Abstract
Glutamate cysteine ligase (GCL) deficiency is a rare autosomal recessive trait that compromises production of glutathione, a critical redox buffer and enzymatic cofactor. Patients have markedly reduced levels of erythrocyte glutathione, leading to hemolytic anemia and in some cases, impaired neurological function. Human glutamate cysteine ligase is a heterodimer comprised of a catalytic (GCLC) and a regulatory subunit (GCLM), which catalyzes the initial rate limiting step in glutathione production. Four clinical missense mutations have been identified within GCLC: Arg127Cys, Pro158Leu, His370Leu, and Pro414Leu. Here, we have evaluated the impacts of these mutations on enzymatic function in vivo and in vitro to gain further insights into the pathology. Embryonic fibroblasts from GCLC null mice were transiently transfected with wild-type or mutant GCLC and cellular glutathione levels were determined. The four mutant transfectants each had significantly lower levels of glutathione relative to wild-type, with the Pro414Leu mutant being most compromised. The contributions of the regulatory subunit to GCL activity were investigated using an S. cerevisiae model system. Mutant GCLC alone could not complement a glutathione-deficient strain and required the concurrent addition of GCLM to restore growth. Kinetic characterizations of the recombinant GCLC mutants indicated that the Arg127Cys, His370Leu, and Pro414Leu mutants have compromised enzymatic activity that can largely be rescued by the addition of GCLM. Interestingly, the Pro158Leu mutant has kinetic constants comparable to wild-type GCLC, suggesting that heterodimer formation is needed for stability in vivo. Strategies that promote heterodimer formation and persistence would be effective therapeutics for the treatment of GCL deficiency.
Glutathione (L-γ-glutamyl-L-cysteinyl-glycine; GSH) is an abundant tripeptide critical for oxidative stress response and detoxification of xenobiotics. It contributes to signalling pathways as well as the regulation of enzymatic activity by altering the accessibility of protein sulfhydryl groups (1, 2). In addition to serving as a redox buffer, glutathione participates in the storage and transport of cysteine (3) and select heavy metals (4). The diverse functions of glutathione are necessary for normal cellular processes (3, 5–7), and disruption of glutathione homeostasis is associated with numerous disease states (8, 9).
Glutathione is synthesized in humans by the sequential action of glutamate cysteine ligase (GCL) and glutathione synthetase (10). GCL catalyzes the formation of an amide linkage between the γ-carboxyl group of glutamate and cysteine to form γ-glutamylcysteine, which is the rate-limiting step in glutathione biosynthesis. In vertebrate systems, GCL typically exists as a heterodimer consisting of a catalytic subunit (73 kDa) and a modifier subunit (31 kDa). The catalytic subunit (GCLC) contains the active site while the modifier subunit (GCLM) participates in regulation of enzymatic activity, enhancing catalytic efficiency upon heterodimer formation (11). GCLC null mice are embryonic lethal (7, 12) while GCLM null mice are viable, producing low levels of glutathione (10%–20% of normal levels), consistent with a regulatory role (6).
Several reports of hereditary GCL deficiency in humans have been communicated (13–17). The disease is marked by hemolytic anemia, low levels of erythrocyte glutathione (typically < 10% of normal levels), and in some cases, neurological disability. In the past decade, specific mutations in the coding region of GCLC have been identified in patients with hereditary GCL deficiency. Of the four clinical mutants identified thus far, three result in a leucine substitution: proline 158 (Pro158Leu), histidine 370 (His370Leu), or proline 414 (Pro414Leu). In each case, GCL activity was markedly reduced in erythrocyte samples with corresponding reductions in glutathione levels. However, the precise mechanism by which the mutation reduced enzymatic activity was not identified beyond speculation that each mutation may impact protein stability. A fourth mutation, resulting in the substitution of an arginine residue at position 127 with a cysteine residue (Arg127Cys), has been shown to directly impair enzymatic activity (14).
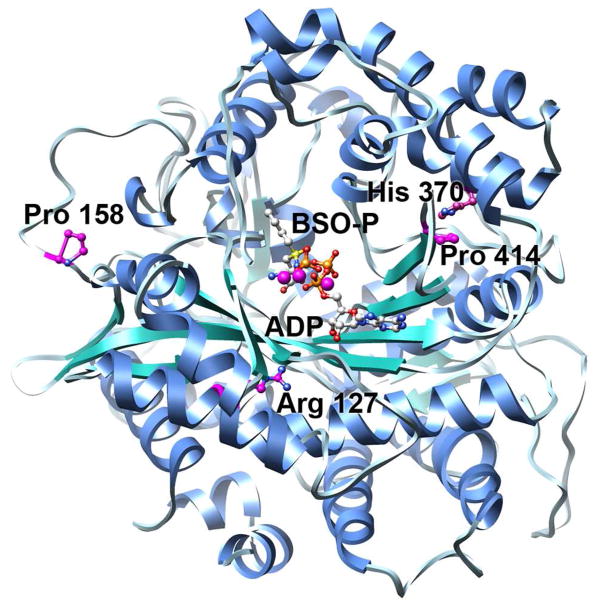
Recently, the crystal structure of the closely related Saccharomyces cerevisiae GCL was reported, allowing a homology model of human GCLC to be generated (Figure 1) (18). Examination of the model provided significant insights to the possible functions of each of these four residues. In the current study, we used GCLC null mouse embryonic fibroblasts (7) and S. cerevisiae devoid of glutamate cysteine ligase (Δgsh1) as model systems to further examine the impacts of these clinical mutations on glutathione production. In addition, we have kinetically characterized each of the human GCLC clinical mutants, either alone or in complex with human GCLM. The results of these studies demonstrate the impact of the clinical mutations responsible for hereditary GCL deficiency on the activity and stability of the enzyme in vitro and in vivo, as well as the critical role of the modifier subunit in enhancing wild-type activity and restoring mutant activity.
Figure 1. Homology model of human GCLC reveals the locations of the four clinical mutations.
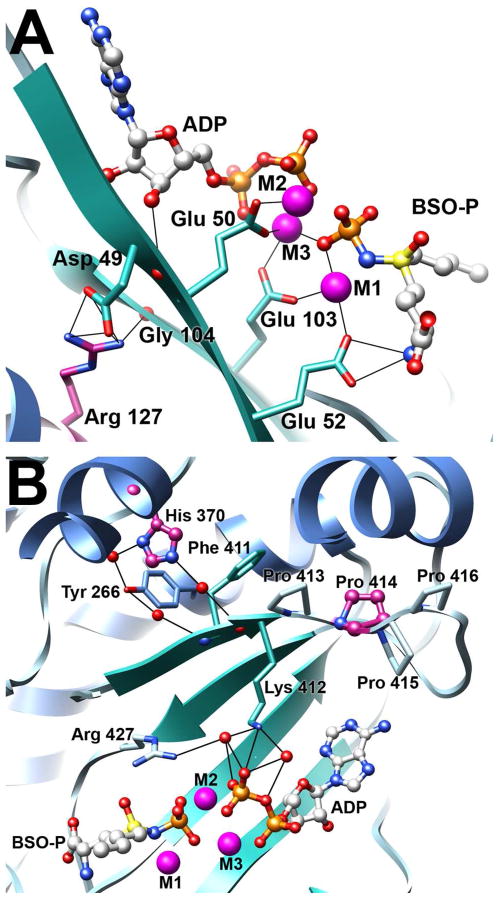
The overall model of hGCLC is shown in ribbon representation with β-strands colored in green, α-helices in blue, and loop regions in grey. ADP and the transition state analogue, phosphorylated BSO (BSO-P), were docked into the previously described model and are shown in ball and stick representation. Carbon atoms are colored in grey, oxygen atoms in red, sulfur atoms in yellow, nitrogen atoms in blue, phosphorus in orange, and magnesium atoms in purple. The locations of the four clinical mutations are highlighted, with carbon atoms colored in purple.
Experimental Procedures
Plasmid construction and manipulations
The coding sequence corresponding to GCLM (NM_002061.2) was amplified from a human cDNA library incorporating the appropriate restriction sites into the designed primers. For expression in S. cerevisiae, the insert was digested with BamH1 and Xho1 and ligated into a complementarily digested p416 ADH vector (19). The bacterial construct was generated using a pET28a vector (Novagen) and the restriction enzymes NheI and SalI. To generate expression vectors for human GCLC (NM_001498.3), the coding sequence was inserted into a p415 ADH (19), a, pCMV6-Entry [C-terminal Myc and DDK Tagged], or a pET24a (Novagen) vector for expression in S. cerevisiae, mouse embryonic fibroblasts, or E. coli, respectively. The point mutations, His370Leu, Pro158Leu, Pro414Leu, Arg127Cys, and Lys412Ala were introduced using the QuikChange site-directed mutagenesis kit (Stratagene) following the manufacturer’s instructions. All constructs were verified by sequencing (Eurofins MWG Operon).
Mammalian tissue culture
A GCLC null mouse embryonic fibroblast cell line, GCLC−/−, was generously provided by Dr. Zhengzheng Shi and cultured in glutathione-containing media to 60% to 70% confluency as described previously [7]. Cells were transiently transfected with wild-type GCLC and each clinical mutant using FuGeneHD transfection reagent (Roche). After 24 hours, the media was replaced with glutathione-deficient media. Cells were cultured an additional 24 hours, released with trypsin, and washed with cold 1X PBS. The cells were counted and evaluated by Western Blot, using polyclonal antibodies raised against full-length recombinant GCLC and GCLM (Covance). Protein levels were normalized against β-tubulin, using an Odyssey Infrared Imaging System (LI-COR) with Alexa Fluor 688 anti-mouse IgG (Invitrogen; β-tubulin) and IRDye 800 anti-rabbit IgG (Rockland; GCLC and GCLM) as secondary antibodies. Human GCLC was consistently observed as a doublet with an approximate molecular weight of 75 kDa. Both bands were used for quantification.
Determination of relative glutathione levels
To determine intercellular glutathione levels, cells were lysed by mechanical disruption (BulletBlender; Next Advance). After centrifugation, glutathione levels were quantified in the cleared lysates using an enzymatic recycling method (20) that employs glutathione reductase and 5,5′-dithio-bis-2-nitrobenzoic acid (Cayman Chemical Company). Glutathione levels were normalized to GCLC protein levels in the transfectants, with wild-type GCLC set equal to 1. Each transfection was performed in triplicate and the mean value and standard error for each mutant determined. Using Prism (Graphpad Software), an ANOVA one-way test with Dunnett’s Multiple Comparison Test was used to determine the statistical significance of the lower glutathione levels observed in the mutant transfectants.
Yeast Spotting Assay
A BY4741 haploid S. cerevisiae strain deficient in glutamate cysteine ligase (Δgsh1; Open Biosystems) requires exogenous glutathione for growth, thus providing a powerful screening tool for the formation of functional human GCL heterodimer. Δgsh1 were transformed with wild-type or mutant GCLC, with or without wild-type GCLM. An empty p416ADH vector was co-transformed with the GCLC alone samples. The transformations were performed as previously described (21). Briefly, Δgsh1 yeast were streaked on YPD (Yeast, Peptone, Dextrose, complete media.) plates and a single colony was selected and grown overnight in liquid culture. Cells were harvested by centrifugation, washed with sterile water followed by 1X Tris-EDTA/0.2M lithium acetate, and resuspended in 1X Tris-EDTA/0.2M lithium acetate. After incubation for 15 minutes at 30ºC, the plasmid constructs and 40% PEG3350/TE/0.1 lithium acetate were added to the cells. Cells were further incubated at 30ºC for 20 minutes, followed by heat shock at 42ºC for 20 minutes. The transformed cells were plated on uracil/leucine deficient plates (Synthetic Defined Media without leu and ura; SD-leu,ura). GCLC only tranformants grew poorly in the absence of glutathione and required the addition of 10 μM glutathione. GCLC with GCLM transformants were restreaked and grown overnight in uracil/leucine deficient media containing 1 μm glutathione. The next day, the yeast were diluted to 0.2, 0.1, and 0.04 OD600 nm and spotted on uracil/leucine deficient plates. Plates were monitored for growth for 72 hours. Data for the 36 hour time point are presented.
Protein expression and purification
The initial purification steps for recombinant human GCLC and GCLM were comparable to those described for S. cerevisiae GCL (18). The GCLC or GCLM construct was used to transform Escherichia coli Rosetta BL21(DE3) cells (Novagen). Cells were grown in 2xYT medium containing 50 μg ml−1 kanamycin at 30°C, and protein production was induced by the addition of 500 μM isopropyl-1-thio-β-D-galactopyranoside once the cells reached an A600nm of 0.6. After induction, cultures were grown overnight at 18 °C. Cells were harvested by centrifugation (20 min, 8000 × g, 4 °C) and stored at −80 °C. Frozen cell pellets were thawed, resuspended in lysis buffer (50 mM sodium phosphate buffer, pH 8.0, 300 mM NaCl, Halt Protease Inhibitor Single Use Cocktail (EDTA-free; Thermo Scientific), 10 mM imidazole), treated with lysozyme (1 mg/ml), and disrupted by sonication. Following centrifugation to remove cellular debris (30 min, 20,000 × g, 4 °C), the supernatant was loaded onto a HisTrap Chelating HP Column (GE Healthcare) equilibrated with lysis buffer. The column was washed to baseline (A280nm), and the remaining bound proteins were eluted using a linear imidazole gradient (10–250 mM). For GCLM, appropriate fractions were pooled and required no additional purification. GCLM was dialyzed against 20 mM Tris, pH 7.4 containing 2 mM DTT, concentrated (Amicon stirred cell 8050, 10-kDa cut-off), flash-frozen in liquid nitrogen, and stored at −80°C.
For GCLC, the protein was dialyzed against 50 mM Tris, pH 8.0 containing 2 mM DTT and subjected to ion-exchange chromatography using a HiTrap Q FF column (GE Healthcare). Pooled fractions were then dialyzed against 50 mM Tris pH 7.4, 300 mM NaCl, 2 mM DTT, and subjected to size-exclusion chromatography using a HiPrep 16/60 column Sephacryl S-100 HR column. After pooling the appropriate fractions, GCLC was dialyzed against 20 mM Tris, pH 7.4 containing 2 mM DTT, concentrated (Amicon stirred cell 8050, 10-kDa cut-off), flash-frozen in liquid nitrogen, and stored at −80°C. The holoenzyme complex was prepared by mixing purified GCLM and GCLC at a ratio of 2:1. Initially, the wild-type heterodimer was isolated from individual subunits by size-exclusion chromatography. Subsequent kinetic studies indicated that the crude 2:1 GCLM/GCLC mixture accurately reflects the catalytic efficiency of the purified heterodimer. Increasing the ratio to 4:1 GCLM/GCLC produced no additional enhancement in activity for wild-type or mutant GCLC, indicating that a slight molar excess of GCLM is sufficient for complete activation. This is consistent with previous studies of mouse GCLC (22).
Kinetic assays
Enzymatic activity was measured using an indirect assay that couples ADP production to NADH oxidation (23). Each wild-type and mutant GCLC was characterized alone or in complex with GCLM. The reaction mixture contained 20 mM MgCl2, 5 mM phosphoenolpyruvic acid, 0.2 mM NADH, and 4 units each of pyruvate kinase and lactate dehydrogenase (Sigma) in 1 ml of buffer (100 mM Tris, pH 8.0, 150 mM KCl). To determine the apparent kinetic constants, two of the substrates were held at or near saturating concentrations and the third was varied. Typically, substrates were held at concentration between 5 to 10-fold over the determined Km value. However, considerable substrate inhibition was observed with excess ATP present, similar to S. cerevisiae GCL (24), necessitating compromises with respect to achieving complete saturation. Thus, ATP concentrations were generally held at 3 to 5-fold over the determined Km value to limit substrate inhibition. Reduction in absorbance at 340 nm was followed over three minutes, and measurements were determined in triplicate. Michaelis-Menten kinetics were observed and apparent Km and Vmax values were determined using Prism (Graph Pad Software). To examine protein stability, human GCLC and GCLM/GCLC heterodimer (~ 1mg/mL) were incubated at 37ºC. At the indicated time, an aliquot was removed and enzymatic activity assayed. The mean and standard error for at least three replicates are presented as relative activity versus time.
Thermal Shift Assay
The ThermoFluor method was used to assess protein stability (25, 26). Each assay was contained 40 μl of PBS, 5 μl of a 250X Sypro Orange (Molecular Probes) and 5 μl of protein (~2 mg/ml) in a 200 μl thin-walled PCR tube sealed with an optical flat cap (Bio-Rad). The sample temperature was increased from 20ºC to 95ºC in 0.5ºC increments using a MyIQ Real-Time PCR Detection system to monitor total fluorescence. Data were initially plotted as arbitrary fluorescence units as a function of temperate. A first derivative curve of this data was generated to determine the Tm value. At least six replicates were performed for each condition. To assess the impact of ligand binding on enzyme stability, hGCLC was incubated with 100 μM L-buthionine-S,R-sulfoximine (BSO), 200 mM MgCl2, and 5 mM ATP at room temperature for 30 minutes, prior to the thermal shift assay. hGCLM with hGCLC were incubated at a ratio of 2:1 overnight at 4ºC to form the heterodimer complex.
Results
Recapitulating homozygous mutations using a GCLC-deficient mammalian model system
To begin examining the molecular basis of human GCLC deficiency, embryonic fibroblasts obtained from GCLC null mice (7), GCLC−/−, were transiently transfected with wild-type or mutant human GCLC. Relative GCLC expression was quantified by western analysis and normalized to β-tubulin. The hGCLC antibody, raised in rabbit against full length recombinant hGCLC, readily detected wild-type and mutant proteins, recognizing a doublet of approximately 75 kDa (Figure 2, inset). This doublet has been observed previously (27, 28), and several reports have suggested that GCLC may be post-translationally modified (27, 29, 30). Efforts to identify the precise identity of the two observed bands are ongoing. Initial studies suggest that the lower band is a 3–5 kDa truncation of the intact protein, as the engineered C-terminal Flag epitope was only detected in the upper band (Supplemental Figure 1). A comparable doublet was observed in HepG2 cells when detecting endogenous hGCLC (data not shown).
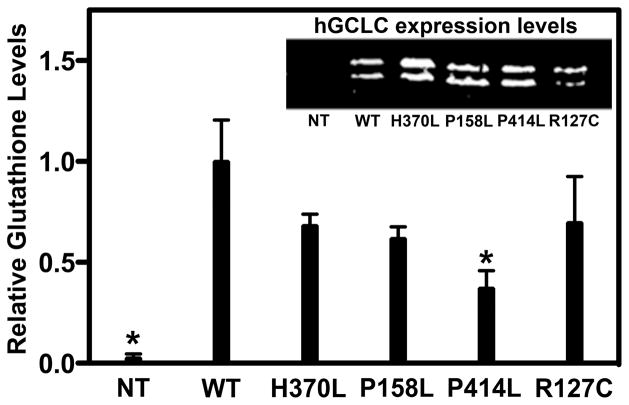
Figure 2. Glutathione production is reduced as a result of each of the four clinical mutations.
Embryonic fibroblasts obtained from GCLC null mice were transiently transfected with wild-type or mutant human GCLC. Inset. hGCLC expression was monitored via western analysis of whole cell lysates. Non-transfected cells (NT) served as a negative control. Main. Total glutathione levels were measured and normalized to protein levels as determined by western analysis (Inset), using β-tubulin (not shown) as a loading control. Mean ± SD values for three replicates are plotted. An ANOVA one-way test with Dunnett’s Multiple Comparison Test was used to analyze the data. Untransfected and Pro414Leu transfected cells had glutathione levels that were statistically reduced relative to wild-type GCLC, and are indicated by an asterisk.
The total glutathione concentrations for the wild-type and mutant transfectants were measured and normalized to GCLC expression. As seen in Figure 2, the mean glutathione level in untransfected cells was negligible. The nominal level observed was the result of the exogenously supplied glutathione needed for growth. The untransfected (<1%) and the Pro414Leu (~37%) transfected cells had glutathione levels that were statistically reduced relative to wild-type GCLC transfectants. Glutathione levels in the His370Leu, the Arg127Cys, and the Pro158Leu transfectants were consistently reduced with levels between 60% and 70% of wild-type. These reproducible but modest reductions are in contrast to more dramatic reductions observed in patient samples. This may reflect differences in cellular GCLC:GCLM ratios as discussed below.
Assessing the contributions of the regulatory subunit to GCL activity in an S. cerevisiae model system
In mammalian systems, GCLC and GCLM are differentially regulated and the relative ratio of the two proteins impacts overall glutathione production (11, 22, 31). The GCLC-null mouse embryonic fibroblast cell line has a basal level of GCLM, and the heterodimer is the major species observed when GCLC is transiently expressed (data not shown). To assess the contributions of GCLM to the overall efficiency of the GCLC mutants, it was necessary to find a system in which neither GCLC nor GCLM is expressed. Since a double null GCLC/GCLM mammalian cell line is not yet available, we identified S. cerevisiae as an alternative eukaryotic system. S. cerevisiae has a single gene, gsh1, that is responsible for the synthesis of γ-glutamylcysteine and is closely related to human GCLC (32, 33). The Δgsh1 strain is unable to produce glutathione and grows only when supplied with an exogenous source or when transfected with GCL. Thus, the Δgsh1 yeast line provides a reasonable system to examine the interplay between the GCLC and GCLM subunits.
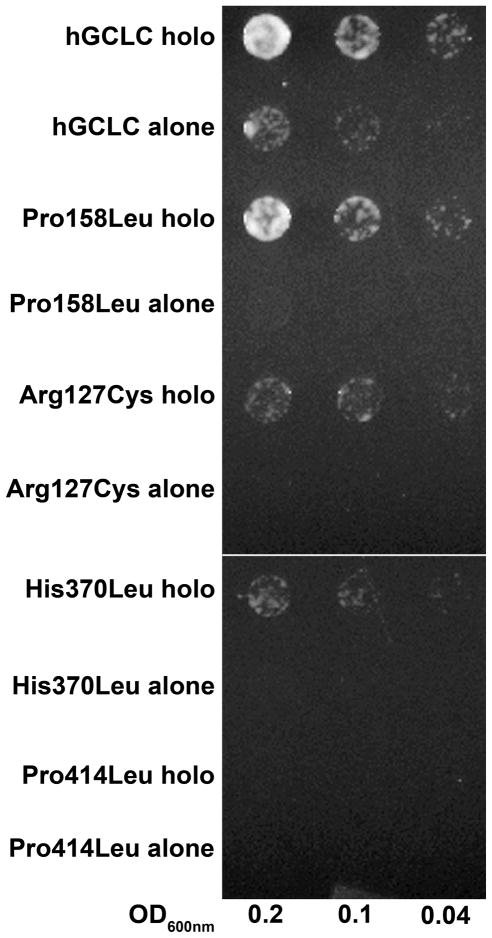
The Δgsh1 yeast line was transformed with either wild-type or mutant GCLC along with the GCLM expression construct or empty vector and plated on the appropriate selection media (SD-leu,ura). Overnight cultures were initiated but required the addition of 1μM glutathione to obtain a reasonable growth rate. Several dilutions of each overnight culture were spotted and monitored for growth. Although protein expression levels for wild-type and GCLC mutants were comparable, wild-type GCLC could only weakly rescue the Δgsh1 strain whereas none of the mutant GCLC transformants grew (Figure 3), consistent with impaired function. Co-expression of wild-type GCLC and GCLM resulted in robust growth as a result of increased glutathione synthesis (data not shown). Strikingly, co-expression of GCLM with Pro158Leu restored growth to levels comparable to wild-type holoenzyme. To a lesser degree, the addition of GCLM partially rescued the Arg127Cys and the His370Leu mutants. In contrast, the Pro414Leu mutant, either alone or co-expressed with GCLM, did not grow without the addition of reduced glutathione.
Figure 3. The modifer subunit enhances the activity of wild-type and mutant hGCLC.
An S. cerevisiae strain deficient in glutamate cysteine ligase (Δgsh1) was transformed with wild-type or mutant GCLC, with or without wild-type GCLM. An empty p416ADH vector was co-transformed with the GCLC alone samples. Transformants were restreaked, grown overnight in uracil/leucine deficient media, diluted to 0.2, 0.1, and 0.04 OD600 nm, spotted on uracil/leucine deficient plates, and grown for 36 hours as described in the text.
Characterization of recombinant human GCLC and GCLM
To further dissect the impacts of human GCLC mutations on enzymatic function, each protein was overexpressed in E. coli and purified to homogeneity (>95% pure based on SDS-PAGE). Typical yields were ~50 mg/L of bacterial culture for human GCLM and ~10 mg/L of bacterial culture for human GCLC. Similar yields were observed for each of the four clinical mutants, suggesting that overall protein folding was not dramatically compromised by the mutations. Apparent steady state kinetic constants for the catalytic subunit alone (Table 1) and the holoenzyme (Table 2) were determined using a coupled assay system that monitored the production of ADP as a measure of enzymatic activity.
Table 1.
Apparent kinetic constants for wild-type and mutant human GCLC
| Glutamate | Cysteine | ATP | ||||
|---|---|---|---|---|---|---|
| Km (mM) | Vmax (μmol min−1 mg−1) | Km (mM) | Vmax (μmol min−1 mg−1) | Km (mM) | Vmax (μmol min−1 mg−1) | |
| Wild-type | 1.14 ± .07 | 1.00 ± .019 | 0.10 ± .02 | 1.41 ± .057 | 2.68 ± .19 | 1.43 ± .047 |
| Pro158Leu | 0.68 ± .09 | 1.18 ± .005 | 0.08 ± .01 | 1.78 ± .047 | 3.57 ± .21 | 2.13 ± .061 |
| Arg127Cys | 1.38 ± .14 | .544 ± .019 | 0.05 ± .01 | .676 ± .014 | 4.47 ± .56 | .837 ± .057 |
| His370Leua | ND | ND | ND | ND | ND | ND |
| Pro414Leua | ND | ND | ND | ND | ND | ND |
Steady state kinetic constants could not be determined.
Table 2.
Apparent kinetic constants for wild-type and mutant human GCLC/GCLM complex
| Glutamate | Cysteine | ATP | ||||
|---|---|---|---|---|---|---|
| Km (mM) | Vmax (μmol min−1 mg−1) | Km (mM) | Vmax (μmol min−1 mg−1) | Km (mM) | Vmax (μmol min−1 mg−1) | |
| Wild-type | 0.46 ± .08 | 1.21 ± .061 | 0.07 ± .01 | 1.23 ± .043 | 0.44 ± .08 | 2.38 ± .260 |
| Pro158Leu | 0.65 ± .06 | 3.31 ± .085 | 0.17 ± .02 | 3.17 ± .085 | 0.30 ±.05 | 4.77 ± .440 |
| Arg127Cys | 1.38 ± .14 | 1.38 ± .052 | 0.12 ± .01 | 1.34 ± .028 | 0.50 ± .09 | 1.67 ± .170 |
| His370Leu | 0.44 ± .04 | 1.22 ± .028 | 0.16 ± .02 | 1.44 ± .047 | 0.26 ± .04 | 1.89 ± .166 |
| Pro414Leu | 0.77 ± .09 | .913 ± .038 | 0.17 ± .02 | .619 ± .024 | 0.82 ± .29 | 1.71 ± .426 |
Human GCLC had kinetic constants within the range of activities reported previously (11, 22, 34–36). The formation of the GCLM/GCLC heterodimer increased Vmax while significantly reducing the apparent Km value for ATP and to a lesser extent, the Km for glutamate (compare columns 1 and 3 in Tables 1 and 2) (22). Noticeable substrate inhibition was observed at increasing concentrations of cysteine or ATP, with estimated Ki values of 10 – 15 mM and 2 – 4 mM respectively (data not shown). The high concentrations of ATP used for Km determinations consistently impacted the observed enzymatic rates and accounted for the slight variability in the observed Vmax values. Therefore, all comparisons of enzymatic activity used Vmax values determined as a function of ATP concentration. The Pro158Leu mutant had modest impact on the enzymatic activity of the catalytic subunit, whereas the Arg127Cys GCLC mutant reduced Vmax by approximately 2-fold (Table 1). Both mutations slightly increased the apparent Km for ATP. Using standard assay conditions, the His370Leu and Pro414Leu mutants have activities that are <20% of wild-type GCLC. The His370Leu and the Pro414Leu mutants exhibited a near linear dependence of rate with respect to ATP concentration over the range tested (data not shown). Each mutant had an estimated Km value for ATP greater than 20 mM, precluding the accurate determination of the apparent kinetic constants for the remaining substrates. As discussed below, the His370Leu and the Pro414Leu mutants are likely to be important for the proper placement of a conserved active site lysine residue. Mutation of this lysine residue to an alanine (Lys412Ala) resulted in a protein with virtually undetectable enzymatic activity.
Formation of the activated heterodimer had differing effects on each of the four clinical mutants. For the Pro158Leu mutant, addition of GCLM increased Vmax by approximately 2-fold while significantly lowering the apparent Km for ATP, similar to wild-type enzyme (Table 2). Modest effects were observed with respect to Km values for the cysteine and glutamate substrates. For the Arg127Cys mutation, formation of the GCLM/GCLC heterodimer resulted in an increase in catalytic activity to a level comparable to wild-type enzyme. Similarly, the addition of GCLM to either His370Leu or Pro414Leu dramatically increased enzymatic activity to near wild-type levels (Table 2), primarily realized through dramatic decreases in the apparent Km values for ATP. As expected, the addition of GCLM to the Lys412Ala mutant did not significantly improve enzymatic function (data not shown). These in vitro kinetic characterizations suggest that formation of the GCLM/GCLC heterodimer would mitigate the detrimental effects of the GCLC clinical mutations.
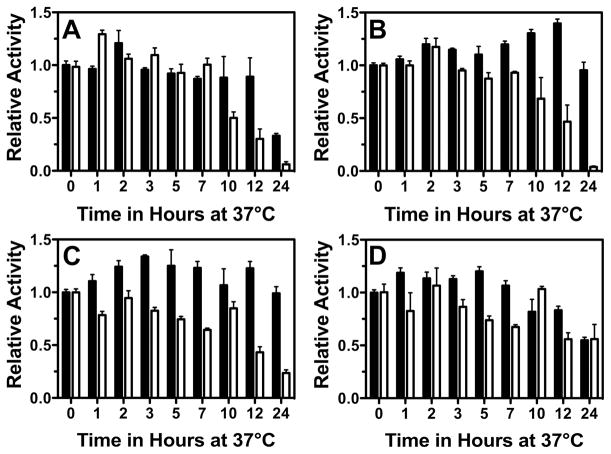
This concept was further supported by the enzymatic stability assays (Figure 5). For the catalytic subunit alone, the wild-type enzyme retains nearly full activity over a 24 hour period (Supplemental Figure 2). In contrast, the Pro414Leu and the His370Leu mutants lost approximately 50% and 75% activity respectively, whereas the Arg127Cys and Pro158Leu mutants lost more than 94% activity over the same time. Thus, although the Pro414Leu and the His370Leu mutants were compromised catalytically (Table 1), they were considerably more stable than the Arg127Cys and Pro158Leu mutants, which had significantly higher enzymatic activity. The addition of the modifier subunit dramatically increased protein stability for the His370Leu, Arg127Cys, and Pro158Leu mutants. However, formation of the Pro414Leu heterodimer provided only modest stabilization, primarily at early time points.
Figure 5. Clinical mutations decrease GCLC protein stability.
Wild-type and mutant GCLC were incubated at 37 ºC and relative enzymatic activity was monitored as a function of time in the absence (white bars) or presence (black bars) of hGCLM. During the 24 hour time course, wild-type enzyme did not show a significant loss of activity (Supplemental Figure 2). Panel A. Arg127Cys. Panel B. Pro158Leu. Panel C. His370Leu. Panel D. Pro414Leu.
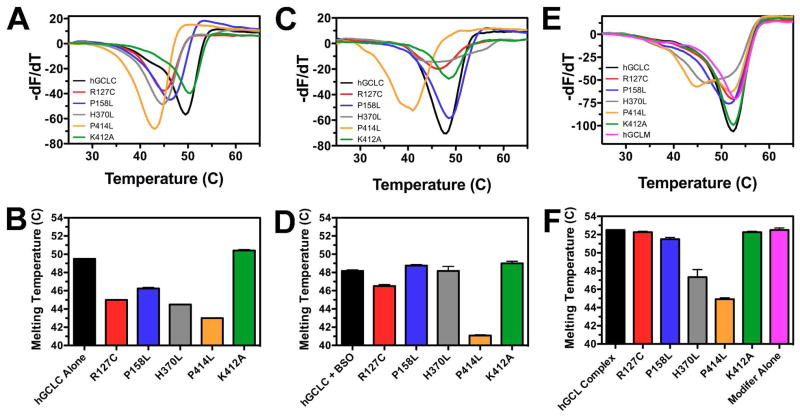
To further examine the impacts of the amino acid substitutions on protein stability, thermal shift assays were performed (Figure 6). The wild-type apoenzyme has a Tm of 49.5ºC, while each of the four clinical mutants has a decreased Tm value (Figure 6A and 6B). The Pro158Leu mutant, with a Tm of 46.3ºC, is the least compromised of the clinical mutants, followed by Arg127Cys (Tm = 45.0ºC). His370Leu and Pro414Leu have Tm values of 44.5ºC and 43.0ºC, respectively, consistent with significantly destabilized protein structures. The Lys412Ala mutant has a slightly elevated Tm (50.4ºC) relative to wild-type hGCLC, demonstrating that an act ive site mutation in and of itself does not necessary impact protein stability.
Figure 6. Human GCLC clinical mutants have reduced thermal stability that can be restored by ligand binding or heterodimer formation.
Using a ThermoFluor assay, thermal denaturation profiles of wild-type and mutant GCLC were collected, and then plotted as a first derivative curve to determine Tm values (Panels A, C, E). Data were obtained for the apoenzyme (Panel A), the BSO-inhibited enzyme (Panel C), and the GCLC/GCLM heterodimer (Panel E), with average Tm values plotted in Panels B, D, and F, respectively.
The impacts of ligand binding and heterodimer formation on thermal denaturation were also examined. As shown in Figures 6C and 6D, addition of the mechanism based inhibitor BSO, along with requisite MgCl2 and ATP, slightly but consistently reduced the Tm (48.2ºC) of wild-type hGCLC. However, formation of the BSO-inhibited enzyme complex significantly stabilized three of the four clinical mutants (Arg127Cys, Pro158Leu, and His370Leu), with each attaining Tm values compared to wild-type hGCLC. Only the Pro414Leu, the most catalytically compromised clinical mutant, was not stabilized by BSO addition (Tm = 41.5ºC). Similarly, formation of the heterodimer stabilized each clinical mutant (Figure 6E and 6F). Human GCLM (pink) had an observed Tm of 52.5ºC, which is slightly higher than that of wild-type hGCLC apoenzyme (49.5ºC). The wild-type heterodimer also exhibited a Tm of 52.5ºC, but a broader transition was observed. This suggests that method cannot resolve individual melting temperatures for the catalytic and regulatory subunit. Nonetheless, formation of the heterodimer with either the Arg127Cys or Pro158Leu mutant resulted in a thermal denaturation profile comparable to wild-type enzyme, demonstrating that complex formation stabilized the compromised mutants. The His370Leu and Pro414Leu mutants displayed two distinct transitions (Figure 6E). The His370Leu mutant exhibited a transition at 47.3ºC and at 51.2ºC, and Pro414Leu had transitions at 44.9ºC and 52.0ºC. Although each transition cannot be unambiguously assigned to a specific unfolding event, it is likely that in each case, the lower transition corresponds to the Tm of the catalytic subunit and the higher transition to that of the regulatory subunit. If that is the case, formation of the heterodimer elevated the Tm of His370Leu and Pro414Leu by 2.8ºC and 1.9ºC respectively.
Discussion
Hereditary GCL deficiency presents a unique opportunity to examine both the molecular mechanism of the disease and the importance of the modifier subunit to the activity of the holoenzyme. Glutathione plays a central role in protecting the cell from endogenous and exogenous threats through oxidative stress remediation and xenobiotic detoxification. The importance of GCL in catalyzing the first and rate-limiting step of glutathione biosynthesis is widely recognized. However, the molecular details of catalysis and the mechanism by which association of the catalytic and modifier subunits enhances catalytic efficiency remain unclear. In the current study, four GCLC missense mutations that result in hereditary glutathione deficiency have been characterized. This work details the molecular basis of this disease and demonstrates the critical role of the modifier subunit in rescuing the activity of severely comprised mutant catalytic subunits.
To provide a quantitative assessment of the differences in glutathione production between the GCLC mutants, two eukaryotic model systems were used. First, we transiently transfected mouse embryonic fibroblast cells obtained from GCLC null mice (GCLC−/−) (7). Consistent with previous studies in which individual clinical mutations were identified (13–16), each of the four mutants had lower levels of total glutathione compared to the wild-type GCLC transfectant when normalized to GCLC protein levels (Figure 2). These results confirm that each mutation is sufficient to decrease glutathione production, even in the presence of basal levels of GCLM.
To assess the contributions of GCLM to catalytic activity, an S. cerevisiae model was employed. S. cerevisiae has a single gene responsible for the efficient biosynthesis of γ-glutamylcysteine, gsh1, and lacks a functional equivalent of GCLM (33). Δgsh1 yeast transformed with either GCLC alone or with both GCLC and GCLM grew on glutathione deficient media, though the presence of GCLM conferred a distinct growth advantage (Figure 3). These results are consistent with observations in mice that GCLM is non-essential but enhances glutathione production (6, 22). Unlike wild-type GCLC, none of the four mutant GCLC subunits identified in hereditary GCL deficiency patients grew in the absence of GCLM, suggesting that overall cellular glutathione levels were likely below 1 μM, the minimal level of exogenous glutathione needed to confer growth to the Δgsh1 strain. In contrast, the presence of GCLM was able to restore growth to three (His370Leu, Pro158Leu, Arg127Cys) of the mutant GCLC transformants. The fourth mutant, Pro414Leu, failed to grow, and is consistent with the more dramatic reduction in glutathione levels observed in the GCLC−/− cell culture system (Figure 2). Thus, the role of the modifier subunit in enhancing catalytic activity appears to be particularly relevant in cases where the catalytic subunit is compromised by a mutation.
Based on the observed differences between wild-type and mutant GCLC in the Δgsh1 yeast and GCLC−/− cells, we performed in vitro kinetic characterizations in the absence (Table 1) and presence (Table 2) of the modifier subunit to further understand the impact of each amino acid mutation on enzyme catalysis and the molecular basis of disease. Consistent with earlier observations with mouse GCL (22), our kinetic studies demonstrate that the modifier subunit enhances the activity of the wild-type subunit and dramatically lowers the Km for ATP. Strikingly, each of the mutant GCLC proteins also displayed increased activity when combined with GCLM, often attaining apparent constants comparable to the wild-type enzyme (Compare Tables 1 and 2), indicating that the mutations do not significantly impact heterodimer formation. In addition, heterodimer formation improved the protein stability of each mutant (Figures 5 and 6). This suggests that therapeutics that promote heterodimer formation in vivo or can mimic the effects of modifier subunit binding may provide effective treatments for GCL deficiency.
To understand the molecular basis of the observed kinetic and phenotypic differences, we examined a homology model of human GCLC (Figures 1 and 4) (18). Previous modeling studies suggested that Arg 127 was located proximal to the substrate binding pocket, in a surface exposed pocket (14). However, these studies necessarily used a more distant homologue as a template. Based on the x-ray structure of the closely related S. cerevisiae GCL, a more robust human GCLC homology model was generated. In our model, Arg 127 is adjacent to the enzyme active site and forms a salt-bridge with Asp 49, as well as a hydrogen bond with the backbone carbonyl oxygen of Gly 104 (Figure 4A). Replacement of Arg 127 with a cysteine residue would likely disrupt this hydrogen bond network, potentially displacing numerous active site residues. For example, the backbone carbonyl of Asp 49 is positioned to form a hydrogen bond with the 3′ hydroxyl group of the ATP ribose. In addition, the β-strands containing Asp 49 and Gly 104 contain conserved glutamate residues critical to function. Disruption of the Arg 127/Asp 49 salt bridge may lead to displacement of Glu 50 and Glu 52, whereas eliminating the Arg 127/Gly 104 hydrogen bond could alter the position of Glu 103. Each of these glutamate residues is involved in magnesium coordination and substrate binding, and is critical for efficient catalysis. Importantly, each of these proposed interactions is conserved in the S. cerevisiae GCL template (18, 24).
Figure 4. Clinical mutations likely disrupt hydrogen bond networks that stabilize the enzyme active site.
The figure illustrates the hydrogen bond networks centered about three of the clinical point mutations. Atoms are colored as in Figure 1 with potential hydrogen bonds illustrated as solid black lines. The three bound magnesium atoms are labeled M1, M2, and M3. Panel A. Arg 127 stabilizes two β-strands within the active site, forming a salt bridge with Asp 49 and a hydrogen bond with the backbone carbonyl of Gly 104. Panel B. His 370 is involved in a hydrogen bond network that positions a β-strand containing Lys 412. Pro 414 is the second of four proline residues in a rigid loop immediately adjacent to Lys 412, which is involved in orienting the β-phosphate of ATP.
The predicted impacts of the Arg127Cys mutation based on modeling studies are supported by the available kinetic data. The initial report of this clinical mutation indicated that the Arg127Cys mutant alone had <5% of wild-type activity and had lower Km values for glutamate and aminobutyrate, a cysteine surrogate. An apparent Km for ATP was not reported (14). In the current study, the Arg127Cys mutant, in the absence of GCLM, has a modestly reduced Vmax as compared to wild-type GCLC alone (Table 1). The apparent Km for ATP increased 2-fold, whereas the apparent Km for glutamate was not significantly altered. Differences in assay conditions including assay temperature, substrate concentrations used, and the location of the engineered histidine tag used for affinity purification may account for these differences. Nonetheless, both reports clearly demonstrate that the Arg127Cys negatively impacts catalysis. Addition of GCLM to Arg127Cys restores enzymatic activity, with the mutant holoenzyme exhibiting kinetic constants comparable to the wild-type heterodimer (Table 2). This restoration of Arg127Cys activity by GCLM is also evident in the yeast spotting assay in which Arg127Cys alone did not grow, but addition of GCLM permitted growth, albeit at a reduced level compared to wild-type (Figure 3). Similarly, the levels of glutathione found in the GCLC−/− cell line transiently transfected with Arg127Cys were consistently lower than wild-type glutathione levels (Figure 2). In vitro assays of protein stability (Figures 5 and 6) indicate that the Arg127Cys mutation compromises protein structure, and that the additional stabilization afforded by heterodimer formation can offset these negative consequences. Thus, the addition of the modifier subunit can partially restore Arg127Cys activity, both in vitro and in vivo.
Our model of human GCLC suggests that histidine 370 and proline 414 are located in close proximity to the ATP-binding region of the active site (Figure 4B). Specifically, both residues appear to be involved in the positioning of a β-strand containing Lys 412, which participates in an extensive hydrogen bond network that is involved in ATP binding and proper orientation of active site residues. The mutation of proline 414 to leucine probably destabilizes a rigid series of proline residues connecting two beta strands resulting in reduction of ATP binding. This is in contrast to a previously reported model that indicated Pro 414 was located at the end of an α-helix, considerably removed from the enzyme active site (13). Histidine 370 appears to participate in an extensive hydrogen bond network with tyrosine 266 and glutamine 402 near the phosphate coordination region. Both His370Leu and Pro414Leu have compromised enzymatic activity in the absence of GCLM, likely as a consequence of poor ATP binding. However, addition of GCLM to either mutant rescued enzyme activity through restoration of the observed Km for ATP (Table 2) and to different extents, improved protein stability (Figures 5 and 6). The His370Leu mutant regained near full activity, whereas the Pro414Leu mutant had somewhat reduced activity. His370Leu stability was more easily rescued by the presence of ligands or heterodimer formation relative to the Pro414Leu mutant. In support of this interpretation, studies with a Lys412Ala mutant indicated that the enzyme has severely compromised catalytic activity, presumably due to its inability to bind ATP, yet has thermal stability equivalent to wild-type enzyme (Figure 6). Overall, our data suggest that formation of the heterodimer can compensate for these destabilizing mutations by ordering the enzyme active site.
These kinetic characterizations agree with the described cell culture studies. Particularly striking is the observation that the most kinetically crippled mutant, Pro414Leu (Table 1), is also the only mutant that failed to grow in combination with GCLM in the yeast spotting assay and that had statistically lower glutathione levels than wild-type in the GCLC−/− cell-based assays. Intriguingly, the only reported clinical case of GCL deficiency with the Pro414Leu mutation was accompanied by progressive motor neuropathy of the lower extremities and psychomotor development (13). Although other cases of neuropathy have been found in patients with GCL deficiency, the suggestion that the Pro414Leu mutation may lead to a more severe version of the disease is consistent with our experimental results.
Proline 158 lies on a solvent-exposed loop far from the enzyme active site. Its remote location and lack of sequence conservation suggests that a mutation of proline 158 to leucine would not have dramatic impact on enzyme catalysis. Our kinetic studies show that this mutation caused a slight, but distinct, increase in GCLC activity in both the catalytic only and holoenzyme forms compared to wild-type (Tables 1 and 2). In contrast, the Pro158Leu mutant exhibited reduced glutathione levels in the GCLC−/− cell line and could not complement the Δgsh1strain, unless the modifier subunit was also present. Once the holoenzyme was formed, the growth of Pro158Leu appeared very similar to that of wild-type GCLC/GCLM heterodimer. This phenotype is very similar to that of Cys152Ala, another mutant in this region of the enzyme recently characterized by our lab (YL and JJB, manuscript in preparation), suggesting that this region of GCLC is particularly sensitive to perturbations.
The apparent inconsistency between limited effect of the clinical mutations on apparent kinetic constants and the significant reduction in glutathione in the yeast and mouse embryonic fibroblast models can be reconciled if protein stability is considered (Figures 5 and 6). The Pro158Leu mutant alone is significantly destabilized. However, once the GCLM subunit was added, protein stability improved dramatically, to levels comparable to wild-type heterodimer. This suggests that the mutant catalytic subunit alone is less persistent in vivo perhaps due to decreased stability or increased turnover. Formation of the heterodimer stabilizes the Pro158Leu mutant and allows for the production of glutathione. These observations are consistent with studies of the equivalent Pro158Leu mutation in mouse GCLC that showed addition of GCLM significantly restored enzymatic function, as assessed by specific activity measurements at 37ºC (37).
Dalton and co-workers have demonstrated that erythrocyte glutathione levels are low in GCLM−/− mice and that GCLM is limiting in most tissues (6, 22). Depending on tissue type, GCLC to GCLM ratios of 1.5 to 7 were typically observed, but the study did not specifically examine the GCLC/GCLM ratio in erythrocytes. However, the observed manifestations of hereditary GCL deficiency combined with the studies reported herein are consistent with limiting GCLM in red blood cells. Patients with GCLC deficiency have very low levels of erythrocyte glutathione and exhibit hemolytic anemia, indicating that glutathione biosynthesis is compromised most notably in erythrocytes, cells in which it is needed to effectively combat oxidative stress. Our kinetic data indicate that for each mutant, formation of the heterodimer dramatically restores GCL activity. These observations suggest that GCLM may be limiting in red blood cells, such that near wild-type enzymatic activity is not realized. In addition, the mutant GCLC may not persist throughout the approximately 120 day lifespan of an erythrocyte (38) when GCLM is limiting.
In summary, our characterizations of the four clinical mutations identified in hereditary GCL deficiency reveal disparate molecular mechanisms that impair glutathione production by reducing the activity of the catalytic subunit of GCL. The critical role of the modifier subunit in enhancing activity of the catalytic subunit is evident in the ability of the modifier subunit to rescue activity of the mutant catalytic enzymes in both our in vitro and in vivo studies. Therapeutic strategies that stimulate GCLM production, stabilize the heterodimer, or mimic heterodimer formation may be effective in the treatment of this disease.
Supplementary Material
Acknowledgments
This work was made possible by NIH Grants GM077289 (JJB), DK079209 (JL) and P20 RR-17675 (National Center for Research Resources).
The authors would like to thank Harrison Roundtree for his early contributions to this work and Joshua Bies for his technical insights.
Footnotes
Supporting Information. Two supplemental figures, illustrating (a) detection of two forms of human GCLC and (b) wild-type GCKC protein stability, may be accessed free of charge via the internet at http://pubs.acs.org.
References
- 1.Lu SC. Regulation of glutathione synthesis. Mol Aspects Med. 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franklin CC, Backos DS, Mohar I, White CC, Forman HJ, Kavanagh TJ. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol Aspects Med. 2009;30:86–98. doi: 10.1016/j.mam.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lieberman MW, Wiseman AL, Shi ZZ, Carter BZ, Barrios R, Ou CN, Chevez-Barrios P, Wang Y, Habib GM, Goodman JC, Huang SL, Lebovitz RM, Matzuk MM. Growth retardation and cysteine deficiency in gamma-glutamyl transpeptidase-deficient mice. Proc Natl Acad Sci U S A. 1996;93:7923–7926. doi: 10.1073/pnas.93.15.7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang W, Ballatori N. Endogenous glutathione conjugates: occurrence and biological functions. Pharmacol Rev. 1998;50:335–356. [PubMed] [Google Scholar]
- 5.Shi ZZ, Habib GM, Rhead WJ, Gahl WA, He X, Sazer S, Lieberman MW. Mutations in the glutathione synthetase gene cause 5-oxoprolinuria. Nat Genet. 1996;14:361–365. doi: 10.1038/ng1196-361. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y, Dieter MZ, Chen Y, Shertzer HG, Nebert DW, Dalton TP. Initial characterization of the glutamate-cysteine ligase modifier subunit Gclm(−/−) knockout mouse. Novel model system for a severely compromised oxidative stress response. J Biol Chem. 2002;277:49446–49452. doi: 10.1074/jbc.M209372200. [DOI] [PubMed] [Google Scholar]
- 7.Shi ZZ, Osei-Frimpong J, Kala G, Kala SV, Barrios RJ, Habib GM, Lukin DJ, Danney CM, Matzuk MM, Lieberman MW. Glutathione synthesis is essential for mouse development but not for cell growth in culture. Proc Natl Acad Sci U S A. 2000;97:5101–5106. doi: 10.1073/pnas.97.10.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human disease. Biomed Pharmacother. 2003;57:145–155. doi: 10.1016/s0753-3322(03)00043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reid M, Jahoor F. Glutathione in disease. Curr Opin Clin Nutr Metab Care. 2001;4:65–71. doi: 10.1097/00075197-200101000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 11.Griffith OW, Mulcahy RT. The enzymes of glutathione synthesis: gamma-glutamylcysteine synthetase. Adv Enzymol Relat Areas Mol Biol. 1999;73:209–267. xii. doi: 10.1002/9780470123195.ch7. [DOI] [PubMed] [Google Scholar]
- 12.Dalton TP, Dieter MZ, Yang Y, Shertzer HG, Nebert DW. Knockout of the mouse glutamate cysteine ligase catalytic subunit (Gclc) gene: embryonic lethal when homozygous, and proposed model for moderate glutathione deficiency when heterozygous. Biochem Biophys Res Commun. 2000;279:324–329. doi: 10.1006/bbrc.2000.3930. [DOI] [PubMed] [Google Scholar]
- 13.Manu Pereira M, Gelbart T, Ristoff E, Crain KC, Bergua JM, Lopez Lafuente A, Kalko SG, Garcia Mateos E, Beutler E, Vives Corrons JL. Chronic non-spherocytic hemolytic anemia associated with severe neurological disease due to gamma-glutamylcysteine synthetase deficiency in a patient of Moroccan origin. Haematologica. 2007;92:e102–105. doi: 10.3324/haematol.11238. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton D, Wu JH, Alaoui-Jamali M, Batist G. A novel missense mutation in the gamma-glutamylcysteine synthetase catalytic subunit gene causes both decreased enzymatic activity and glutathione production. Blood. 2003;102:725–730. doi: 10.1182/blood-2002-11-3622. [DOI] [PubMed] [Google Scholar]
- 15.Ristoff E, Augustson C, Geissler J, de Rijk T, Carlsson K, Luo JL, Andersson K, Weening RS, van Zwieten R, Larsson A, Roos D. A missense mutation in the heavy subunit of gamma-glutamylcysteine synthetase gene causes hemolytic anemia. Blood. 2000;95:2193–2196. [PubMed] [Google Scholar]
- 16.Beutler E, Gelbart T, Kondo T, Matsunaga AT. The molecular basis of a case of gamma-glutamylcysteine synthetase deficiency. Blood. 1999;94:2890–2894. [PubMed] [Google Scholar]
- 17.Hirono A, Iyori H, Sekine I, Ueyama J, Chiba H, Kanno H, Fujii H, Miwa S. Three cases of hereditary nonspherocytic hemolytic anemia associated with red blood cell glutathione deficiency. Blood. 1996;87:2071–2074. [PubMed] [Google Scholar]
- 18.Biterova EI, Barycki JJ. Mechanistic details of glutathione biosynthesis revealed by crystal structures of Saccharomyces cerevisiae glutamate cysteine ligase. J Biol Chem. 2009;284:32700–32708. doi: 10.1074/jbc.M109.025114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mumberg D, Muller R, Funk M. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene. 1995;156:119–122. doi: 10.1016/0378-1119(95)00037-7. [DOI] [PubMed] [Google Scholar]
- 20.Eyer P, Podhradsky D. Evaluation of the micromethod for determination of glutathione using enzymatic cycling and Ellman’s reagent. Anal Biochem. 1986;153:57–66. doi: 10.1016/0003-2697(86)90061-8. [DOI] [PubMed] [Google Scholar]
- 21.Gietz RD, Schiestl RH, Willems AR, Woods RA. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast. 1995;11:355–360. doi: 10.1002/yea.320110408. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Shertzer HG, Schneider SN, Nebert DW, Dalton TP. Glutamate cysteine ligase catalysis: dependence on ATP and modifier subunit for regulation of tissue glutathione levels. J Biol Chem. 2005;280:33766–33774. doi: 10.1074/jbc.M504604200. [DOI] [PubMed] [Google Scholar]
- 23.Huang CS, Chang LS, Anderson ME, Meister A. Catalytic and regulatory properties of the heavy subunit of rat kidney gamma-glutamylcysteine synthetase. J Biol Chem. 1993;268:19675–19680. [PubMed] [Google Scholar]
- 24.Biterova EI, Barycki JJ. Structural basis for feedback and pharmacological inhibition of Saccharomyces cerevisiae glutamate cysteine ligase. J Biol Chem. 2010;285:14459–14466. doi: 10.1074/jbc.M110.104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ericsson UB, Hallberg BM, Detitta GT, Dekker N, Nordlund P. Thermofluor-based high-throughput stability optimization of proteins for structural studies. Analytical biochemistry. 2006;357:289–298. doi: 10.1016/j.ab.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 26.Pantoliano MW, Petrella EC, Kwasnoski JD, Lobanov VS, Myslik J, Graf E, Carver T, Asel E, Springer BA, Lane P, Salemme FR. High-density miniaturized thermal shift assays as a general strategy for drug discovery. J Biomol Screen. 2001;6:429–440. doi: 10.1177/108705710100600609. [DOI] [PubMed] [Google Scholar]
- 27.Sun WM, Huang ZZ, Lu SC. Regulation of gamma-glutamylcysteine synthetase by protein phosphorylation. Biochem J. 1996;320(Pt 1):321–328. doi: 10.1042/bj3200321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan N, Meister A. Amino acid sequence of rat kidney gamma-glutamylcysteine synthetase. J Biol Chem. 1990;265:1588–1593. [PubMed] [Google Scholar]
- 29.Backos DS, Fritz KS, Roede JR, Petersen DR, Franklin CC. Posttranslational modification and regulation of glutamate-cysteine ligase by the alpha,beta-unsaturated aldehyde 4-hydroxy-2-nonenal. Free Radic Biol Med. 2011;50:14–26. doi: 10.1016/j.freeradbiomed.2010.10.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ochi T. Hydrogen peroxide increases the activity of gamma-glutamylcysteine synthetase in cultured Chinese hamster V79 cells. Arch Toxicol. 1995;70:96–103. doi: 10.1007/BF02733669. [DOI] [PubMed] [Google Scholar]
- 31.Gipp JJ, Bailey HH, Mulcahy RT. Cloning and sequencing of the cDNA for the light subunit of human liver gamma-glutamylcysteine synthetase and relative mRNA levels for heavy and light subunits in human normal tissues. Biochem Biophys Res Commun. 1995;206:584–589. doi: 10.1006/bbrc.1995.1083. [DOI] [PubMed] [Google Scholar]
- 32.Ohtake Y, Yabuuchi S. Molecular cloning of the gamma-glutamylcysteine synthetase gene of Saccharomyces cerevisiae. Yeast. 1991;7:953–961. doi: 10.1002/yea.320070907. [DOI] [PubMed] [Google Scholar]
- 33.Spector D, Labarre J, Toledano MB. A genetic investigation of the essential role of glutathione: mutations in the proline biosynthesis pathway are the only suppressors of glutathione auxotrophy in yeast. J Biol Chem. 2001;276:7011–7016. doi: 10.1074/jbc.M009814200. [DOI] [PubMed] [Google Scholar]
- 34.Tu Z, Anders MW. Identification of an important cysteine residue in human glutamate-cysteine ligase catalytic subunit by site-directed mutagenesis. Biochem J. 1998;336(Pt 3):675–680. doi: 10.1042/bj3360675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tu Z, Anders MW. Expression and characterization of human glutamate-cysteine ligase. Arch Biochem Biophys. 1998;354:247–254. doi: 10.1006/abbi.1998.0676. [DOI] [PubMed] [Google Scholar]
- 36.Fraser JA, Saunders RD, McLellan LI. Drosophila melanogaster glutamate-cysteine ligase activity is regulated by a modifier subunit with a mechanism of action similar to that of the mammalian form. J Biol Chem. 2002;277:1158–1165. doi: 10.1074/jbc.M106683200. [DOI] [PubMed] [Google Scholar]
- 37.Yang Y, Chen Y, Johansson E, Schneider SN, Shertzer HG, Nebert DW, Dalton TP. Interaction between the catalytic and modifier subunits of glutamate-cysteine ligase. Biochem Pharmacol. 2007;74:372–381. doi: 10.1016/j.bcp.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 38.D’Alessandro A, Righetti PG, Zolla L. The red blood cell proteome and interactome: an update. J Proteome Res. 2010;9:144–163. doi: 10.1021/pr900831f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.