Abstract
Migraine is a neurovascular disorder characterized by recurrent episodic headaches, and is caused by abnormal processing of sensory information due to peripheral and/or central sensitization. The exact pathophysiological mechanism underlying migraine is not fully understood; however, cortical spreading depression (CSD) is thought to provide the basis for migraine aura and may serve as a trigger of migraine pain. CSD depends on neuronal–glial cell communication, which is mediated by intercellular transfer of messengers through connexin-containing gap junctions, as well as messengers released into the extracellular space by non-junctional connexin-containing hemichannels. These processes are believed to be important in peripheral sensitization within the trigeminal ganglion and to lead to central sensitization. The novel benzopyran compound tonabersat binds selectively to a unique site in the brain. In preclinical studies, tonabersat markedly reduced CSD and CSD-associated events and inhibited gap-junction communication between neurons and satellite glial cells in the trigeminal ganglion. Together, these findings suggest that tonabersat should have clinical application in preventing migraine attacks.
Keywords: Connexins, cortical spreading depression, gap junctions, tonabersat, trigeminovascular
Introduction
Migraine is a disorder of cerebral nerve and blood vessel dysfunction that affects approximately 10–15% of the US and European populations (1, 2). The characteristic repeated, episodic headaches last several hours to days, are often throbbing, frequently unilateral and generally moderate to severe, are associated with sensitivity to light, sound or movement and may involve nausea or vomiting (3, 4). In up to 30% of patients, the migraine attack is preceded or accompanied by aura—transient, focal, usually visual, neurological symptoms (3). The pathophysiological basis of migraine is genetic in that many patients have first-degree relatives who also are migraineurs (3). A 3 : 1 preference for women over men suggests that female hormones may play some aetiological role (2). Not surprisingly, symptoms are often disabling and result in missed work days, work impairment and lost productivity, as well as interference with other normal daily activities (2). Despite the prevalence and social and economic burden of migraine, the exact pathophysiological mechanisms of migraine are not known, although abnormal processing of sensory signals leading to peripheral and central sensitization may be important (3, 5).
Pathophysiology
Cortical spreading depression (CSD), which is characterized by a wave of depolarization—a decrease in direct current (d.c.) potential measured on the brain surface—followed by hyperpolarization that spreads slowly across the cortex, is thought to be a physiological trigger for migraine (6). CSD was identified in the 1940s in exposed rabbit cortex that was subjected to 1–5 s of repetitive electrical stimulation or to a few light touches with a glass rod (7). Depression of spontaneous electrical activity spreads slowly and gradually from the stimulated area and is followed by slow recovery of electrical activity in the opposite direction. During CSD, a large proportion of cortical neurons are depolarized, with massive efflux of potassium ions from intracellular to extracellular compartments (8, 9). This ionic alteration creates an ‘all-or-none’ process that propagates slowly as a wave across the cortex at a rate of 2–5 mm/min.
CSD probably provides the pathophysiological basis for migraine aura, a neurological disturbance usually arising in the visual cortex that may appear shortly before or during a migraine attack (8). This hypothesis is based largely on two consistent observations: (i) CSD moves across the cortex at a rate matching the propagation of aural symptoms (6, 8) and (ii) functional magnetic resonance imaging (MRI) of patients during visual aura shows slowly propagating changes in cerebral blood flow in the visual cortex that match the changes during CSD—an increase in blood flow during depolarization and a decrease during hyperpolarization.
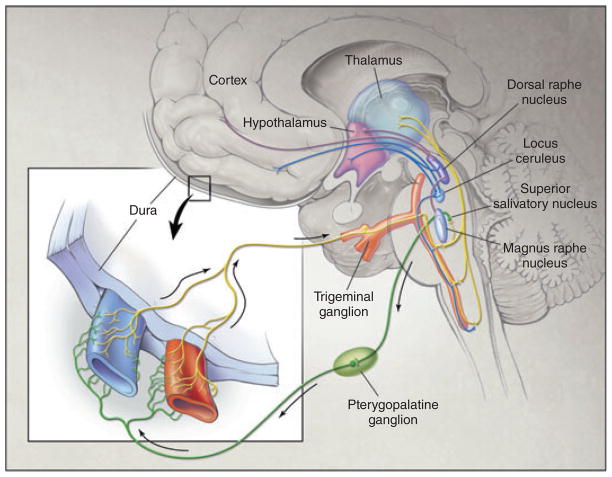
CSD is also a likely trigger of migraine pain, although the exact mechanism remains to be elucidated. The slow movement of the CSD wave across the cortex may promote the release of potassium, arachidonic acid, hydrogen ions and nitric oxide (NO) (10). Achievement of critical levels of these substances is thought to cause sensitization and activation of trigeminal neurons in the afferent loop. This, in turn, activates second-order neurons in the trigeminocervical complex, which transmit sensory signals to the brainstem, and parasympathetic efferents, which project from the sphenopalatine ganglion. The physiological consequences of the release of vasoactive molecules from trigeminal afferents and parasympathetic efferents are vasodilation, plasma leakage and mast cell degranulation within the dura mater (Fig. 1) (11).
Figure 1.
Pathophysiology of migraine. The trigeminovascular input from the meningeal vessels passes through the trigeminal ganglion and synapses in the trigeminocervical complex. The peripheral release of molecules results in vasodilation, plasma leakage and mast cell degranulation within the dura mater, while central release can cause activation of second-order neurons leading to the pain of migraine attacks. Trigeminal activation can mediate parasympathetic responses via the pterygopalatine ganglion. Adapted from Goadsby et al. (11).
Astrocytes, a subset of glial cells, have been implicated in the mechanisms of CSD based on two critical functions: (i) maintaining the milieu around active neurons, which includes regulating extracellular potassium, pH and neurotransmitter levels, particularly glutamate and γ-aminobutyric acid and (ii) propagating intercellular calcium waves (12, 13). The astrocytes reside close to neurons in separate but highly interactive networks. Whereas synapses connect the neuronal network, gap junctions connect the astrocyte network, forming a syncytium-like organization (14). Ranging from a few to several hundred, these gap junctions are clusters of tightly packed intercellular channels that allow direct biochemical and electrical communication between neighbouring cells (15). The gap junctions are essential to the formation of a functional syncytium of astrocytes.
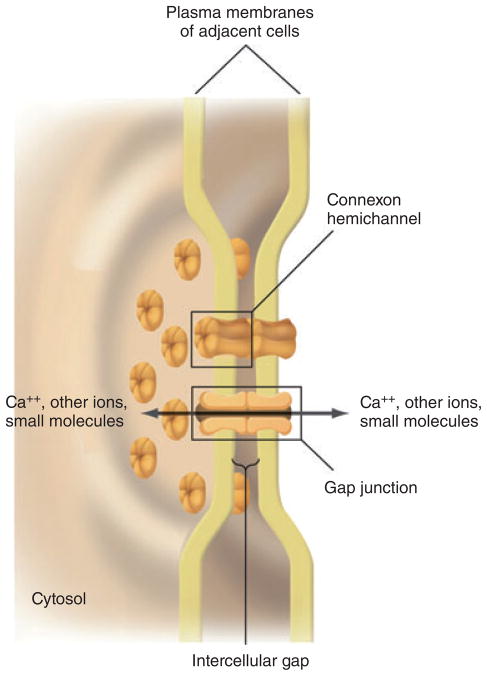
Gap junctions are formed between the cell membranes of two adjacent cells, each of which contributes one hemichannel. Each hemichannel is composed of a hexamer of connexins, termed a connexon, arranged around a central pore (Fig. 2) (12, 16, 17). The hemichannel is generally closed before docking with a hemichannel from an adjacent cell. The interaction between the two hemichannels opens both, forming the gap junction and allowing intercellular passage of ions and small molecules (16). Twenty-one different connexins have been identified in humans and, depending on the subset expressed in any one cell type, they influence the selectivity and conductance of the gap junctions (15). Approximately 1.0–1.5 nm in diameter, gap junctions allow transport of molecules up to about 1 kDa in size (16). The nature of the connexins, however, determines the transport of specific molecules, based on size, shape and charge, through the gap junction.
Figure 2.
Gap junctions. A three-dimensional drawing showing the interacting plasma membranes of two adjacent cells connected by gap junctions. Both membranes contain connexin-containing hemichannels that join across the intercellular gap to form a gap-junction channel connecting the two cells. Adapted from Lodish et al. (17).
Of the at least 11 connexins identified in the central nervous system (CNS), connexins 26, 29, 30, 32, 36 and 43 are found at ultrastructurally defined gap junctions in neurons and glial cells (18). Astrocytes express at least three different connexins—26, 30 and 43—at gap junctions (18). Although connexin 43 is the predominant member expressed in astrocytes, the three connexins are heterogeneously distributed throughout the CNS (16, 18). For example, astrocytes in subcortical areas contain large amounts of each of the three connexins, whereas astrocytes in the cerebral cortex express low levels of connexin 26 and moderate levels of connexin 30. Because permeability properties of each connexin vary with respect to specific ions and molecules, the different levels of expression of each connexin may reflect regional differences in the functional requirements of astrocyte gap junctions (18).
Experimental studies suggest that both intercellular movement of messengers by gap junctions and extracellular paracrine/autocrine messengers may help to propagate calcium waves (19, 20). As noted, the large ionic gradient shifts associated with CSD lead to profound depletion of spontaneous and evoked neuronal activity (19). Calcium waves are a manifestation of this process, in which intracellular calcium increases spread from cell to neighbouring cell. The release of adenosine triphosphate (ATP) by non-junctional connexin hemichannels may be one mechanism underlying extracellular messenger-mediated calcium wave propagation (13). In support of this mechanism, the connexin hemichannel activator quinine can stimulate ATP release and propagation of intercellular calcium waves in cultured astrocytes, whereas these processes are inhibited by the gap-junction blocker flufenamic acid (13). Studies with such blockers only ambiguously distinguish between mechanisms, however, because the hemichannels may be the source of the extracellular signal (in this case ATP) or, as part of a gap junction, they may still be able to transfer a messenger that triggers ATP release by the second cell (16).
Interestingly, N-methyl-D-aspartate (NMDA) receptor antagonists block CSD but, unlike the gap-junction blockers, do not inhibit calcium wave propagation (12). Astrocytes are known to express several types of glutamate receptors, including NMDA receptors (12). Therefore, the generation and propagation of CSD may depend on neuronal activation and calcium influx triggered by NMDA receptors. If this is the case, gap-junction-mediated propagation of calcium waves may represent the advancing front of CSD, triggering secondary depolarization of surrounding neurons to release potassium ions and glutamate into the extracellular space. Glutamate may then stimulate cytosolic calcium oscillations in astrocytes, providing a feedback loop that allows CSD propagation (12). Glutamate release from astrocytes also appears to be mediated by the opening of connexin hemichannels (21).
Neuronal–glial cell communications through gap junctions and extracellular paracrine/autocrine processes are believed to be important in development of peripheral sensitization within the trigeminal ganglion (22). Peripheral sensitization, which is characterized by increased neuronal excitability and a lowered threshold for activation, may possibly trigger a migraine attack. Moreover, activation and sensitization of the trigeminovascular afferent fibres appear crucial for initiation of migraine pain and for subsequent central sensitization, in which increased excitability of second-order neurons leads to pain and allodynia. Increased gap-junction communication between neurons and satellite glial cells was observed in the trigeminal ganglion in response to chemical activation of sensory trigeminal nerves (22). Thus, by allowing ions and other small molecules to pass between these cells, increased gap-junction communication can modulate the excitability of both neuronal and glial cells under pathophysiological conditions.
Increased neuronal–glial signalling by way of gap junctions is common in neuroinflammatory CNS disorders, such as cerebral ischaemia and Alzheimer’s disease, and may have underlying pathological significance (23). In addition, increased neuronal–glial signalling by way of gap junctions has been observed in animal models of chronic pain (24).
Tonabersat
Our current understanding of the mechanism underlying migraine suggests that gap-junction inhibitors may represent a viable pharmacological strategy for prevention. Tonabersat (SB-220453), a mechanistically novel benzoylamino-benzopyran compound, binds selectively and with high affinity to a unique stereoselective site in rat and human brains (25, 26). Tonabersat does not induce contraction of human isolated blood vessels, including middle meningeal artery, coronary artery and saphenous vein (27). By contrast, the 5-HT1B/1D agonist sumatriptan, which effectively reverses migraine attacks, causes marked concentration-dependent contractions of all three vessels. Neither agent displays a positive or negative inotropic effect in isolated atrial or ventricular trabeculae. Because tonabersat does not contract the middle meningeal artery, its antimigraine effects cannot be attributed to direct cerebral vasoconstriction. The lack of activity on these isolated vessels suggests that tonabersat is unlikely to affect the cardiovascular system adversely.
Tonabersat inhibits the CSD that is believed to underlie the aura of migraine. In anaesthetized cats, tonabersat administered 90 min before KCl-induced CSD dose-dependently reduced the number of CSD events and the duration of CSD activity at both cortical sites (28). Tonabersat also inhibited CSD-induced repetitive pial vasodilation but left systemic haemodynamics unchanged and did not influence the amplitude (i.e. change in d.c. potential) or time to repolarization after the first CSD event in this study. A second study obtained comparable results when CSD was measured by repetitive diffusion-weighted MRI, which detects the transient changes in cerebral water during CSD (29). Tonabersat 10 mg/kg intraperitoneally significantly reduced the mean number of CSD events and the duration of CSD activity compared with vehicle control. Sumatriptan 0.3 mg/kg intravenously did not affect the number of CSD events and produced only a minor decrease in the duration of CSD activity.
In the anaesthetized cat model, CSD evoked by KCl application involved an increase in NO production, detected electrochemically by a NO-selective electrode placed on the cortical surface of the suprasylvian gyrus (30). In vehicle-treated animals, KCl induced a median of 4.5 peaks in NO activity with a total of 59 min of NO activity, consistent, respectively, with the number of CSD events and duration of CSD activity. Animals pre-treated with tonabersat 10 mg/kg intraperitoneally had a median of one NO peak and 5 min of NO activity. This study demonstrated that tonabersat inhibits the CSD-associated release of NO.
In a subsequent study, CSD was evoked in anaesthetized rats by application of KCl to the pia of the parietal cortex, and plasma guanosine monophosphate (cGMP) levels were measured 3 days later as a marker of post-CSD NO metabolism (31). Tonabersat 10 mg/kg intraperitoneally significantly reduced the median number of extracellular d.c. depolarizations compared with vehicle control; sumatriptan 0.3 mg/kg intravenously had no effect. Similarly, tonabersat reduced the CSD load—defined by integrating each depolarization and adding the integrated values; again sumatriptan had no effect. Cortical and brainstem cGMP levels measured 3 days after the brief period of CSD activity were highly correlated with the CSD load, especially cGMP in the brainstem. Tonabersat abolished the increases in cGMP levels in the brainstem as well as in the ipsilateral and contralateral cortex. Sumatriptan did not affect ipsilateral cortical cGMP, but partially reduced cGMP at the other two sites. Thus, this study provided additional evidence that tonabersat inhibits CSD-associated increases in NO.
Tonabersat also attenuated trigeminal nerve stimulation-induced neurovascular responses in anaesthetized cats (32). In this model, stimulation of the trigeminal ganglion nerve produces a frequency-dependent increase in carotid blood flow and concomitant reduction in carotid vascular resistance, with little effect on blood pressure or heart rate. Administration of tonabersat either intraduodenally or intravenously reduced the trigeminal nerve stimulation-induced neurovascular response in a time- and dose-dependent manner. For example, 2 h after intraduodenal administration of 10 mg/kg, the carotid vascular reflex fell by 55%. Tonabersat may therefore modulate afferent or efferent parts of the pathway from sensory trigeminal ganglia to parasympathetic ganglia. As noted, activation of sensory trigeminal afferents and efferents is also important in the aetiology of migraine.
Evidence for tonabersat as a modulator of gap-junction communication between trigeminal ganglion neurons and satellite glial cells was provided in a recent study in rats (33). Injection of the cytokine tumour necrosis factor-alpha in the V2 region (whisker pad) lowered the threshold for activation of nociceptive neurons in the V1 region (eyebrow) in response to a capsaicin injection there. Moreover, the injection of both substances greatly increased neuron–satellite glial cell communication by way of gap junctions in both regions, as measured by movement of True Blue dye. This change in gap-junction activity was accompanied by increased expression of the gap-junction protein connexin 26 (measured immunohistochemically) in both neurons and satellite glia in the V1 and V2 regions. The levels of active p38 mitogen-activated protein (MAP) kinase, which is a protein implicated in sensitization of neurons and glial cells, also increased. Significantly, pretreatment of rats with tonabersat inhibited gap-junction communication between neurons and satellite glia, and blocked increases in connexin 26 and active p38 MAP kinase. These effects suggest that tonabersat can inhibit gap-junction communication between trigeminal neurons and glial cells, and thus may play a role in decreasing peripheral sensitization of trigeminal nerves. It will be of clinical importance to determine if tonabersat can inhibit increased gap-junction communication within the CNS and prevent central sensitization.
Conclusion
The exact mechanism underlying the aetiology of migraine is yet to be elucidated. According to current understanding, CSD provides the pathophysiological basis for migraine aura, and subsequently, CSD or a related event triggers processes leading to peripheral sensitization within the trigeminal ganglion as well as central sensitization. Neuronal–glial cell communication is important in CSD propagation, which is mediated by connexin-containing gap junctions and non-junctional connexin-containing hemichannels. Accordingly, connexin-containing gap junctions may be a viable target for migraine prophylaxis by inhibiting neuronal–glial communication and thereby interfering with CSD propagation and, possibly, the development of peripheral sensitization. In experimental studies, tonabersat inhibited gap-junction activity, CSD and CSD-associated events. As tonabersat does not cause constriction of cerebral or coronary vessels, it would not be expected to cause any adverse cardiovascular events. Based on data from these preclinical studies, it is likely that tonabersat will be beneficial for the prophylactic treatment of migraine.
Acknowledgments
B. Weichman, PhD (BMW Associates, Skillman, NJ, USA), and P. Kontur, PhD and M. Kersting PhD (JL Shapiro Associates Inc., Edison, NJ, USA) assisted with manuscript preparation with financial support from Minster Pharmaceuticals.
Footnotes
Competing interests
The authors have received grant support from Minster Pharmaceuticals for basic science studies on tonabersat.
References
- 1.Durham PL. Calcitonin gene-related peptide (CGRP) and migraine. Headache. 2006;46 (Suppl 1):S3–8. doi: 10.1111/j.1526-4610.2006.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Unger J. Migraine headaches: a historical perspective, a glimpse into the future, and migraine epidemiology. Dis Mon. 2006;52:367–84. doi: 10.1016/j.disamonth.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Goadsby PJ. Recent advances in understanding migraine mechanisms, molecules and therapeutics. Trends Mol Med. 2007;13:39–44. doi: 10.1016/j.molmed.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Calabresi P, Galletti F, Rossi C, Sarchielli P, Cupini LM. Antiepileptic drugs in migraine: from clinical aspects to cellular mechanisms. Trends Pharmacol Sci. 2007;28:188–95. doi: 10.1016/j.tips.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Dodick D, Silberstein S. Central sensitization theory of migraine: clinical implications. Headache. 2006;46 (Suppl 4):S182–91. doi: 10.1111/j.1526-4610.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- 6.Goadsby PJ. Emerging therapies for migraine. Nature Clin Pract Neurol. 2007;3:610–9. doi: 10.1038/ncpneuro0639. [DOI] [PubMed] [Google Scholar]
- 7.Leao AAP. Spreading depression of activity in the cerebral cortex. J Neurophysiol. 1944;7:359–90. doi: 10.1152/jn.1947.10.6.409. [DOI] [PubMed] [Google Scholar]
- 8.Rogawski MA. Common pathophysiologic mechanisms in migraine and epilepsy. Arch Neurol. 2008;65:709–14. doi: 10.1001/archneur.65.6.709. [DOI] [PubMed] [Google Scholar]
- 9.Netsiri C, Bradley DP, Takeda T, Smith MI, Papadakis N, Hall LD. A delayed class of BOLD waveforms associated with spreading depression in the feline cerebral cortex can be detected and characterised using independent component analysis (ICA) Magn Reson Imaging. 2003;21:1097–110. doi: 10.1016/s0730-725x(03)00199-1. [DOI] [PubMed] [Google Scholar]
- 10.Moskowitz MA. Pathophysiology of headachenpast and present. Headache. 2007;47 (Suppl 1):S58–63. doi: 10.1111/j.1526-4610.2007.00678.x. [DOI] [PubMed] [Google Scholar]
- 11.Goadsby PJ, Lipton RB, Ferrari MD. Migraine—current understanding and treatment. N Engl J Med. 2002;346:257–70. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- 12.Benarroch EE. Neuron-astrocyte interactions: partnership for normal function and disease in the central nervous system. Mayo Clin Proc. 2005;80:1326–38. doi: 10.4065/80.10.1326. [DOI] [PubMed] [Google Scholar]
- 13.Stout CE, Costantin JL, Naus CCG, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;277:10482–8. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- 14.Giaume C, McCarthy KD. Control of gap-junctional communication in astrocytic networks. Trends Neurosci. 1996;19:319–25. doi: 10.1016/0166-2236(96)10046-1. [DOI] [PubMed] [Google Scholar]
- 15.Laird DW. Life cycle of connexins in health and disease. Biochem J. 2006;394:527–43. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett MVL, Contreras JE, Bukauskas FF, Sáez JC. New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci. 2003;26:610–7. doi: 10.1016/j.tins.2003.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell JE. Molecular cell biology. 5. New York: W. H. Freeman & Co; 2004. [Google Scholar]
- 18.Nagy JI, Dudek FE, Rash JE. Update on connexins and gap junctions in neurons and glia in the mammalian nervous system. Brain Res Rev. 2004;47:191–215. doi: 10.1016/j.brainresrev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Chen G, Dunbar RL, Gao W, Ebner TJ. Role of calcium, glutamate neurotransmission, and nitric oxide in spreading acidification and depression in the cerebellar cortex. J Neurosci. 2001;21:9877–87. doi: 10.1523/JNEUROSCI.21-24-09877.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grafstein B, Liu S, Cotrina ML, Goldman SA, Nedergaard M. Meningeal cells can communicate with astrocytes by calcium signaling. Ann Neurol. 2000;47:18–25. [PubMed] [Google Scholar]
- 21.Ye Z-C, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci. 2003;23:3588–96. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thalakoti S, Patil VV, Damodaram S, Vause CV, Langford LE, Freeman SE, Durham PL. Neuron–glia signaling in trigeminal ganglion: implications for migraine pathology. Headache. 2007;47:1008–23. doi: 10.1111/j.1526-4610.2007.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kielian T, Esen N. Effects of neuroinflammation on glia–glia gap junctional intercellular communication: a perspective. Neurochem Int. 2004;45:429–36. doi: 10.1016/j.neuint.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Ohara PT, Vit JP, Bhargava A, Jasmin L. Evidence for a role of connexin 43 in trigeminal pain using RNA interference in vivo. J Neurophysiol. 2008;100:3064–73. doi: 10.1152/jn.90722.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan WN, Evans JM, Hadley MS, Herdon HJ, Jerman JC, Parsons AA, et al. Identification of (−)-cis-6-acetyl-4S-(3-chloro-4-fluoro-benzoylamino)-3,4-dihydro-2,2-dimethyl-2H-benzo[b]pyran-3S-ol as a potential antimigraine agent. Bioorg Med Chem Lett. 1999;9:285–90. doi: 10.1016/s0960-894x(98)00728-8. [DOI] [PubMed] [Google Scholar]
- 26.Upton N, Raval P, Herdon H, Jerman J, Parsons AA, Chan WN, Thompson M. SB-220453, a mechanistically novel benzopyran compound, inhibits trigeminal nerve ganglion (TGN) stimulation-induced carotid vasodilatation. Cephalalgia. 1999;19:351. [Google Scholar]
- 27.MaassenVanDenBrink A, van den Broek RWM, de Vries R, Upton N, Parsons AA, Saxema PR. The potential anti-migraine compound SB-220453 does not contract human isolated blood vessels or myocardium; a comparison with sumatriptan. Cephalalgia. 2000;20:538–45. doi: 10.1046/j.1468-2982.2000.00078.x. [DOI] [PubMed] [Google Scholar]
- 28.Smith MI, Read SJ, Chan WN, Thompson M, Hunter AJ, Upton N, Parsons AA. Repetitive cortical spreading depression in a gyrencephalic feline brain: inhibition by the novel benzoylamino-benzopyran SB-220453. Cephalalgia. 2000;20:546–53. doi: 10.1046/j.1468-2982.2000.00092.x. [DOI] [PubMed] [Google Scholar]
- 29.Bradley DP, Smith MI, Netsiri C, Smith JM, Bockhorst KHJ, Hall LD, et al. Diffusion-weighted MRI used to detect in vivo modulation of cortical spreading depression: comparison of sumatriptan and tonabersat. Exp Neurol. 2001;172:342–53. doi: 10.1006/exnr.2001.7809. [DOI] [PubMed] [Google Scholar]
- 30.Read SJ, Smith MI, Hunter AJ, Upton N, Parsons AA. SB-220453, a potential novel antimigraine agent, inhibits nitric oxide release following induction of cortical spreading depression in the anaesthetized cat. Cephalalgia. 2000;20:92–9. doi: 10.1046/j.1468-2982.2000.00022.x. [DOI] [PubMed] [Google Scholar]
- 31.Read SJ, Hirst WD, Upton N, Parsons AA. Cortical spreading depression produces increased cGMP levels in cortex and brain stem that is inhibited by tonabersat (SB-220453) but not sumatriptan. Brain Res. 2001;891:69–77. doi: 10.1016/s0006-8993(00)03191-7. [DOI] [PubMed] [Google Scholar]
- 32.Parsons AA, Bingham S, Raval P, Read S, Thompson M, Upton N. Tonabersat (SB-220453) a novel benzopyran with anticonvulsant properties attenuates trigeminal nerve-induced neurovascular reflexes. Br J Pharmacol. 2001;132:1549–57. doi: 10.1038/sj.bjp.0703932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durham PL, Damodaram S, Thalakoti S, Freeman SE, Garrett FG. Tonabersat inhibits trigeminal ganglion neuronal–satellite glial cell signaling. Headache. 2008;48:S5. doi: 10.1111/j.1526-4610.2008.01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]