. The optimal use of the SF-36 will be explored using data from previous clinical trials in North America and Europe as well as planned cross-sectional and longitudinal cohort studies. The role of organ-or symptom-specific tools (e.g., fatigue severity scores and sino-nasal symptom scores) will also be studied. The results of such studies will inform clinicians and researchers on the determinants of HRQOL and domains that may be particularly important measures of the efficacy of future therapies in AAV.
MEASURING PATIENT-REPORTED OUTCOMES IN VASCULITIS
Consideration of HRQOL as an important domain of interest contrasts with the historical approach of outcome assessment in vasculitis, which was primarily based on items that physicians considered relevant in terms of disease activity, disease extent, and damage. However, beyond HRQOL, little is known and published about the patient’s perspective of burden of disease in systemic vasculitis. Despite the development and improvement of physician-administered instruments measuring disease extent, activity, and damage, there are no vasculitis-specific instruments covering patient-estimated burdens of disease. Because patients’ subjective experiences are important, particularly in making treatment decisions, such patient-reported burden of disease should be included in the outcome measurement process. It was identified as an important component for measurement at the OMERACT 8 conference. Therefore the Vasculitis Working Group sought to develop an instrument that assessed the patient’s view of the burden of disease, a concept that was felt likely to be an additional domain separate from, but complementing, HRQOL.
After a systematic literature review, analysis of existing data, and input from a panel of physicians with expertise in the care of vasculitis, a questionnaire was developed to collect data regarding patients’ view of vasculitis-related health outcomes or burden of disease. Several hundred patients with various forms of vasculitis from 3 countries in North America and Europe were surveyed with this preliminary instrument. Data included details on patient demographics, clinical data, and ratings on Likert scales of 40 vasculitis-related items as well as open text comments. Preliminary analysis revealed, perhaps not surprisingly, that patients’ views of the vasculitis-related health outcomes differ substantially from physicians’ views32.
The discussion and feedback at OMERACT 9 to this PRO project was extremely positive. The group felt that PRO was an important domain to be included in any proposed core set of outcomes in vasculitis. There was strong consensus to consider collecting data within focus groups of patients with various types of vasculitis to further identify and select items of importance. Another option is to utilize the Patient-Reported Outcomes Measurement System (PROMIS; http://www.nihpromis.org) to add additional items, particularly those regarding fatigue and sleep. It was also suggested that persons with specific organ manifestations may be best able to identify the specific burdens associated with those manifestations (e.g., renal failure). The possibility of conducting a patient Delphi process for item selection was also discussed. These steps could be informative for item reduction, item weighting, and overall revision of the instrument. Further consideration will also be given to whether vasculitis type-specific instruments/modules for this relatively heterogeneous group of diseases are needed for PRO assessment. Future work will include establishing whether such a PRO instrument provides additional information separate from, but complementary to, HRQOL instruments in vasculitis.
OTHER DOMAINS AND AREAS OF INTEREST
As future research sheds light on the utility of biologic/biochemical, imaging, or other biomarkers in vasculitis, these will need to undergo rigorous evaluation under the OMER-ACT filter to determine whether they should be included as core set measures. Further, as disease management focuses on limiting patient burden of disease, participation and function are additional important domains that will need to be put on the research agenda. As the core set is further developed, the group will also explore the utility of applying the principles of the International Classification of Functioning (ICF) for documenting the manifestations of disease33.
SUMMARY
The OMERACT Vasculitis Working Group has made significant progress since its initial participation in OMERACT 7. Based on the work to date, Figure 1 depicts a framework for the domains of interest identified as being important in vas-culitis outcomes research. Through the OMERACT process, consensus has been reached to move forward with research on key domains and subsequently propose a core set of domains for inclusion in clinical studies of vasculitis (the inner circle in Figure 1). Table 1 outlines the specific research agenda for these domains. Other domains of interest are also felt to be important, but further work is required to determine whether such domains should be included in the core set, or whether they would complement the information obtained from the core set (the second circle in Figure 1). Finally, future research agendas will explore other domains, which may lead to additional useful outcome measures that may add to or complement the core set (the outer circle in Figure 1). The OMERACT Vasculitis Working Group plans to address the specified research agenda in Table 1 in an effort to have the initial 4 key domains, which were identified at OMERACT 8 and endorsed at OMERACT 9, evaluated through the OMERACT filter for inclusion in a core set of vasculitis outcomes at a future OMERACT meeting.
Acknowledgments
The OMERACT Vasculitis Working Group is supported by the Vasculitis Clinical Research Consortium through The National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (UO1 AR51874) and the National Center for Research Resources/NIH: U54 RR01949703. The Vasculitis Clinical Research Consortium is part of the NIH Rare Diseases Clinical Research Network (www.RareDiseasesNetwork.org/vcrc).
The authors thank Dr. John Kirwan and Dr. Sarah Hewlett for their advice on the patient-reported outcomes project, Dr. Vibeke Strand for her ongoing help and advice, especially during the OMERACT 9 meeting, and the many patients and research colleagues within OMERACT who have provided crucial feedback.
References
- 1.Hoffman GS, Cid MC, Hellmann DB, Guillevin L, Stone JH, Schousboe J, et al. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum. 2002;46:1309–18. doi: 10.1002/art.10262. [DOI] [PubMed] [Google Scholar]
- 2.Jayne D, Rasmussen N, Andrassy K, Bacon P, Tervaert JW, Dadoniene J, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med. 2003;349:36–44. doi: 10.1056/NEJMoa020286. [DOI] [PubMed] [Google Scholar]
- 3.Wegener’s Granulomatosis Etanercept Trial (WGET) Research Group. Etanercept plus standard therapy for Wegener’s granulomatosis. N Engl J Med. 2005;352:351–61. doi: 10.1056/NEJMoa041884. [DOI] [PubMed] [Google Scholar]
- 4.De Groot K, Rasmussen N, Bacon PA, Tervaert JW, Feighery C, Gregorini G, et al. Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2005;52:2461–9. doi: 10.1002/art.21142. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman GS, Cid MC, Rendt-Zagar KE, Merkel PA, Weyand CM, Stone JH, et al. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med. 2007;146:621–30. doi: 10.7326/0003-4819-146-9-200705010-00004. [DOI] [PubMed] [Google Scholar]
- 6.Jayne DR, Gaskin G, Rasmussen N, Abramowicz D, Ferrario F, Guillevin L, et al. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol. 2007;18:2180–8. doi: 10.1681/ASN.2007010090. [DOI] [PubMed] [Google Scholar]
- 7.Merkel PA, Seo P, Aries P, Neogi T, Villa-Forte A, Boers M, et al. Current status of outcome measures in vasculitis: focus on Wegener’s granulomatosis and microscopic polyangiitis. Report from OMERACT 7. J Rheumatol. 2005;32:2488–95. [PubMed] [Google Scholar]
- 8.Seo P, Luqmani RA, Flossmann O, Hellmich B, Herlyn K, Hoffman GS, et al. The future of damage assessment in vasculitis. J Rheumatol. 2007;34:1357–71. [PubMed] [Google Scholar]
- 9.Boers M, Brooks P, Strand CV, Tugwell P. The OMERACT filter for Outcome Measures in Rheumatology. J Rheumatol. 1998;25:198–9. [PubMed] [Google Scholar]
- 10.Luqmani RA, Bacon PA, Moots RJ, Janssen BA, Pall A, Emery P, et al. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM. 1994;87:671–8. [PubMed] [Google Scholar]
- 11.Luqmani RA, Exley AR, Kitas GD, Bacon PA. Disease assessment and management of the vasculitides. Baillieres Clin Rheumatol. 1997;11:423–46. doi: 10.1016/s0950-3579(97)80052-0. [DOI] [PubMed] [Google Scholar]
- 12.Stone JH, Hoffman GS, Merkel PA, Min YI, Uhlfelder ML, Hellmann DB, et al. A disease-specific activity index for Wegener’s granulomatosis: modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides (INSSYS) Arthritis Rheum. 2001;44:912–20. doi: 10.1002/1529-0131(200104)44:4<912::AID-ANR148>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 13.Flossmann O, Bacon P, de Groot K, Jayne D, Rasmussen N, Seo P, et al. Development of comprehensive disease assessment in systemic vasculitis. Ann Rheum Dis. 2007;66:283–92. doi: 10.1136/ard.2005.051078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukhtyar C, Lee R, Brown D, Carruthers D, Dasgupta B, Dubey S, et al. Modification and validation of the Birmingham Vasculitis Activity Score (Version 3) Ann Rheum Dis. 2008 Dec 3; doi: 10.1136/ard.2008.101279. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Merkel PA, Cuthbertson D, Hellmich B, Hoffman GS, Jayne DJ, Kallenberg CGM, et al. Comparison of disease activity measures for ANCA-associated vasculitis. Ann Rheum Dis. 2009;68:103–6. doi: 10.1136/ard.2008.097758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahr AD, Neogi T, Lavalley MP, Davis JC, Hoffman GS, McCune WJ, et al. Assessment of the item selection and weighting in the Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis. Arthritis Rheum. 2008;59:884–91. doi: 10.1002/art.23707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seo P. Wegener’s granulomatosis: managing more than inflammation. Curr Opin Rheumatol. 2008;20:10–6. doi: 10.1097/BOR.0b013e3282f18bef. [DOI] [PubMed] [Google Scholar]
- 18.Seo P, Min YI, Holbrook JT, Hoffman GS, Merkel PA, Spiera R, et al. Damage caused by Wegener’s granulomatosis and its treatment: prospective data from the Wegener’s Granulomatosis Etanercept Trial (WGET) Arthritis Rheum. 2005;52:2168–78. doi: 10.1002/art.21117. [DOI] [PubMed] [Google Scholar]
- 19.Exley AR, Bacon PA, Luqmani RA, Kitas GD, Gordon C, Savage CO, et al. Development and initial validation of the Vasculitis Damage Index for the standardized clinical assessment of damage in the systemic vasculitides. Arthritis Rheum. 1997;40:371–80. doi: 10.1002/art.1780400222. [DOI] [PubMed] [Google Scholar]
- 20.Seo P, Jayne D, Luqmani R, Merkel PA. Assessment of damage in vasculitis: Expert ratings of damage. Rheumatology. 2009;48:823–7. doi: 10.1093/rheumatology/kep103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seo P, Merkel PA, Specks U, Hoffman GS, Langford CA, Spiera R, et al. Damage in ANCA-associated vasculitis: Preliminary evidence for the ANCA-associated Vasculitis Index of Damage (AVID) [abstract] Arthritis Rheum. 2006;54 (Suppl):S487. [Google Scholar]
- 22.Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118:622–9. doi: 10.7326/0003-4819-118-8-199304150-00009. [DOI] [PubMed] [Google Scholar]
- 23.Fortin PR, Abrahamowicz M, Neville C, du Berger R, Fraenkel L, Clarke AE, et al. Impact of disease activity and cumulative damage on the health of lupus patients. Lupus. 1998;7:101–7. doi: 10.1191/096120398678919813. [DOI] [PubMed] [Google Scholar]
- 24.Alarcon GS, McGwin G, Jr, Uribe A, Friedman AW, Roseman JM, Fessler BJ, et al. Systemic lupus erythematosus in a multiethnic lupus cohort (LUMINA). XVII. Predictors of self-reported health-related quality of life early in the disease course. Arthritis Rheum. 2004;51:465–74. doi: 10.1002/art.20409. [DOI] [PubMed] [Google Scholar]
- 25.Tandon PK, Stander H, Schwarz RP., Jr Analysis of quality of life data from a randomized, placebo-controlled heart-failure trial. J Clin Epidemiol. 1989;42:955–62. doi: 10.1016/0895-4356(89)90160-1. [DOI] [PubMed] [Google Scholar]
- 26.Tugwell P, Bombardier C, Buchanan WW, Goldsmith C, Grace E, Bennett KJ, et al. Methotrexate in rheumatoid arthritis. Impact on quality of life assessed by traditional standard-item and individualized patient preference health status questionnaires. Arch Intern Med. 1990;150:59–62. doi: 10.1001/archinte.150.1.59. [DOI] [PubMed] [Google Scholar]
- 27.Chang SW, Fine R, Siegel D, Chesney M, Black D, Hulley SB. The impact of diuretic therapy on reported sexual function. Arch Intern Med. 1991;151:2402–8. [PubMed] [Google Scholar]
- 28.Laupacis A, Wong C, Churchill D. The use of generic and specific quality-of-life measures in hemodialysis patients treated with erythropoietin. The Canadian Erythropoietin Study Group. Control Clin Trials. 1991;12 (Suppl 4):168S–79S. doi: 10.1016/s0197-2456(05)80021-2. [DOI] [PubMed] [Google Scholar]
- 29.Guyatt GH, King DR, Feeny DH, Stubbing D, Goldstein RS. Generic and specific measurement of health-related quality of life in a clinical trial of respiratory rehabilitation. J Clin Epidemiol. 1999;52:187–92. doi: 10.1016/s0895-4356(98)00157-7. [DOI] [PubMed] [Google Scholar]
- 30.Hurst NP, Kind P, Ruta D, Hunter M, Stubbings A. Measuring health-related quality of life in rheumatoid arthritis: validity, responsiveness and reliability of EuroQOL (EQ-5D) Br J Rheumatol. 1997;36:551–9. doi: 10.1093/rheumatology/36.5.551. [DOI] [PubMed] [Google Scholar]
- 31.Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ. 1998;316:736–41. doi: 10.1136/bmj.316.7133.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herlyn K, Seo P, Hellmich B, Merkel PA. Patient-reported outcome assessment in vasculitis provides important data and a unique perspective [abstract] Clin Exp Rheumatol. 2007;25:S–117. doi: 10.1002/acr.20276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stucki G, Boonen A, Tugwell P, Cieza A, Boers M. The World Health Organisation International Classification of Functioning, Disability and Health: a conceptual model and interface for the OMERACT process. J Rheumatol. 2007;34:600–6. [PubMed] [Google Scholar]


