Abstract
The activation of the human A3 adenosine receptor (AR) by a wide range of N6-substituted adenosine derivatives was studied in intact CHO cells stably expressing this receptor. Selectivity of binding at rat and human ARs was also determined. Among N6-alkyl substitutions, small N6-alkyl groups were associated with selectivity for human A3ARs vs. rat A3ARs, and multiple points of branching were associated with decreased hA3AR efficacy. N6-Cycloalkyl-substituted adenosines were full (≤5 carbons) or partial (≥6 carbons) hA3AR agonists. N6-(endo-Norbornyl)adenosine 13 was the most selective for both rat and human A1ARs. Numerous N6-arylmethyl analogues, including substituted benzyl, tended to be more potent in binding to A1 and A3 vs. A2AARs (with variable degrees of partial to full A3AR agonisms). A chloro substituent decreased the efficacy depending on its position on the benzyl ring. The A3AR affinity and efficacy of N6-arylethyl adenosines depended highly on stereochemistry, steric bulk, and ring constraints. Stereoselectivity of binding was demonstrated for N6-(R-1-phenylethyl)adenosine vs. N6-(S-1-phenylethyl)adenosine, as well as for the N6-(1-phenyl-2-pentyl)adenosine, at the rat, but not human A3AR. Interestingly, DPMA, a potent agonist for the A2AAR (Ki = 4 nM), was demonstrated to be a moderately potent antagonist for the human A3AR (Ki = 106 nM). N6-[(1S,2R)-2-Phenyl-1-cyclopropyl]adenosine 48 was 1100-fold more potent in binding to human (Ki = 0.63 nM) than rat A3ARs. Dual acting A1/A3 agonists (N6-3-chlorobenzyl- 29, N6-(S-1-phenylethyl)- 39, and 2-chloro-N6-(R-phenylisopropyl)adenosine 53) might be useful for cardioprotection.
Keywords: Purines, Nucleosides, GPCR, Cyclic AMP, Receptor binding, Structure–activity relationships
1. Introduction
The structure–activity relationships for adenosine derivatives as agonists at the ARs have been studied in both binding and functional assays [1,2]. Several of the four subtypes of adenosine receptors have cytoprotective effects when activated. The activation of the A3AR by selective agonists has been demonstrated to be both cardioprotective and cerebroprotective [3,4]. In previous studies, a number of structural determinants for A3AR activation have been identified [5–9], leading to the general conclusion that the ability of an adenosine derivative to activate the A3AR is highly structure sensitive. In this study, we further evaluated the binding affinity and functional properties of a wide range of N6-substituted adenosine derivatives at both human and rat A3ARs stably expressed in CHO cells.
The affinity and efficacy of many of these of N6-substituted adenosine derivatives have been evaluated at rat A1 and/or A2AARs [10]. However, at A3ARs, the structure–activity relationships of a wide variety of N6-substituted adenosine derivatives has not been fully evaluated. Recent studies [5,6,8,9] suggested that various adenosine derivatives, previously assumed to be full/partial agonists at A3ARs, are indeed antagonists. Thus, the efficacy of adenosine derivatives at the A3AR appears to be more dependent on small structural changes than at other subtypes. In an effort to develop potent and selective A3 receptor agonists and antagonists, the affinity and/or functional potency of these compounds for human and rat A3ARs were measured. In order to ascertain the selectivity within the same species, we also determined the binding affinity of selected adenosine derivatives at human and/or rat A1 and A2AARs.
2. Materials and methods
2.1. Materials
[125I]N6-(4-Amino-3-iodobenzyl)adenosine-5′-N-methyluronamide ([125I]I-AB-MECA; 2000 Ci/mmol), [3H]R-PIA (34 Ci/mmol), [3H]DPCPX (120 Ci/mmol), and [3H]cyclic AMP (40 Ci/mmol) were from Amersham Pharmacia Biotech. [3H]CGS21680 (47 Ci/mmol) was from Perkin-Elmer Life Sciences. ENBA, NECA, CPA, CHA, DPMA (N6-[2-(3.5-dimethoxyphenyl)-2-(2-methylphenylethyl)] adenosine), ADAC, R- and S-PIA, N6-benzyl-NECA, and N6-benzyladenosine (Bn-Ado) were purchased from Sigma-RBI. Other N6-substituted adenosine derivatives were the kind gift of Dr. Ray A. Olsson (University of South Florida) and Dr. John W. Daly (NIDDK). All other chemicals were from standard commercial sources and of analytical grade.
2.2. Cell culture and membrane preparation
The CHO cells expressing recombinant human and rat A3ARs were cultured in DMEM and F12 (1:1) supplemented with 10% fetal bovine serum, 100 units/mL penicillin, 100 µg/mL streptomycin, 2 µmol/mL glutamine, and 800 µg/mL geneticin. After harvest and homogenization, cells were centrifuged at 500 g for 10 min, and the pellet was resuspended in 50 mM Tris–HCl buffer (pH 8.0) containing 10 mM MgCl2, 1 mM EDTA. The suspension was homogenized with an electric homogenizer for 10 s, and was then re-centrifuged at 20,000 g for 20 min at 4°. The resultant pellets were resuspended in buffer in the presence of 3 U/mL adenosine deaminase, and the suspension was stored at −80° prior to the binding experiments. The membranes from rat forebrain and striatum were prepared as previously described [11]. Striatal and forebrain tissues from Wistar rats were homogenized in ice-cold 50 mM Tris–HCl buffer, pH 7.4, using an electric homogenizer. The homogenate was centrifuged at 20,000 g for 10 min at 4°, and the pellet was washed in fresh buffer. The final pellet was stored at −80° until the binding experiments. The protein concentration was measured using the Bradford assay [12].
2.3. Binding assay
For A3AR binding experiments, the procedures used were similar to those previously described [13]. Briefly, each tube contained 100 µL of membrane suspension, 50 µL of [125I]I-AB-MECA (final concentration 0.5 nM), and 50 µL of increasing concentrations of compounds in Tris–HCl buffer (50 mM, pH 8.0) containing 10 mM MgCl2, 1 mM EDTA. Non-specific binding was determined using 10 µM Cl-IB-MECA. The mixtures were incubated at 25° for 60 min. Binding reactions were terminated by filtration through Whatman GF/B filters under reduced pressure using an MT-24 cell harvester (Brandell). Filters were washed three times with ice-cold buffer. Radioactivity was determined in a Beckman 5500B γ-counter. The binding of [3H]R-PIA to the rat forebrain and recombinant human A1ARs and the binding of [3H]CGS21680 to rat striatal and recombinant human A2AARs were performed as previously described [11].
2.4. Cyclic AMP accumulation assay
Intracellular cyclic AMP levels were measured with a competitive protein binding method [14]. CHO cells expressing recombinant human and rat A3ARs were harvested by trypsinization. After centrifugation and resuspension in medium, cells were planted in 24-well plates in 1.0 mL medium/well. After 24 hr, the medium was removed and cells were washed three times with 1 mL/well of DMEM, containing 50 mM HEPES, pH 7.4. Cells were then treated with agonists and/or test compounds in the presence of rolipram (10 µM) and adenosine deaminase (3 U/mL).After 45 min forskolin (10 µM) was added to the medium, and incubation was continued for an additional 15 min. The reaction was terminated by removing the medium, and cells were lysed upon the addition of 200 µL/well of 0.1 M ice-cold HCl. The cell lysate was resuspended and stored at −20°. For determination of cyclic AMP production, protein kinase A (PKA) was incubated with [3H]cyclic AMP (2 nM) in K2HPO4/EDTA buffer (K2HPO4, 150 mM; EDTA, 10 mM), 20 µL of the cell lysate, and 30 µL 0.1 M HCl, or 50 µL of cyclic AMP solution (0–16 pmol/200 µL for standard curve). Bound radioactivity was separated by rapid filtration through Whatman GF/C filters and washed once with cold buffer. Bound radioactivity was measured by liquid scintillation spectrometry.
2.5. Statistical analysis
Binding and functional parameters were estimated using GraphPAD Prism software (GraphPAD). IC50 values obtained from competition curves were converted to Ki values using the Cheng–Prusoff equation [15]. Data were expressed as means ± standard error.
3. Results
3.1. Activation of A3ARs by N6-substituted adenosine derivatives
The activation of A3ARs by a wide range of the N6-substituted, halo-containing, and other adenosine derivatives (Tables 1 and 2) was examined by measuring their effects on forskolin-stimulated cyclic AMP accumulation in CHO cells stably expressing the human A3AR. The efficacy of each of these adenosine derivatives was evaluated at a fixed concentration of 10 µM, and in some cases full concentration–response curves were measured. As shown in Fig. 1A, the non-selective agonist NECA 56 and the selective A3AR agonist Cl-IB-MECA 59 both inhibited maximally the forskolin-stimulated cyclic AMP production by approximately 50%. Bn-Ado 21 was demonstrated to be less efficacious, while benzyl-NECA 57 [16] showed similar maximal efficacy as the full agonist Cl-IB-MECA. The A1AR agonist, CHA 10, was shown to be less efficacious than Cl-IB-MECA, while no significant activation by the A2AAR agonist DPMA 46 [17] was demonstrated. 2-Cl-R-PIA 53 [18] was also less than fully efficacious.
Table 1.
Binding affinities of adenosine derivatives at rat A1 and A2AARs and at human and rat A3ARs and maximal agonist effects at human A3ARs expressed in CHO cells
| Compound |
N6 substitution |
Ki (rA1AR) (nM)a |
Ki (rA2AAR) (nM)a |
Ki (rA3AR) (nM)a |
Ki (hA3AR) (nM)a |
Percent activation (hA3AR) |
|---|---|---|---|---|---|---|
| N6-Substituted analogues | ||||||
| Alkyl | ||||||
| 1 | CH3 | 60 ± 11 | >10000 | 6390 ± 1630 | 9.3 ± 0.4 | 96 ± 3 |
| 2 | CH3O | 223 ± 32 | >10000 | 997 ± 343 | 28.6 ± 4.7 | 107 ± 13 |
| 3 | CH2CH3 | 4.9 ± 0.2 | 8900 ± 770 | 1050 ± 140 | 4.7 ± 1.9 | 102 ± 6 |
| 4 | CH2C(CH3)3 | 17 ± 6 | >10000 | 1870 ± 320 | 306 ± 80 | 76 ± 4 |
| 5 | CH(CH3)2 | 1.9 ± 0.1 | 2030 ± 510 | 201 ± 38 | 18.3 ± 5.5 | 111 ± 4 |
| 6 | CH(CH2CH3)2 | 0.8 ± 0.2 | 471 ± 200 | 147 ± 40 | 55.1 ± 9.5 | 99 ± 6 |
| 7 | CH(CH((CH3)2)2 | 3.8 ± 0.8 | 2170 ± 490 | 753 ± 384 | 3760 ± 840 | 21 ± 2 |
| Cycloalkyl | ||||||
| 8 | Cyclobutyl | 0.7 ± 0.1 | 1740 ± 170 | 144 ± 64 | 6.4 ± 1.0 | 100 ± 7 |
| 9 CPA | Cyclopentyl | 0.45 ± 0.04b | 462c | 240 ± 36c | 72 ± 12 | 97 ± 4 |
| 10 CHA | Cyclohexyl | 0.9 ± 0.2 | 514c | 167 ± 26c | 73 ± 23 | 76 ± 2 |
| 11 | Cyclooctyl | 1.7 ± 0.1 | 6200 ± 1120 | 498 ± 197 | 411 ± 55 | 49 ± 5 |
| 12 | exo-2-Norbornyl | 0.7 ± 0.2 | 3400 ± 140 | 253 ± 30 | 85 ± 31 | 114 ± 6 |
| 13 ENBA | (S)-endo-2-Norbornyl | 0.34 ± 0.06 | 477 ± 72 | 282 ± 101 | 915 ± 299 | 23 ± 10 |
| 14 | 7-Norbornyl | 0.48 ± 0.01 | >10000 | 229 ± 76 | 112 ± 25 | 103 ± 1 |
| 15 | 1-Adamantyl | 73 ± 3 | 14800 ± 3500 | >10000 | >10000 | 0 |
| 16 | 2-Adamantyl | 46 ± 5 | >10000 | >10000 | >10000 | 0 |
| 17 | Cyclopropylmethyl | 0.8 ± 0.3 | 1370 ± 410 | 608 ± 242 | 10.2 ± 4.1 | 108 ± 4 |
| 18 | Dicyclopropylmethyl | 0.8 ± 0.2 | 590 ± 30 | 772 ± 200 | 41.3 ± 5.3 | 31 ± 6 |
| 19 | Cyclohexylmethyl | 19 ± 7 | >10000 | 2550 ± 1610 | 263 ± 73 | 38 ± 3 |
| Aryl-containing | ||||||
| 20 | Phenyl | 3.3 ± 0.3 | 663c | 802 ± 279c | 14.9 ± 3.1 | 102 ± 9 |
| 21 | Benzyl | 175 ± 20 | 285c | 120 ± 20c | 41.3 ± 5.3 | 55 ± 3 |
| 22 | 2-Phenylethyl | 24.0 ± 8.8d | 161c | 240 ± 58c | 2.1 ± 0.4 | 84 ± 5 |
| 23 | 2-Phenylethoxy | 225 ± 45 | >10000 | 8660 ± 2900 | 88.7 ± 7.4 | 73 ± 6 |
| 24 ADAC | 4-[[[4-[[[(2-Aminoethyl)amino]-carbonyl]-methyl]aniline]-carbonyl]methyl]phenyl | 0.85c | 210c | 185 ± 64 | 13.3 ± 3.0 | 103 ± 4 |
| 25 Metrifudil | 2-Methylbenzyl | 59.6 ± 14.3e | 24.1 ± 1.8e | 35 ± 15 | 47.2 ± 10.8 | 100 ± 3 |
| 26 | 2-Methoxybenzyl | 36 ± 2 | 761 ± 460 | 29 ± 11 | 32.5 ± 4.6 | 81 ± 8 |
| 27 | 2-Fluorobenzyl | 6 ± 1 | 551 ± 282 | 60 ± 8 | 339 ± 5 | 67 ± 7 |
| 28 | 2-Chlorobenzyl | 17 ± 3 | 93 ± 16 | 13 ± 1 | 17.3 ± 3.2 | 95 ± 1 |
| 29 | 3-Chlorobenzyl | 45 ± 10 | >10000 | 35 ± 20 | 4.4 ± 1.7 | 80 ± 3 |
| 30 | 4-Chlorobenzyl | 61 ± 3 | 5120 ± 1230 | 96 ± 38 | 47.5 ± 4.1 | 96 ± 2 |
| 31 | 2-Pyridylmethyl | 225 ± 5 | >10000 | 111 ± 32 | 115 ± 33 | 73 ± 15 |
| 32 | 3-Pyridylmethyl | 115 ± 4 | 3220 ± 1210 | 288 ± 67 | 4.5 ± 1.1 | 100 ± 6 |
| 33 | 4-Pyridylmethyl | 70 ± 5 | 3860 ± 1520 | 67 ± 9 | 80.1 ± 20.0 | 99 ± 12 |
| 34 | 2-Furanylmethyl | 95 ± 9 | >10000 | 301 ± 72 | 21.8 ± 3.9 | 54 ± 10 |
| 35 | 2-Thienylmethyl | 36 ± 7 | 734 ± 60 | 112 ± 33 | 59.8 ± 25.7 | 92 ± 2 |
| 36 | 3-Thienylmethyl | 45 ± 9 | 3300 ± 450 | 297 ± 53 | 26.3 ± 8.2 | 97 ± 8 |
| 37 | 1-Naphthylmethyl | 13 ± 3 | 1120 ± 580 | 2.7 ± 0.5 | 25.2 ± 9.7 | 67 ± 8 |
| 38 | R-1-Phenylethyl | 3.4 ± 0.4 | 1300 ± 620 | 60 ± 25 | 113 ± 22 | 76 ± 10 |
| 39 | S-1-Phenylethyl | 195 ± 20 | >10000 | 1110 ± 460 | 68.7 ± 17.3 | 83 ± 5 |
| 40 | R-1-Indanyl | 65 ± 10 | 2480 ± 740412f | 79 ± 12 | 233 ± 27 | 82 ± 5 |
| 41 R-PIA | R-1-Phenyl-2-propyl | 1.2 ± 0.1 | 124c | 158 ± 52c | 8.7 ± 0.9 | 102 ± 6 |
| 42 S-PIA | S-1-Phenyl-2-propyl | 49.3c | 1820c | 920 ± 311c | 68 ± 12 | 97 ± 3 |
| 43 | R-1-Phenyl-2-pentyl | 3.34 ± 0.66d | >10000 | 76 ± 18 | 70.9 ± 26.2 | 92 ± 9 |
| 44 | S-1-Phenyl-2-pentyl | 282 ± 17d | >10000 | 1810 ± 530 | 37 ± 13 | 101 ± 10 |
| 45 | R-1-Phenyl-isopentyl | 8.89 ± 1.97d | 3250 ± 580 | 103 ± 43 | 96 ± 17 | 77 ± 4 |
| 46 DPMA | 2-(3,5-Dimethoxy-phenyl)-2-(2-methylphenylethyl | 142c | 4.4c | 3570 ± 1700c | 106 ± 22 | 0 |
| 47 | (1R,2S)-2-Phenyl-1-cyclopropyl | 15.2 ± 3.2d | 3040 ± 490 | 358 ± 33 | 24.1 ± 10.9 | 87 ± 4 |
| 48 | (1S,2R)-2-Phenyl-1-cyclopropyl | 11.8 ± 2.4d | 560 ± 232 | 694 ± 157 | 0.63 ± 0.17 | 117 ± 9 |
| 49 | cis-(1R,2R)-2-Phenylcyclohexyl | 15 ± 6 | >10000 | 1170 ± 30 | 1450 ± 241 | 73 ± 12 |
| 50 | trans-(1R,2S)-2-Phenylcyclohexyl | 6 ± 3 | 672 ± 51 | 279 ± 41 | 559 ± 96 | 72 ± 9 |
| Compound | Substitution |
Ki (rA1AR) (nM) |
Ki (rA2AAR) (nM) |
Ki (rA3AR) (nM) |
Ki (hA3AR) (nM) |
Percent activation (hA3AR) |
| Other halo analogues | ||||||
| 51 CADO | 2-Chloro | 6.7 ± 1.0b | 63e | 1890 ± 900e | 87 ± 24 | 100 ± 7 |
| 52 FADO | 2-Fluoro | 68.3 ± 18.9d | 28c | 4590 ± 2410 | 99 ± 13 | 31 ± 3 |
| 53 | 2-Chloro-R-PIA | 0.86 ± 0.14d, 1.4 ± 0.1g | 1070 ± 250 | 34 ± 10 | 13.1 ± 0.9 | 76 ± 13 |
| 54 | 5′-Chloro-5′-deoxyadenosine | 20 ± 1b | 62.7 ± 14.4 | 4590 ± 2410 | 107 ± 6 | 9 ± 4 |
All A3AR experiments were performed using adherent CHO cells stably transfected with cDNA encoding the human or rat A3 receptor. Percent activation of the human A3AR was determined at 10 µM. Unless otherwise noted, Ki values at A1AR are from Daly et al. [10]. Binding at A1 and A2AARs was carried out as described in Section 2. Values from the present study are means ± SEM, N = 3–5.
Data from Daly and Padgett [19].
Data from Van Galen et al. [16].
Ki values at the A1AR determined in the present study.
Data from Siddiqi et al. [20].
Data from Trivedi et al. [17].
Data from Thompson et al. [18].
Table 2.
Binding affinities of reference adenosine agonists at rat A1 and A2A receptors and at human and rat A3 receptors and maximal agonist effects at human A3 receptors expressed in CHO cells
| Compound | Ki (rA1AR) (nM)a | Ki (rA2AAR) (nM)a | Ki (rA3AR) (nM)a | Ki (hA3AR) (nM)a | Percent activation (hA3AR) |
|---|---|---|---|---|---|
| 55 CCPA | 0.6b | 950b | 237 ± 71b | 38 ± 6c | 0d |
| 56 NECA | 6.3b | 10.3b | 113 ± 34b | 35 ± 12 | 103 ± 6 |
| 57 N6-Bn-NECA | 87 ± 14b | 95 ± 25b | 6.8 ± 2.5b | 10.8 ± 1.5 | 107 ± 8 |
| 58 CGS21680 | 2600b | 15b | 584 ± 32b | 114 ± 16d | 98 ± 5 |
| 59 Cl-IB-MECA | 820e | 470e | 0.33 ± 0.08e | 1.4 ± 0.3d | 100 |
| 60 MRS 542 | 18.5e | 38.5e | 1.41 ± 0.17e | 1.8 ± 0.1d | 0d |
All A3AR experiments were performed using adherent CHO cells stably transfected with cDNA encoding the human or rat A3 receptor. Binding at A1 and A2AARs was carried out as described in Section 2. Percent activation of the human A3AR was determined at 10 µM. Values from the present study are means ± SEM, N = 3.
Data from Van Galen et al. [16].
Data from Gao and Jacobson [9].
Data from Gao et al. [8].
Data from Kim et al. [21].
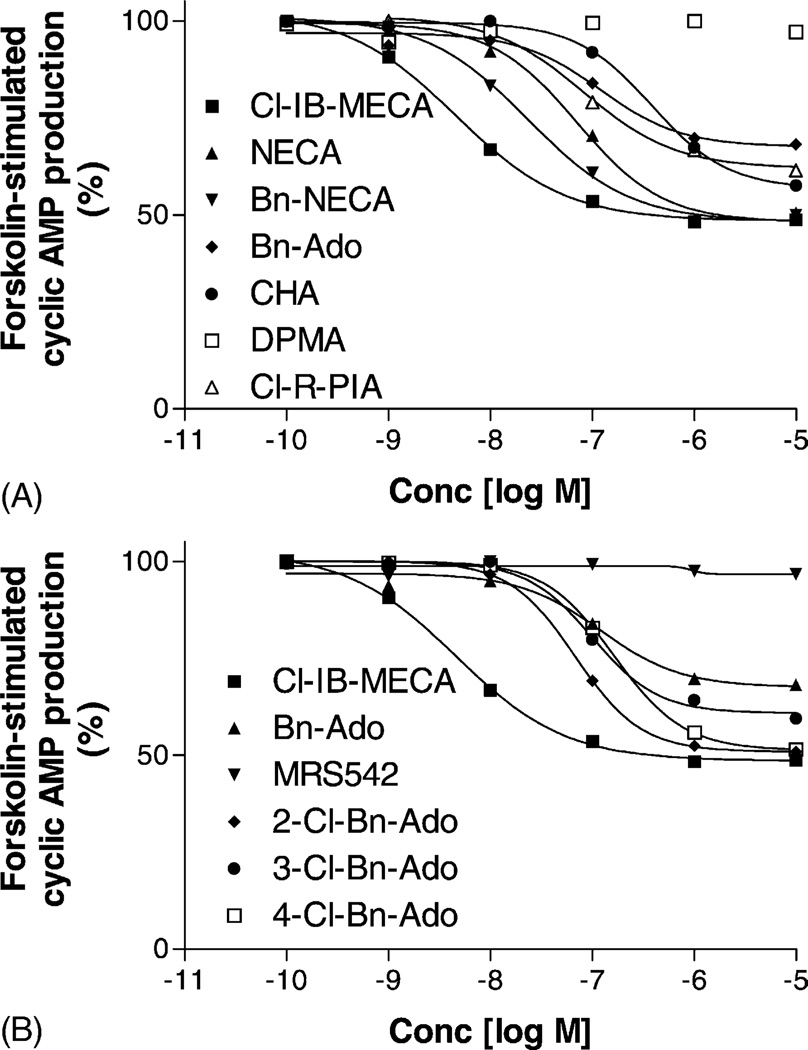
Fig. 1.
Inhibition of forskolin-stimulated cyclic AMP production in CHO cells stably transfected with the human A3AR, induced by various agonists (A) and by N6-benzyl derivatives (B). All experiments were performed in the presence of 10 µM rolipram and 3 U/mL adenosine deaminase. Forskolin (10 µM) was used to stimulate cyclic AMP levels. The level of cyclic AMP corresponding to 100% was 220 ± 30 pmol/mL. The data shown were from one experiment performed in duplicate and are typical of three independent experiments giving similar results. IC50 values were (in nM): 10, CHA, 354 ± 116; 21, Bn-Ado, 120 ± 34; 28, 2-Cl-Bn-Ado, 81 ± 19; 29, 3-Cl-Bn-Ado, 103 ± 22; 30, 4-Cl-Bn-Ado, 146 ± 31; 53, Cl-R-PIA, 76± 21; 56, NECA, 62 ± 17; 57, Bn-NECA, 18.2 ± 3.4; 59, Cl-IB-MECA, 3.2 ± 1.4.
Various fluoro and chloro substitutions of the N6-benzyl group may increase the efficacy of Bn-Ado 21. Chloro substitutions at the 2- or 4-position converted Bn-Ado 21 into full agonists 28 and 30, while substitution at the 3-position increased efficacy only to a limited extent (Fig. 1B). The 2-fluorobenzyl substituent of 27 [19] also significantly increased the efficacy. In contrast, the chloro substituent at the 2-position of the adenine moiety might convert the full or partial agonists into antagonists, which have been demonstrated earlier [8,9].
Other N6-substituents that significantly decreased the efficacy (Tables 1 and 2) included: dicyclopropylmethyl 18, 2-phenylethyl 22, 2-phenylethoxy 23, 2-methoxybenzyl 26, 3-chlorobenzyl 29, 2-pyridylmethyl 31, 2-furanylmethyl 34, 1-naphthylmethyl 37 R- and S-1-phenylethyl 38 and 39, R-phenyl-isopentyl 45, and (1R,2S)-2-phenyl-1-cyclopropyl 47. The 3-pyridylmethyl derivative 32 was a potent, full agonist. 5′-Chloro-5′-deoxyadenosine 54 displayed low efficacy at the human A3AR. It should be noted that some compounds tested in this study could not be clearly identified as full or partial agonists due to low affinity for the human A3AR, although the efficacy observed at 10 µM was reduced. These include the following N6 substitutions: 2,2-dimethylpropyl 4, 2,4-dimethyl-3-pentyl 7, cyclooctyl 11, (S)-endo-2-norbornyl 13, 1-adamantyl 15, 2-adamantyl 16, cyclohexylmethyl 19, cis- and trans-(1R,2S)-2-phenyl-cyclohexyl 49 and 50.
3.2. Binding of the N6-substituted adenosine derivatives to A1, A2A, and A3ARs
We initially measured the affinity and potency of the adenosine derivatives for the human A3AR. We also examined the selectivity and species differences by determining the binding affinity of these compounds for rat A1, A2A, and A3ARs.
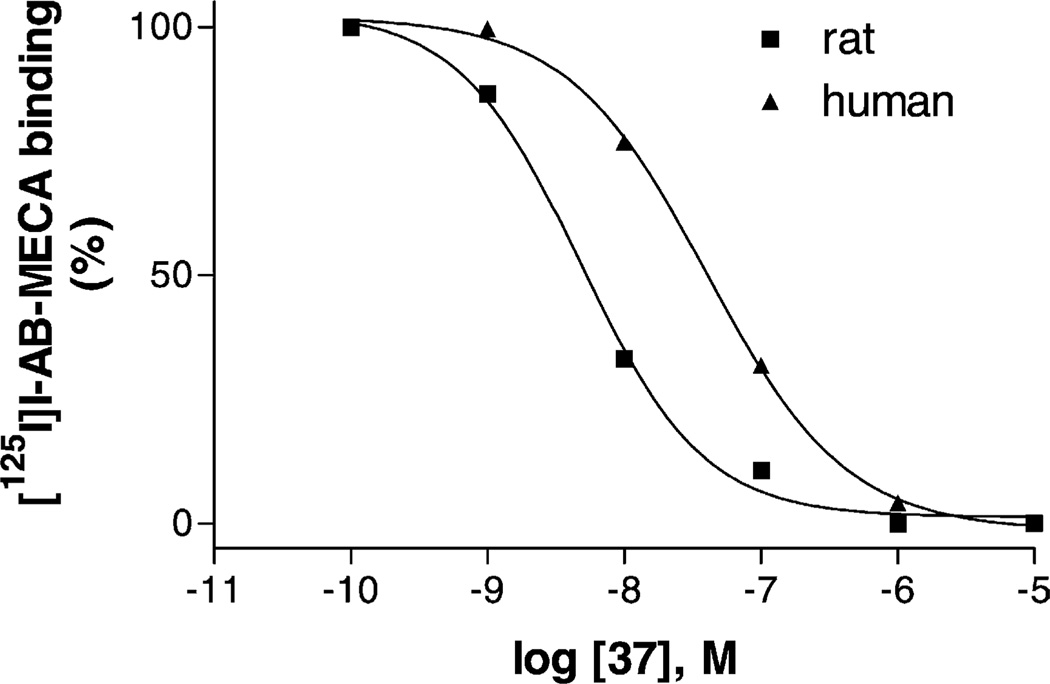
Fig. 2 shows that two compounds were found to be more potent at human than at rat A3ARs. N6-Methyladenosine 1 and N6-[(1S,2R)-2-phenyl-1-cyclopropyl]adenosine 48 were 687- and 1100-fold more potent, respectively, in binding to human A3ARs vs. rat A3ARs. A number of other compounds, including the simplest substituted adenosine derivatives CADO and FADO, were also found to be more potent for human than for the rat A3AR (Table 1). Small N6-substituents (1–3) were consistently associated with selectivity for human A3ARs vs. rat A3ARs.
Fig. 2.
Competition for radioligand binding ([125I]I-AB-MECA) at A3ARs in two species, by two representative adenosine analogues 1 and 48, which were more potent at human than at rat A3ARs. The procedures were described in “Section 2”. The data points are from a representative experiment performed in duplicate. The mean Ki values calculated from three independent experiments are listed in the text.
The reverse species dependence was also noted. Fig. 3 shows that 1-naphthylmethyladenosine 37 was approximately 10-fold more potent for rat than for human A3ARs. Some other compounds, including ENBA 13 and 2-fluorobenzyladenosine 27, were also shown to be more potent for rat than for human A3ARs (Table 1).
Fig. 3.
Competition for radioligand binding ([125I]I-AB-MECA) at A3ARs in two species, by an adenosine analogue which was more potent at rat than at human A3ARs. The procedures were described in “Section 2”. The data points are from a representative experiment performed in duplicate. The mean Ki values calculated from three independent experiments are listed in the text.
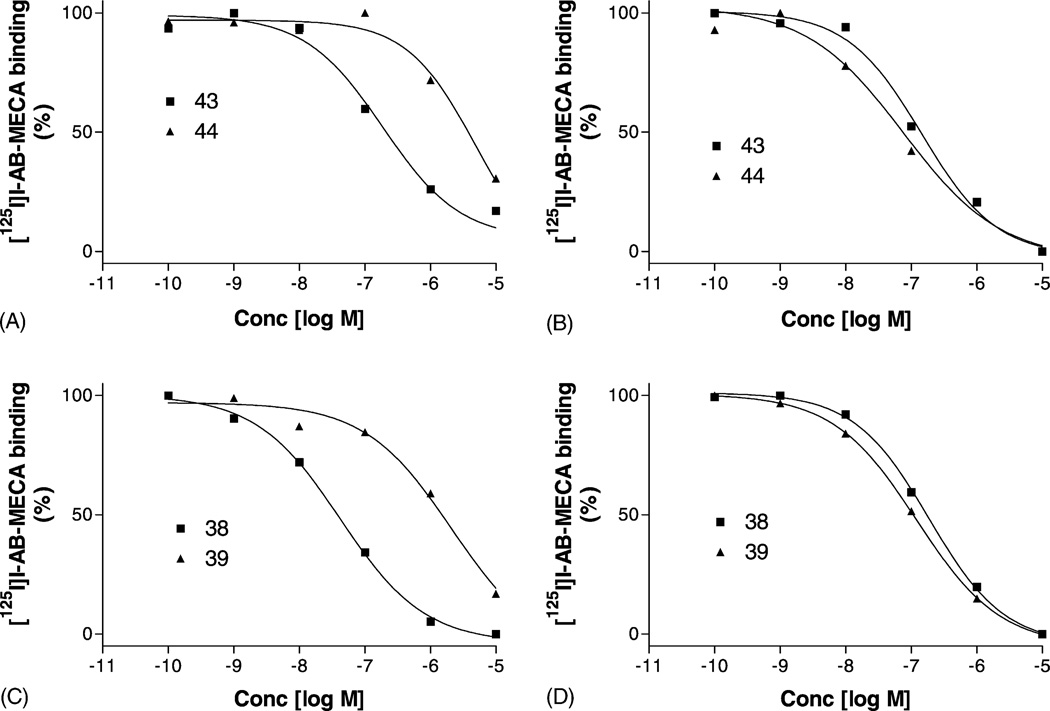
Stereoselectivity of binding of adenosine derivatives has been established at A1 and A2AARs [10], but not fully investigated at A3ARs. Fig. 4 shows compounds 38 and 39 N6-(R- and S-1-phenylethyl), as well as compounds 43 and 44 N6-(R- and S-1-phenyl-2-pentyl) showed stereoselectivity for rat but not for human A3ARs. In the case of compounds 38 and 39, the potency of the R-isomer was similar for rat and human, while the S-isomer was 16-fold more potent at the human than at the rat A3AR. Similarly, in the case of compounds 43 and 44, the potency of the R-isomer was similar for rat and human, while the S-isomer was 48-fold more potent for human than for rat A3ARs (Table 1).
Fig. 4.
Competition for radioligand binding ([125I]I-AB-MECA) at A3ARs by pairs of R- and S-diastereoisomers (N6-(1-phenyl-2-pentyl)adenosines (A) and (B); N6-(1-phenylethyl)adenosines (C) and (D) at rat (A) and (C) and at human (B) and (D) A3ARs, demonstrating that stereoselectivity of binding was species dependent. The procedures were described in “Section 2”. The data points are from a representative experiment performed in duplicate. The mean Ki values calculated from three independent experiments are listed in the text.
Several compounds, including N6-cyclooctyl 11, N6-(exo-norbornyl) 12, ENBA 13, and N6-(7-norbornyl) 14, were found to be over 200-fold selective for rat A1 vs. rat A2A and A3ARs (Table 3). We further tested the possible selectivity of these compounds for the human A1AR. However, as shown in Table 3, most of these compounds only showed less than 100-fold selectivity for human A1 vs. human A2A and A3ARs. Surprisingly, ENBA was demonstrated to be the most potent and most selective compound for both human and rat A1ARs. Similar to CGS21680, DPMA, shown to be selective for the rat A2AAR, was shown to be non-selective at human A1, A2A, and A3ARs (Tables 1 and 3). Compound 29 (3-chlorobenzyl) was demonstrated to be over 100-fold selective for both human and rat A1 and A3 vs. A2AARs. Compounds 38 (R) and 39 (S), as well as 43 (R) and 44 (S), diastereoisomers which lost stereospecificity of binding at the human A3AR, were further evaluated at the human A1AR. In contrast to the human A3AR, stereospecific binding was preserved at the human A1AR (Table 3).
Table 3.
Comparison of binding affinity of selected adenosine derivatives at human A1, A2A, and A3ARs (N6-substituent or compound abbreviation in parentheses)
| Ki (nM) | |||
|---|---|---|---|
| hA1 | hA2A | hA3 | |
| 11 (cyclooctyl) | 6.4 ± 1.4 | >10000 | 411 ± 55 |
| 12 (exo-norbornyl) | 2.4 ± 0.8 | >10000 | 85 ± 31 |
| 13 ENBA | 0.38 ± 0.19 | >10000 | 915 ± 299 |
| 14 (7-norbornyl) | 2.1 ± 0.5 | >10000 | 112 ± 25 |
| 29 (3-chlorobenzyl) | 34.9 ± 9.6 | >10000 | 4.4 ± 1.7 |
| 38 (R-1-phenylethyl) | 28.6 ± 9.8 | 1750 ± 540 | 113 ± 22 |
| 39 (S-1-phenylethyl) | 94.5 ± 29.5 | >10000 | 68.7 ± 17.3 |
| 43 (R-1-phenyl-2-pentyl) | 16.9 ± 5.2 | 1120 ± 320 | 70.9 ± 26.2 |
| 44 (S-1-phenyl-2-pentyl) | 113 ± 18 | 6840 ± 2350 | 37 ± 13 |
| 46 DPMA | 168 ± 29 | 153 ± 26 | 106 ± 22 |
| 58 CGS21680 | ND | 43 ± 18 | 114 ± 16 |
| 59 Cl-IB-MECA | 1240 ± 320 | 5360 ± 2470 | 1.4 ± 0.3 |
Values from the present study are means ± SEM, N = 3.
4. Discussion
4.1. Efficacy
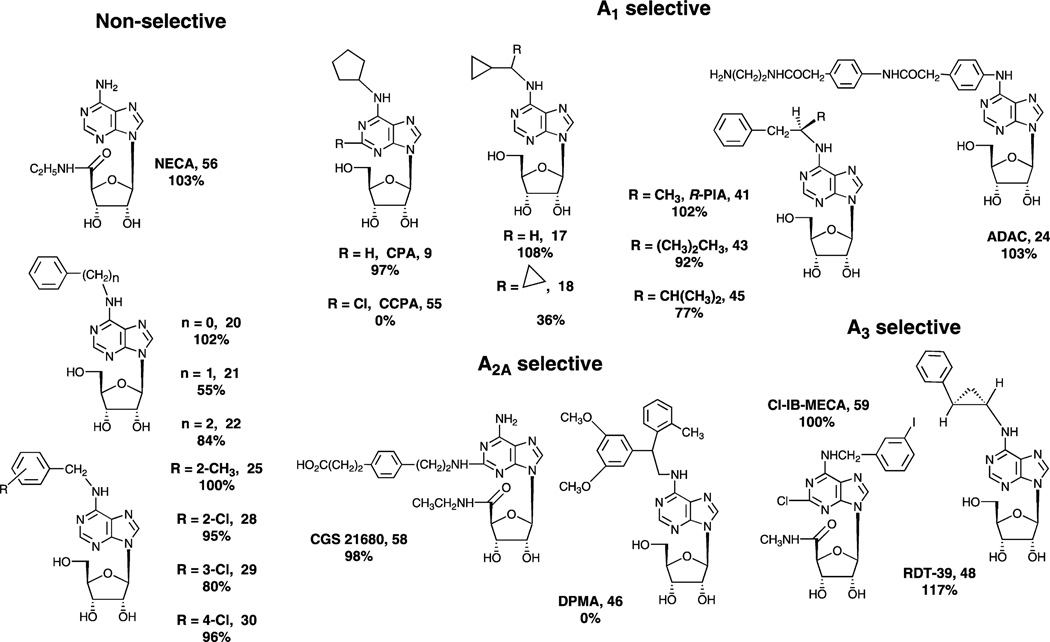
It has been previously demonstrated that N6-(3-iodobenzyl) adenosine was a partial agonist for the human A3AR [8]. However, it was not known whether the decrease in efficacy was due to the benzyl group or its iodo substitution. Here, we further demonstrated the N6-benzyl group itself decreased the maximal efficacy. Furthermore, it was demonstrated in this study that a number of other structural modifications of adenosine (Fig. 5) contributed variously to affinity and efficacy. The full agonist of the A1AR, CHA, was shown to be a partial agonist of the A3AR. A potent and full agonist for the A2AAR, DPMA, was demonstrated to be a moderately potent antagonist of the A3AR.
Fig. 5.
Structures of representative adenosine derivatives and their efficacy (% of inhibition of adenylate cyclase at 10 µM compared to the effect of 10 µM Cl-IB-MECA) as agonists of the human A3AR.
In previous studies, it has been demonstrated that 2-chloro substitution of the adenine ring may both increase the affinity and decrease the efficacy of the adenosine derivatives for the human A3AR [6,8,9]. Here, we further demonstrated that the chloro substituent might alternately decrease, increase, or have no effect on the efficacy of the adenosine derivatives depending on the position of substitution. For example, R-PIA was a full agonist, while a chloro group substituted at the 2-position of the adenine moiety (2-Cl-R-PIA) significantly decreased the efficacy. In contrast, a chloro substituent at the 2- (28) or 4- (30) positions of the benzyl group of Bn-Ado (21, itself having 55% of maximal efficacy) significantly increased the efficacy, converting the partial agonist 21 into a full agonist. Chloro substitution at the 3-position of the benzyl group (29) also significantly increased the efficacy, although to a lesser extent. Similarly, a fluoro substituent at the 2-position of the benzyl ring (27) also induced a modest increase of the efficacy.
In contrast to the N6-benzyl group, which decreased the efficacy, the N6-phenyl group did not significantly influence the efficacy, thus N6-phenyladenosine 20 was still a full agonist. Also, similar to Cl-IB-MECA [8,21], the benzyl group did not influence the efficacy of NECA, and thus, N6-benzyl-NECA [16] was a full agonist.
The appending of an additional cyclopropyl group to compound 17, obtaining compound 18, which only caused a slight change of its affinity, dramatically diminished its maximal A3AR efficacy. Similarly, bridging the methylene group in compound 25 to give 40, thus introducing a ring constraint, also reduced A3 efficacy. A comparison of compounds 17 and 19 suggested that the enlargement of a ring attached to the N6-methyl group appeared to lower both affinity and efficacy at the human A3AR, while lengthening the chain between N6 and the phenyl group seemed to decrease efficacy, with minimum efficacy observed with compound 21. Compared with compound 22, the introduction of an oxygen (hydroxylamine linkage of compound 23) seemed to induce a larger decrease in its affinity for all three AR subtypes, and a smaller decrease of its maximal A3AR efficacy. This is in contrast to compound 2, which showed enhanced potency at the rat A3AR as a result of the oxygen inclusion [22]. Branching at the terminal alkyl position (compound 45 vs. compound 43) did not change the A3 affinity but diminished the A3 efficacy. A 5′-Cl-5′-deoxy substitution (compound 54), already reported for adenosine agonists [5,22], greatly reduced A3AR efficacy.
4.2. Selectivity
N6-(2-Methylbenzyl)adenosine (25) [20] was found to have comparable affinity at rat A1, A2A, and A3ARs, while N6-(2-methoxybenzyl)adenosine 26 was found to be selective for both A1 and A3 but not A2AARs. N6-(2-Fluorobenzyl) adenosine (27) was >10-fold selective for A1 over A2A or A3ARs, while N6-(2-chlorobenzyl)adenosine (28) was shown to have similar affinity for A1 and A3ARs. N6-(3-Chlorobenzyl)adenosine (29) was found to be extremely selective for A1 and A3 vs. A2AARs. N6-(Cyclooctyl)adenosine (11), the N6-norbornyladenosines (12–14), and the N6-(1- and 2-adamantyl)adenosine derivatives (15 and 16) were demonstrated to be selective for the A1AR.
Some compounds shown to be selective agonists for a particular adenosine receptor subtype in the rat, were less selective among human ARs. For example, DPMA was selective for the A2AAR only in the rat. R-PIA was selective for the rat A1AR, but had similar affinity for human A1 and A3ARs [2]. Compared with R-PIA (41), the substitution of the propyl group by a pentyl group (43) induced a dramatic decrease in A2A affinity, but only minor change in A1 and A3AR affinity. CGS21680 58, originally shown to be a selective agonist for rat A2AAR, was demonstrated to be equipotent at human A2A and A3ARs [2].
4.3. Species difference: human and rat A3ARs
The identity in amino acid sequence between human and rat A3ARs is only 72% [23], which is much lower that the interspecies homology of A1, A2A, and A2BARs. It is known that almost all non-nucleoside antagonists of the human A3AR showed extremely low affinity for rat A3 receptors. For example, MRS 1220 [24], MRE 3008F20 [25], and (5-[[(4-pyridyl)amino]carbonyl]amino-8-methyl-2-(2-furyl)-pyrazolo[4,3-e]1,2,4-triazolo[1,5-c]pyrimidine hydrochloride) (Ki = 0.01 nM) [26] displayed exceptionally high affinity for the human A3AR, but extremely low affinity for the rat A3AR (Ki > 1000 nM). Adenosine derivatives were presumed to display less variability of affinity than non-nucleoside antagonists between human and rat A3ARs. However, as shown in the present study, species differences in binding were characterized for many of these compounds. For example, N6-[(1S,2R)-2-phenyl-1-cyclopropyl]adenosine 48 was demonstrated to be over 1000-fold more potent for human than rat A3AR. A small N6-substituent was demonstrated to be extremely important for the species difference between human and rat A3ARs. For example, N6-methyladenosine 1 was demonstrated to be approximately 700-fold more potent at human A3ARs vs. rat A3ARs. A number of other compounds, including 3 and 22, were also demonstrated to be over 100-fold more potent at human than at rat A3ARs. Interestingly, several compounds were found to be more potent at the rat A3AR (compounds 7, 13, 27, 37, and 40). Compounds 38 and 39 (N6-R- and S-1-phenylethyl) and compounds 43 and 44 (N6-R- and S-1-phenyl-2-pentyl) showed stereoselectivity for rat but not for human A3ARs. In each case the R-isomer was equipotent at rat and human A3ARs, while the S-isomer was more potent at human by 20- and 50-fold for 39 and 44, respectively.
In conclusion, it was demonstrated in this study that a number of substitutions of adenosine contributed differently to affinity and efficacy. A number of full agonists for A1 and A2AARs were demonstrated to be partial agonists or antagonists for the hA3AR. Among N6-alkyl substitutions, small N6-alkyl groups were associated with selectivity for human A3ARs vs. rat A3ARs, and multiple points of branching were associated with decreased hA3AR efficacy. N6-Cycloalkyl-substituted adenosines were full (≤5 carbons) or partial (≥6 carbons) hA3AR agonists. N6-(endo-Norbornyl)adenosine 13 was the most selective for both rat and human A1ARs. Numerous N6-arylmethyl analogues, including substituted benzyl, tended to be more potent in binding to A1 and A3 vs. A2AARs (with variable degrees of partial to full A3AR agonism). A chloro substituent decreased the efficacy depending on its position on the benzyl ring. The A3AR affinity and efficacy of N6-arylethyl adenosines depended highly on stereochemistry, steric bulk, and ring constraints. The identification of dual acting A1/A3 agonists, such as 29, 39, and 53, might be useful for cardioprotection [3].
Acknowledgments
We are grateful to Prof. Ray A. Olsson and Dr. John W. Daly for the gift of adenosine agonists and for helpful discussions. We thank Dr. Gary L. Stiles (Duke University) for the gifts of CHO cells expressing the human and rat A3 receptors. N.M. thanks the Cystic Fibrosis Foundation (Bethesda, MD) for financial support.
Abbreviations
- ADAC
N6-[4-[[[4-[[[(2-aminoethyl)amino]carbonyl]-methyl]aniline]carbonyl]methyl]phenyl]adenosine
- Bn-Ado
N6-benzyladenosine
- Cl-IB-MECA
2-chloro-N6-(3-iodobenzyl)-5′-N-methylcarbamoyladenosine
- CGS21680
2-[p-(2-carboxyethyl)phenyl-ethylamino]-5′-N-ethylcarboxamidoadenosine
- CHA
N6-cyclohexyladenosine
- DBXRM
N-methyl-1,3-dibutylxanthine-7-β-d-ribofuronamide
- CPA
N6-cyclopentyladenosine
- DPMA
N6-[2-(3.5-dimethoxyphenyl)-2-(2-methylphenylethyl)]adenosine
- CADO
2-chloroadenosine
- ENBA
(S)-endo-2-norbornyladenosine
- FADO
2-fluoroadenosine
- GPCR
G protein-coupled receptor
- Tris
tris(hydroxymethyl)aminomethane
- I-AB-MECA
N6-(4-amino-3-iodobenzyl)-5′-N-methylcarboxamidoadenosine
- MRS 542
2-chloro-N6-(3-iodobenzyl)adenosine
- MRS 1220
N-[9-chloro-2-(2-furanyl)[1,2,4]triazolo[1,5-c]quinazolin-5-yl]benzene-acetamide
- NECA
5′-N-ethylcarboxamidoadenosine
- PIA
N6-[phenylisopropyl]adenosine
- RDT-39
N6-[(1S,2R)-2-phenyl-1-cyclopropyl]adenosine
References
- 1.Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 2.Klotz KN, Hessling J, Hegler J, Owman C, Kull B, Fredholm BB, Lohse MJ. Comparative pharmacology of human adenosine receptor subtypes—characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch Pharmacol. 1998;357:1–9. doi: 10.1007/pl00005131. [DOI] [PubMed] [Google Scholar]
- 3.Liang BT, Jacobson KA. A physiological role of the adenosine A3 receptor: sustained cardioprotection. Proc Natl Acad Sci USA. 1998;95:6995–6999. doi: 10.1073/pnas.95.12.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Von Lubitz DK, Lin RC, Popik P, Carter MF, Jacobson KA. Adenosine A3 receptor stimulation and cerebral ischemia. Eur J Pharmacol. 1994;263:59–67. doi: 10.1016/0014-2999(94)90523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Tilburg EW, von Frijtag Drabbe Künzel J, de Groote M, Vollinga RC, Lorenzen A, IJzerman AP. N6,5′-Disubstituted adenosine derivatives as partial agonists for the human adenosine A3 receptor. J Med Chem. 1999;42:1393–1400. doi: 10.1021/jm981090+. [DOI] [PubMed] [Google Scholar]
- 6.van Tilburg EW, van der Klein PA, von Frijtag Drabbe Künzel J, de Groote M, Stannek C, Lorenzen A, IJzerman AP. 5′-O-Alkyl ethers of N,2-substituted adenosine derivatives: partial agonists for the adenosine A1 and A3 receptors. J Med Chem. 2001;44:2966–2975. doi: 10.1021/jm001114o. [DOI] [PubMed] [Google Scholar]
- 7.Gao ZG, Chen A, Barak D, Kim SK, Müller CE, Jacobson KA. Identification by site-directed mutagenesis of residues involved in ligand recognition and activation of the human A3 adenosine receptor. J Biol Chem. 2002;277:19056–19063. doi: 10.1074/jbc.M110960200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao ZG, Kim SK, Biadatti T, Chen W, Lee K, Barak D, Kim SG, Johnson CR, Jacobson KA. Structural determinants of A3 adenosine receptor activation: nucleoside ligands at the agonist/antagonist boundary. J Med Chem. 2002;45:4471–4484. doi: 10.1021/jm020211+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao ZG, Jacobson KA. 2-Chloro-N6-cyclopentyladenosine, adenosine A1 receptor agonist, antagonizes the adenosine A3 receptor. Eur J Pharmacol. 2002;443:39–42. doi: 10.1016/s0014-2999(02)01552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daly JW, Padget W, Thompson RD, Kusachi S, Bungi WJ, Olsson RA. Structure–activity relationships for N6-substituted adenosines at a brain A1-adenosine receptor with a comparison to an A2-adenosine receptor regulating coronary blood flow. Biochem Pharmacol. 1986;35:2467–2481. doi: 10.1016/0006-2952(86)90042-0. [DOI] [PubMed] [Google Scholar]
- 11.Gao ZG, Van Muijlwijk-Koezen JE, Chen A, Muller CE, IJzerman AP, Jacobson KA. Allosteric modulation of A3 adenosine receptors by a series of 3-(2-pyridinyl)isoquinoline derivatives. Mol Pharmacol. 2001;60:1057–1063. [PMC free article] [PubMed] [Google Scholar]
- 12.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 13.Olah ME, Gallo-Rodriguez C, Jacobson KA, Stiles GL. 125I-4-Aminobenzyl-5′-N-methylcarboxamidoadenosine, a high affinity radioligand for the rat A3 adenosine receptor. Mol Pharmacol. 1994;45:978–982. [PMC free article] [PubMed] [Google Scholar]
- 14.Nordstedt C, Fredholm BB. A modification of a protein-binding method for rapid quantification of cAMP in cell-culture supernatants and body fluid. Anal Biochem. 1990;189:231–234. doi: 10.1016/0003-2697(90)90113-n. [DOI] [PubMed] [Google Scholar]
- 15.Cheng Y-C, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 16.Van Galen PJM, van Bergen AH, Gallo-Rodriguez C, Melman N, Olah ME, IJzerman AP, Stiles GL, Jacobson KA. A binding site model and structure–-activity relationships for the rat A3 adenosine receptor. Mol Pharmacol. 1994;45:1101–1111. [PMC free article] [PubMed] [Google Scholar]
- 17.Trivedi BK, Blankley CJ, Bristol JA, Hamilton HW, Patt WC, Kramer WJ, Johnson SA, Bruns RF, Cohen DM, Ryan MJ. N6-Substituted adenosine receptor agonists: potential antihypertensive agents. J Med Chem. 1991;34:1043–1049. doi: 10.1021/jm00107a025. [DOI] [PubMed] [Google Scholar]
- 18.Thompson RD, Secunda S, Daly JW, Olsson RA. Activity of N6-substituted 2-chloroadenosines at A1 and A2 adenosine receptors. J Med Chem. 1991;34:3388–3390. doi: 10.1021/jm00116a007. [DOI] [PubMed] [Google Scholar]
- 19.Daly JW, Padgett W. Agonist activity of 2- and 5′-substituted adenosine analogs and their N6-cycloalkyl derivatives at A1- and A2-adenosine receptors coupled to adenylate cyclase. Biochem Pharmacol. 1992;43:1089–1093. doi: 10.1016/0006-2952(92)90616-q. [DOI] [PubMed] [Google Scholar]
- 20.Siddiqi SM, Jacobson KA, Esker JL, Olah ME, Ji X, Melman N, Tiwari KN, Secrist JA, III, Schneller SW, Cristalli G, Stiles GL, Johnson CR, IJzerman AP. Search for newpurine- and ribose-modified adenosine analogues as selective agonists and antagonists at adenosine receptors. J Med Chem. 1995;38:1174–1188. doi: 10.1021/jm00007a014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim HO, Ji X-d, Siddiqi SM, Olah ME, Stiles GL, Jacobson KA. 2-Substitution of N6-benzyladenosine-5′-uronamides enhances selectivity for A3-adenosine receptors. J Med Chem. 1994;37:3614–3621. doi: 10.1021/jm00047a018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mogensen JP, Roberts SM, Bowler AN, Thomsen C, Knutsen LJ. The synthesis of new adenosine A3 selective ligands containing bioisosteric isoxazoles. Bioorg Med Chem Lett. 1998;8:1767–1770. doi: 10.1016/s0960-894x(98)00302-3. [DOI] [PubMed] [Google Scholar]
- 23.Salvatore CA, Jacobson MA, Taylor HE, Linden J, Johnson RG. Molecular cloning and characterization of the human A3 adenosine receptor. Proc Natl Acad Sci USA. 1993;90:10365–10369. doi: 10.1073/pnas.90.21.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobson KA, Park KS, Jiang J-l, Kim Y-C, Olah ME, Stiles GL, Ji X-d. Pharmacological characterization of novel A3 adenosine receptor-selective antagonists. Neuropharmacology. 1997;36:1157–1165. doi: 10.1016/s0028-3908(97)00104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varani K, Merighi S, Gessi S, Klotz KN, Leung E, Baraldi PG, Cacciari B, Romagnoli R, Spalluto G, Borea PA. [3H]MRE 3008F20: a novel antagonist radioligand for the pharmacological and biochemical characterization of human A3 adenosine receptors. Mol Pharmacol. 2000;57:968–975. [PubMed] [Google Scholar]
- 26.Maconi A, Pastorin G, Da Ros T, Spalluto G, Gao ZG, Jacobson KA, Baraldi PG, Cacciari B, Varani K, Moro S, Borea PA. Synthesis, biological properties, and molecular modeling investigation of the first potent, selective, and water-soluble human A3 adenosine receptor antagonist. J Med Chem. 2002;45:3579–3582. doi: 10.1021/jm020974x. [DOI] [PMC free article] [PubMed] [Google Scholar]