Abstract
There is evidence that abnormal cerebral development during childhood is a risk factor for various cognitive and psychiatric disorders. There is not, however, sufficient normative data available on large samples of typically developing children, especially within the narrow preadolescent age range. We analyzed high resolution MRI images from 126 normally developing children between ages 6 and 10 years. Age related differences in cortical thickness and in the volumes of major subcortical structures were assessed. Thinner cortices were observed in the occipital, parietal and somatosensory regions as well as in distinct regions of the temporal and frontal lobes with increasing age. Among the major subcortical sructures analyzed in this study, only the thalamus showed increased volume with age after accounting for intracranial volume. Within the age range studied age-related cortical and subcortical differences were similar for boys and girls except for the right insula, where girls showed slight increase in thickness with age. The findings reveal age-associated changes in brain anatomy, providing information about the trajectory of normal brain development during late childhood.
Keywords: Brain development, neurodevelopment, cortical thickness analysis
1. Introduction
In the first months after birth there is enormous growth of gray matter that is regionally specific. Occipital regions grow faster than prefrontal regions (Gilmore et al., 2007b). The overall brain size increases by 100% during the first year of postnatal life, resulting in a brain at one year of age that is already about 73% of adult size, and at 2 years age about 80–90% of adult size (Knickmeyer et al., 2008). During late childhood (ages 6–10) the brain reaches approximately 95% of the volume of the adult brain. Throughout this period, volumetric changes are small. However, the brain undergoes a critical fine-tuning process to reach the cognitive performance of an adult brain (Caviness et al., 1996). Especially during the periadolescent period (8–10 in girls and 9–11 in boys), the second phase of neuronal rearrangements takes place (Andersen, 2003). In this phase of brain development, significant overshoot of synapses and receptors occur, which is followed by their pruning or competitive elimination.
Studies of normal cortical development are important, not only in terms of generating basic information about brain changes during development but also are critical for identification of deviation from normal development to predict risk for psychopathology and cognitive impairments. Alterations in brain morphology are associated with neuropsychiatric (depression, schizophrenia, anxiety disorders) and neurodevelopmental (autism, ADHD) disorders (Damsa et al 2009; Del Arco and Mora 2009; Garrett et al 2008; Geuze et al 2005; Kates et al 2004; Kates et al 2002; Koenigs and Grafman 2009; Kyriakopoulos and Frangou 2009; Mitchell et al 2009; Shaw and Rabin 2009; Szeszko et al 2005; Verhoeven et al 2009; White et al 2008). The vulnerability hypothesis suggests that the risk of developing these disorders may, in part, be a consequence of pre-existing alterations in brain morphology (Gilbertson et al 2002), which has been supported in studies of patients prodromal for psychiatric disorders (Bhojraj et al; Witthaus et al). For instance, in a study of patients with schizophrenia, Akbarian et al (1996) reported abnormal NMDA activity in the prefrontal and frontal lobes in patients and suggested that these could be attributed to a developmental defect in the formation of corticocortical and thalamocortical connections. Abnormal development of brain regions in presence of neurodevelopmental or neuropsychiatric disorders can only be understood only if normal trajectories of brain development are established.
With a few notable exceptions, there has been surprisingly little attention directed at normal brain development in exclusively preadolescent subjects and this is especially true for studies examining cortical thickness. Using MRI, several studies have examined changes in the developing brain from early childhood through adulthood by measuring lobar volumes or gray matter density and a few studies used cortical thickness to quantify those changes. For example, Giedd et al (1999) studied volumetric changes in the brain lobes and the white matter using a combination of cross-sectional and longitudinal data from subjects between ages 4 to 21. They documented linear increases in total brain white matter (WM) and nonlinear, region-specific changes in the gray matter (GM). Frontal and parietal GM increased until 11–12 years while the temporal lobe GM grew until 16 years and the occipital lobe GM increased linearly over the whole age range.
Although studies of age-associated changes in lobar volumes provide information about the overall brain development, they do not provide critical information about the development of functionally distinct regions. In order to address this problem, several groups have developed techniques to measure gray matter density at the voxel scale. Sowell et al (1999) used voxel based morphometry (VBM) to study differences in GM between late childhood and adolescence. They studied nine normally developing children (7–10 yrs) and nine normal adolescents (12–16 yrs) and reported significantly less GM in dorsal parietal, motor and prefrontal areas among adolescent as compared to preadolescent children. Similarly, Wilke et al (2007) used VBM to investigate typical brain development between 5 and 18 years on a cross-sectional data. They also found significant GM loss in parietal and occipital areas but they reported an increase in GM in the frontal and temporal areas.
A slightly different approach to quantify age associated changes in GM was proposed by Sowell et al (2001) who studied brain growth and GM density in typically developing children (N=14, 7–11 years), adolescents (N=11, 12–16 years) and adults (N=10, 23–30 years). Their findings suggested that as compared to children, adolescents had significantly less gray matter density in dorsal frontal and parietal lobes. However, between adolescence and adulthood, the loss in gray matter density accelerated in the frontal lobe while it slowed down in the parietal areas. Using the same approach, Gogtay et al (2004) studied age-associated changes in the brain from longitudinally acquired MRI data. They investigated brain development in preadolescent and adolescent children who were scanned two to four times in two-year intervals (ages 4–21). They reported loss of gray matter density with age in different regions of the neocortex, which progressed linearly in most cortical regions. However, a nonlinear trajectory was seen in the postcentral gyrus and dorsolateral prefrontal cortex (DLPFC), with an early increase between approximately 4 and 8 years followed by a decrease between 8 and 21 years. They suggested that this loss in gray matter density could be attributed to synaptic pruning together with trophic glial and vascular changes and possible cell shrinkage
In general, these studies described the brain developmental trajectory between early childhood and young adulthood using a variety of techniques and metrics. The only two studies that investigated brain development restricted to the preadolescent period in typically developing children have been published by Sowell et al (2004) and Caviness et al (1996). In the study published by Sowell et al (2004), measures of cortical thickness were used instead of GM density to quantify age-associated changes between 5 and 11 years in a population of 45 children. Each subject was scanned twice and the local changes in mean cortical thickness were studied between the two time points. The data for the first and second time points were obtained from children that spanned an age range of 5.3 – 9.5 years and 7.4 – 11.8 years, respectively. Cortical thinning was reported between the two time points in occipital, parietal and frontal lobes. They also observed thickening in the language areas. They suggested that the reduction in cortical thickness could partly be accounted for by the proliferation of myelin into the periphery of the cortical neuropil. Thus, those regions would appear as gray matter in younger subjects and white matter in older subjects. On the other hand, Caviness et al (1996) studied volumetric changes in some of the subcortical structures, white matter and the neocortex between 7 and 11 years. Interestingly, Caviness et al (1996) did not detect any age-associated changes in the volumes of the structures they studied. They suggested that their small sample size (30 children), cross-sectional sample and their technique resulted in limited sensitivity and the failure to detect any changes.
Here, we assess age related, regionally specific changes in cortical thickness during a critical period in brain development when a new phase of neuronal rearrangements takes place (Andersen, 2003, Caviness et al., 1996). We investigated age-related, regionally specific differences in cortical thickness as well as volumes of major subcortical regions in a group of 126 typical, healthy preadolescent children between 6 and 10 years of age using high resolution T1 weighted MRI images. In order to identify brain regions that followed a linear trajectory of cortical development during this late childhood period, we first analyzed the whole age range. Then, the same analyses were carried out for shorter age windows, each one spanning approximately 2 years, allowing detection of potential non-linear changes. We used sliding time windows with partial overlaps to study the early, intermediate and late development in this age range. It is especially of interest to identify regions where the second wave of brain development takes place during the periadolescent period, which was described by Andersen (2003).
Our findings, which are presented at a higher spatial and temporal resolution than the previous reports, indicate age related differences in cortical thickness in occipital, parietal, sensorimotor and posterior temporal areas. Moreover we observed that the cortical development shifted to frontotemporal, prefrontal and frontal areas during the periadolescent period.
2. Results
2.1. Age related differences in cortical thickness
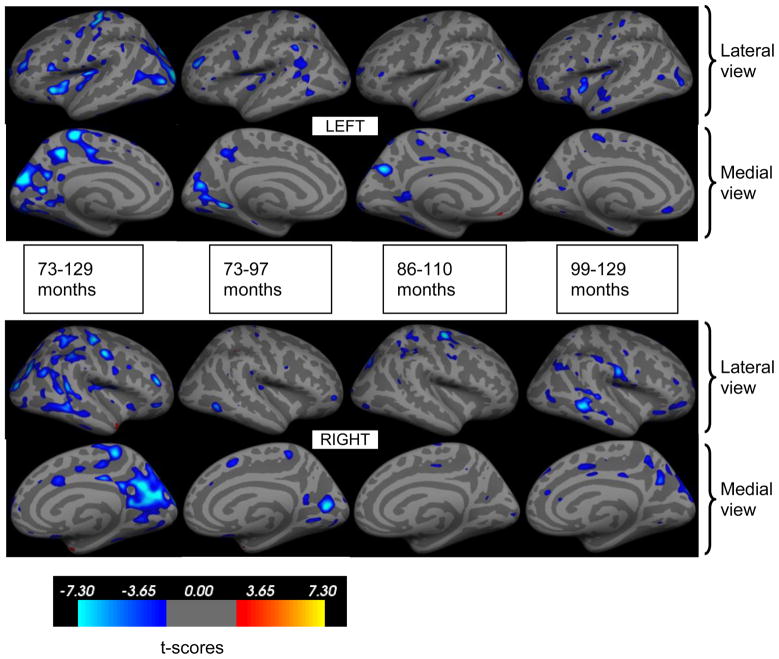
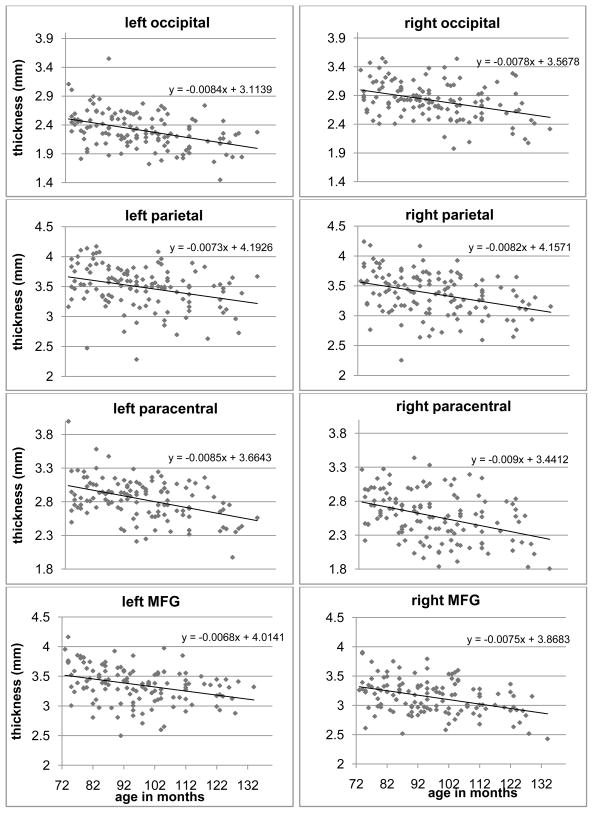
Several cortical areas demonstrated significant age related differences in thickness (Fig.1, blue overlays). In all brain regions shown in Fig.1, cortical thickness was negatively associated with age. Furthermore, as shown in Table 1, regionally specific age related differences in cortical thickness were observed based on the developmental stage. Age associated cortical thinning was apparent at younger ages in the visual cortex and posterior temporal lobe, followed by the somatosensory cortex. At older ages, age related cortical thinning appeared mostly in the temporal and frontal lobes. Fig.2 illustrate scatter plots of age-associated differences in cortical thickness from representative areas in occipital, parietal, sensorimotor and frontal areas. Linear trend lines show the typical thinning pattern in those areas.
Figure 1.
Inflated brain surfaces with statistically significant reduction in cortical thickness with age shown with blue overlay (p<0.05, FDR corrected). Upper row is the lateral and medial surfaces of the left hemisphere and lower row is the right hemisphere. Each column shows changes in cortical thickness during the specified age range. The color bar indicates t-scores.
Table 1.
t-scores for cortical regions that showed age associated reductions in thickness across the age ranges studied.
| t-scores | ||||||||
|---|---|---|---|---|---|---|---|---|
| 73–134 months | 73–97 months | 85–109 months | 97–134 months | |||||
| L | R | L | R | L | R | L | R | |
| Occipital | −6.11 | −6.55 | −3.40 | −3.40 | −3.90 | −3.09 | ||
|
| ||||||||
| Cuneus | −6.05 | −5.17 | −4.63 | −5.68 | −4.24 | |||
|
| ||||||||
| Lingual gyrus | −3.90 | −3.57 | −6.59 | −3.47 | ||||
|
| ||||||||
| Precuneus | −5.88 | −5.70 | −3.64 | −3.52 | −5.17 | −3.11 | −4.18 | |
|
| ||||||||
| Supramarginal gyrus | −3.82 | −3.26 | −2.70 | |||||
|
| ||||||||
| Parietal | −6.21 | −4.15 | −3.62 | −4.05 | −4.79 | |||
|
| ||||||||
| Precentral | −5.67 | −5.51 | −3.46 | −3.00 | −2.83 | −5.54 | −3.18 | −3.28 |
|
| ||||||||
| Paracentral | −7.38 | −5.04 | −3.67 | −3.61 | −2.86 | −3.22 | ||
|
| ||||||||
| Postcentral | −2.54 | −2.53 | −3.02 | −3.55 | −4.55 | |||
|
| ||||||||
| Posterior cingulate | −3.13 | −4.44 | −3.35 | −3.45 | ||||
|
| ||||||||
| Anterior cingulate | −3.36 | |||||||
|
| ||||||||
| Superior temporal sulcus | −4.59 | −2.88 | −3.25 | −3.42 | −3.72 | |||
|
| ||||||||
| Middle temporal gyrus | −3.34 | −4.03 | −3.39 | −3.56 | −5.00 | |||
|
| ||||||||
| Fusiform gyrus | −3.76 | −3.60 | −4.13 | −2.88 | ||||
|
| ||||||||
| Insula | −5.74 | −3.58 | −3.10 | −2.97 | −4.13 | −3.03 | ||
|
| ||||||||
| Pars opercularis | −4.32 | −2.57 | ||||||
|
| ||||||||
| Pars triangularis | −3.07 | |||||||
|
| ||||||||
| Pars orbitalis | −3.76 | −3.19 | ||||||
|
| ||||||||
| Orbitofrontal cortex | −2.76 | −2.86 | −4.00 | −3.04 | ||||
|
| ||||||||
| Superior frontal gyrus | −2.98 | −3.23 | −3.18 | |||||
|
| ||||||||
| Middle Frontal Gyrus | −4.55 | −5.10 | −4.56 | −3.70 | −2.81 | −3.33 | −3.06 | |
The highest t-scores from the largest cluster in each cortical area were recorded. For each age group, results from the left and right hemispheres are reported side by side. The shaded cells indicate that there were no statistically significant changes that can be associated with age (FDR corrected p<0.05). The list is organized in posterior to anterior direction.
Figure 2.
Scatter plots that illustrate age-associated differences in cortical thickness from several representative areas (MFG: Middle frontal gyrus). Linear trend lines were also plotted and the slopes and intercepts were printed in the insets.
Significant age associated cortical thinning was observed bilaterally in all major regions of the brain across the entire age range that we studied (73 to 129 months). The primary visual cortex, fusiform and lingual gyri in the occipital lobe; precuneus and posterior regions in the parietal lobule; precentral, paracentral and postcentral gyri in the sensorimotor cortex; insula, middle frontal gyrus (MFG) and inferior frontal gyrus (IFG) in the frontal lobe as well as posterior cingulate in the limbic system have all followed a similar trajectory of thinning bilaterally during this period. On the other hand, unilateral thinning was evident in the right temporal lobe, specifically in the superior temporal sulcus (STS) and middle temporal gyrus (MTG).
Analyses performed on shorter age ranges showed that between 73 and 97 months, statistically significant age related thinning was observed bilaterally in the insula and MFG in the frontal lobe, precentral and paracentral gyri in the sensorimotor cortex, precuneus in the parietal lobule, primary visual cortex and lingual gyrus in the occipital lobe. On the other hand, supramarginal gyrus in the parietal lobe and STS in the temporal lobe showed significant age associated thinning only in the left hemisphere.
Between 85 and 109 months, age related cortical thinning was observed bilaterally in the paracentral and precentral gyri in the sensorimotor cortex, and occipital and parietal lobes. It should be noted that the cortical thinning was spatially more extensive in the right hemisphere. In contrast, the precuneus, fusiform gyrus and posterior cingulate showed cortical thinning only on the left hemisphere.
Between 97–129 months, age related bilateral cortical thinning was observed in the insula and MFG in the frontal lobe, precentral and postcentral gyri in the sensorimotor areas, MTG in the temporal lobes, precuneus and posterior parietal areas as well as the primary visual cortex. The orbitofrontal cortex and superior temporal areas showed thinning only on the left hemisphere while superior frontal gyrus, parietal lobe and cingulate showed thinning only on the right hemisphere.
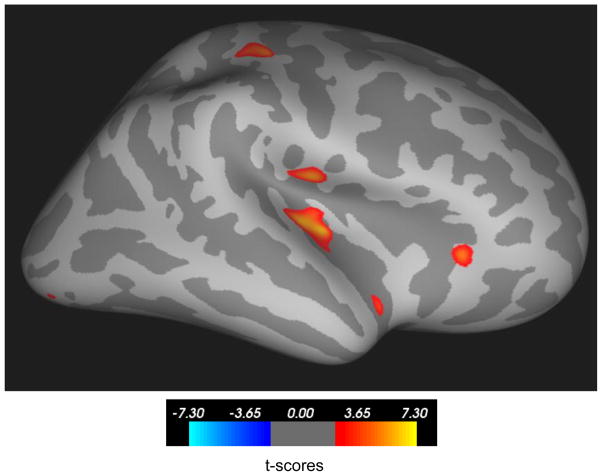
2.2. Sex differences in cortical development
Only a few cortical areas showed differences in developmental profiles between boys and girls. The main differences could be observed in some focal regions in the right insula and in the sensory areas (Fig.3, p<0.05 FDR corrected). These areas showed greater thickness in older ages among girls but not boys. Examination of sex differences within smaller age ranges revealed differences that were restricted to the 85 to 109 months interval. Girls displayed significant age associated thinning in the left medial orbitofrontal cortex (p<0.05, FDR corrected) while boys did not. From table 1 it can be seen that this region was also significant in the whole group analysis.
Figure 3.
Right insula thickness increased in girls and remained almost unchanged in boys between ages 6 and 10 (p<0.05, FDR corrected). The color bar indicates t-scores.
2.3. Volumetric changes in subcortical gray matter and cerebral white matter
As shown in Table 2, only the volume of the thalami was significantly and positively associated with child age after controlling for intracranial volume (ICV). The effect was stronger in the left than in the right hemisphere. Similarly, cerebral white matter showed significant increase in volume with age.
Table 2.
Results of linear regression analyses to study the relationship between the volumes of cortical structures and age in months. Age and ICV were entered as independent variables and the volume of each structure was entered as the dependent variable. Only cerebral white matter and the thalami showed significant increase in volume with age. Intracranial volume (ICV) did not show any significant correlation with age (Pearson’s CC=0.098, p=0.14). It can be seen that ICV plays an important role on the overall size of each structure. R values demonstrate that for most cases the regression model sufficiently explains the inter-subject variance in the volumes of these structures.
| Age in months | ICV | ||
|---|---|---|---|
| t-scores | R | ||
| Left cerebral WM | 3.14 (p=0.002) | 15.75 (p<0.000) | 0.83 |
| Right cerebral WM | 3.69 (p=0.000) | 15.28 (p<0.000) | 0.82 |
| Left thalamus | 3.36 (p=0.001) | 10.59 (p<0.000) | 0.72 |
| Right thalamus | 1.85 (p=0.067) | 9.65 (p<0.000) | 0.67 |
| Left putamen | −0.31 (p=0.755) | 7.53 (p<0.000) | 0.56 |
| Right putamen | −0.90 (p=0.369) | 8.03 (p<0.000) | 0.59 |
| Left hippocampus | 0.143 (p=0.887) | 10.19 (p<0.000) | 0.68 |
| Right hippocampus | 0.858 (p=0.392) | 10.12 (p<0.000) | 0.68 |
| Left amygdala | −0.24 (p=0.809) | 10.67 (p<0.000) | 0.70 |
| Right amygdala | 1.22 (p=0.225) | 11.39 (p<0.000) | 0.72 |
| Left caudate | −0.17 (p=0.864) | 6.82 (p<0.000) | 0.53 |
| Right caudate | 0.098 (p=0.922) | 6.54 (p<0.000) | 0.51 |
Age-associated changes in subcortical structures did not differ by sex. However, a difference was observed in the cerebral white matter. Although boys and girls both showed an increase in the volume of white matter with age, a regression analysis revealed that left cerebral white matter volume correlated with age more strongly among girls (t=2.8, p=0.006) as compared to boys (t=1.9, p=0.061). On the other hand, age associated increases in cerebral white mater in the right hemisphere were statistically significant for both girls (t=2.7, p=0.008) and boys (t=2.5, p=0.013).
Nonlinear models were also explored for age-associated differences in the volumes of these subcortical structures. However, quadratic and cubic models resulted in poorer model fit as demonstrated by lower R values. For the left thalamus, R was approximately 0.29 for both quadratic and cubic models compared to R=0.72 for the linear model. Similarly, the R values dropped to approximately 0.20 for nonlinear model compared to R=0.67 for the right thalamus. Nonlinear effects of age were not detected in any of the other subcortical structures.
These results also show that the overall brain size did not significantly change with age during this period, as demonstrated by a lack of correlation between ICV and age. However, there is substantial variability in brain sizes between individuals of a certain age, resulting in similar magnitude of variations in the size of the subcortical structures. By accounting for this major confounding effect in the regression analysis, the magnitude of age-associated differences could be evaluated.
3. Discussion
We observed age-related differences in cortical thickness and volume of thalami in a cross-sectional sample of 126 typically developing preadolescent children. The current findings based on a previously understudied narrow age range provide new information about normal or expected neurodevelopmetal trajectories.
Age-related cortical thinning or gray matter density losses in typically developing children are generally associated with cortical maturation (Gogtay et al, 2004, and Sowell et al, 2001, 2003 and 2004). The exact mechanisms for the loss in cortical thickness during typical neurodevelopment are not clearly known. One of the major processes that take place during neurodevelopment is synaptic pruning, which is preceded by an overproduction of neurons and of synaptic connections to allow for plasticity and adaptation to the environment. In the mature brain, a gradual, regionally specific pruning of the overabundant synaptic connections allows for greater efficiency. Gogtay et al (2004) suggested that maturational thinning of the cortex could be driven partially by synaptic pruning together with trophic glial and vascular changes and possible cell shrinkage. Sowell et al (2004) also argued that the reduction in cortical thickness could partially be accounted for by the proliferation of myelin into the periphery of the cortical neuropil. Thus, those regions would appear as gray matter in younger subjects and white matter in older subjects, leading to reduced thickness measurements in the cortex.
3.1. Age-associated differences in cortical thickness
In the presented study, age associated cortical thinning, a putative index of cortical maturation, was seen bilaterally in several brain regions, which is consistent with the earlier findings reported by others with a much broader age range (Gogtay et al, 2004, Giedd et al, 2009 and Sowell et al, 1999, 2001, 2003 and 2004). Substantial age related thinning was observed in motor and somatosensory cortices and the primary visual cortex. In addition to these major areas, specific areas such as precuneus and lingual gyrus showed significant thinning with age. Occipital areas and precuneus, appear to be following a continuous maturational trajectory between 6 and 10 years. Similarly, the sensorimotor areas generally show significant age associated thinning between 6 and 10 years; however, the thinning in the postcentral areas was significant only during the late period. Another important region that followed a consistent thinning trajectory was the insula. In the frontal lobe, MFG appears to be maturing during the entire age window. Similarly, pars orbitalis in the inferior frontal gyrus showed thinning when the whole group was analyzed but we failed to detect changes in shorter time windows, possibly due to loss of statistical power.
In general, we observed that the motor and sensory areas continued their development across the entire age range as indicated by decreases in cortical thickness. On the other hand, the development of frontal areas and frontal temporal lobes were more pronounced during the late time window (periadolescent period).
It should be noted that the regions that show cortical maturation in our study are more focal compared to previously published studies due to several factors. First, we focused on a narrow age range of preadolescent children. Second, there are also several differences in our data processing and analysis compared to previous studies. For instance, we did not use the large spatial averaging filter that was used in earlier investigations. In previously published studies, the GM density or cortical thickness was averaged over a 15mm diameter spherical kernel to produce the maps. Moreover, we used a conservative correction scheme for multiple comparisons, which was not applied by others. Third, the image resolution used in our scans is higher compared to some of the earlier studies (1mm3 versus 4.5 mm3 in Gogtay et al, 2004).
3.2. Age-associated differences in subcortical structures
Analysis of all major subcortical structures showed that only cerebral white matter volume and the volumes of the thalami were associated with child age with linear increases between 6 and 10 years. This is an interesting finding since the thalamus is a critical structure that acts as a relay between various subcortical areas and the cerebral cortex, particularly motor and sensory circuits. During typical development in this age range, motor skills, cognitive abilities, attention and behavioral controls improve, which could be explained by the concurrent maturation of these thalamo-cortical systems. The increase in volume could possibly be explained by ongoing synaptogenesis, myelination and neuronal proliferation that takes place to accommodate these cognitive developments.
Interestingly, the left thalamus was more strongly associated with age compared to the right side. The reason for this asymmetric development is not clearly known at this point and should be explored further. It is possible that the developing motor skills and handedness plays a role in this asymmetric growth.
When we studied age-associated changes using linear, quadratic and cubic models, we observed that linear models represented the development of the thalami more accurately than the nonlinear models. Furthermore, neither linear nor nonlinear models sufficiently explained the variability in other subcortical structures.
3.3. Sex differences in brain development
Evaluation of sex differences in age-associated cortical changes revealed significant thickening in the insula for girls, but not boys. Insula is associated with important adaptive functions such as motor control, emotional experience, self awareness as well as pain perception and differentiation. Prior work has demonstrated gender differences in the functioning of the posterior insula in the perception of pain (Moulton, et al., 2006). However, it is not clear how cortical thickening in this specific area might contribute to cognitive and behavioral differences seen between males and females.
Sex differences were additionally observed in the association between age and cerebral white matter in the left hemisphere. Even though there were substantial age associated increases in the white matter volume in both boys and girls, the association was stronger for girls potentially indicating that the girls have an accelerated myelination and increased synaptic proliferation during this period. These findings contrast with a prior study including a broader age range of 4 to 21 years Giedd et al (1999) that suggested greater increases in white matter volume in boys as compared to girls emphasizing the importance of evaluating developmental changes within focused developmental windows.
3.4. Conclusion
In this study, we demonstrated age associated cortical changes at a high temporal and spatial resolution using cross-sectional data acquired from a carefully selected population of typically developing children. The increased resolutions were afforded by the increased statistical power using the data from a relatively large population of children within a tight age range. Previously published studies generally used smaller subject populations and wider age ranges.
We have used cortical thickness as a measure of age-associated changes in the brain during late childhood period, which was also proposed by Sowell et al (2004). This could be used as a metric that reflects actual physical changes in the brain more closely compared to measurements of GM density from T1 weighted MR images. Although Gogtay et al (2004) noted that GM density and cortical thickness measurements were highly correlated in their studies, the techniques to measure GM density usually sacrifice spatial specificity. For example, in voxel based morphometry (VBM) intra-subject registration of brain images cannot account for the individual variations in sulcal and gyral anatomy and large spatial smoothing is usually applied to filter out some of the individual variations.
In this study, cross-sectional data from a large population of subjects was analyzed to observe age-associated differences in cortical thickness as well as volumes of subcortical structures during late childhood. A longitudinal study of brain development during late childhood was published by Sowell et al (2004), in which changes in cortical thickness between two time points were reported. Each subject was scanned twice with approximately two years between imaging sessions. The first scans were acquired from children between ages 5.3 and 9.5 years and the second scans were acquired when they were between 7.4 and 11.8 years old. Their results revealed cortical development in outstanding detail. However, because each time point spanned a wide range of ages and there was overlap of these time windows, cortical development was not demonstrated within the early, intermediate and late periods that we studied.
Our data are among the few to characterize normative neurodevelopmental changes that occur during the preadolescent period. This is a critical gap in our understanding of normative neurodevelopment, which has primarily focused on infancy and adolescents/adulthood with limited investigation of developmental changes during early and middle childhood. Not only is it important to characterize normative development, but also this information may improve our ability to predict susceptibility for future cognitive and psychiatric disorders. For example, schizophrenia has been considered a neurodevelopmental disorder for over 20 years. Weinberger (1987) suggested that if a lesion that occurs early in life affects a brain region that has yet to mature functionally, it might increase the risk for developing a neuropsychiatric disorder later in life. He suggested that the periadolescent period is critical for the appearance of the clinical symptoms of schizophrenia because this is the period when certain brain regions and functions begin to mature that are critical to meeting the challenges of adult life (Weinberger 1987, Lewis and Levitt 2002). Jaaro-Peled et al (2009) also highlighted the fact that aberrant postnatal brain development is a critical factor in schizophrenia. They noted that these deviations from the normal trajectories of brain maturation caused by susceptibility genes and environmental insults during early neurodevelopment initiate neurophysiological changes over a long period of time, leading to the onset of full-blown disease mainly after puberty. They provided several important findings that suggested that abnormal brain development during late childhood and early adulthood could be one of the contributing factors in schizophrenia. For instance, they noted that aberrant synaptic elimination during this period could account for the timing of schizophrenia. They also cited disturbances in myelination, especially in prefrontal and frontal areas, as another potential factor in the onset of this disease. Since these processes continue through late childhood and mostly complete during young adulthood, this period becomes important in the prodromal stages of this disease. Another important study by Pantelis et al (2003) examined whether progressive structural changes occur in prodromal subjects and they reported significant baseline differences in regional gray-matter volume between those who subsequently developed a psychotic illness and those who did not. Even though the mean age of the subjects was 19.3 (±3.7), it is possible that those structural changes occurred earlier.
Therefore, the studies that establish the trajectories of typical brain development during childhood could aid in studies that explore relationships between aberrant development in certain brain regions during late childhood and various neuropsychiatric disorders that might develop later in life. However, one must be careful when such associations are explored because deviations from normal developmental trajectories could be one of many complex factors that lead to mental health disorders; many individuals with similar aberrant brain developments could lead a life free from such disorders.
4. Experimental procedures
4.1. Subjects
126 children (59 girls and 67 boys) between the ages of 74 and 129 months (~6 – 10 years) were recruited for this study (mean age 96.4 months). These children were born at one of two hospitals in the greater Los Angeles area (UC Irvine Medical Center, or Long Beach Memorial Medical Center) and were recruited from ongoing protocols of development. The potential participant’s mother was contacted by phone and the study was explained. If they agreed to participate, a visit was scheduled. Subjects were paid $50 for their visit and were reimbursed for their travel costs.
In order to study a cohort that would represent the general population, 10% of the children were left-handed. Handedness was determined using a modified version of the Edinburgh Handedness Inventory, (Oldfield, 1971). Our low risk sample was comprised of only singletons with a stable neonatal course (Median Apgar = 9, Range 7 to 10) and without known congenital, chromosomal, or genetic anomalies (e.g., trisomy 21), neonatal illness (e.g., respiratory distress, mechanical ventilation over 48 hours or sepsis). Participants had no evidence of intraventricular hemorrhage (determined by ultrasound), periventricular leukomalacia, and/or low-pressure ventriculomegaly in the newborn period and normal neurologic findings at the current assessment. All children were typically developing and in the appropriate grade for their age. The study was approved by the Institutional Review Board for the protection of human subjects and written consents were obtained from the parents.
4.2. MR Imaging protocol
MRI scans were acquired on a 3T Philips Achieva system. After the subject lay down on the scanner bed and head coil was fitted, tight padding was placed around the head in order to minimize head motion. Ear protection was given to all children. To further increase compliance and reduce motion, children were fitted with headphones and allowed to watch the movie of their choice while in the scanner. They were instructed to stay still while in the scanner and were asked to inform us immediately if they wish to stop by using a squeeze ball that alerts the operator. Usually, the parents stayed in the scanner room with the child to keep them calm and alert us if they observe any signs of distress. They were also given ear protection if they decided to stay in the scanner room.
In the beginning of a scanning session, the calibration and pilot scans were performed, which took less than a minute. These were followed by a high resolution T1 weighted anatomical scan, which was acquired using a 3D MPRAGE pulse sequence that covered the whole brain. The images were acquired in the sagittal orientation with FOV=240×240mm2, 1mm3 isotropic voxel dimensions, 150 slices, TR=11ms, TE=3.3ms, inversion pulse delay =1100ms, flip angle=18°. No signal averaging and no SENSE acceleration were used. Acquisition time for this protocol was 7 minutes.
The images were reviewed by the MR operator immediately after the scan was completed. If there were visible signs of motion artifacts, the subject was asked if he or she could stay for an additional scan. If the subject agreed, he or she was reminded to stay still as much as possible and a second scan was acquired.
4.3. Image processing
Cortical surface reconstruction and volumetric segmentation was performed with the FreeSurfer image analysis software suite, which is available for download online (http://surfer.nmr.mgh.harvard.edu/). Streamlined image processing procedures are provided in this software package, which first begins by applying intensity normalization prior to segmentation to minimize errors in identifying the boundaries (Sled et al., 1998). This is followed by removal of non-brain tissues (Segonne et al., 2004). Then, the images are transformed into the Talairach space and subcortical white matter and subcortical gray matter structures are segmented (Fischl et al., 2002; Fischl et al., 2004a). Pial and white matter surfaces are located by finding the highest intensity gradient, which defines the transition from one tissue class to the other (Dale et al., 1999; Dale and Sereno, 1993; Fischl and Dale, 2000). Once the preprocessing steps are completed, surface inflation is applied to each individual brain (Fischl et al., 1999a) and the inflated brains are registered to a spherical atlas. This procedure utilizes individual cortical folding patterns to achieve accurate registration of cortical geometry across subjects (Fischl et al., 1999b). Cortical thickness is calculated as the closest distance from the gray matter / white matter surface to the pial surface at each vertex on the tessellated surface (Fischl and Dale, 2000). Procedures for the measurement of cortical thickness have been validated against histological analysis (Rosas et al., 2002) and manual measurements (Kuperberg et al., 2003; Salat et al., 2004).
The cortical surface images generated by the FreeSurfer software were visually inspected for errors in segmentation and corrections were made as needed.
4.4. Analysis of differences in cortical thickness related to age and sex
Age related differences in cortical thickness were analyzed at each and every node on the cortical surface using a linear regression model. Spatially normalized cortical thickness maps of each subject were entered into a regression model. Children’s ages in months were entered as a continuous variable and sex was entered as a categorical variable. All statistical tests were thresholded at p <0.05, corrected for multiple comparisons using False Discovery Rate (FDR).
We first analyzed the cortical maturation across the whole age range, studying image data from all 126 subjects. Then, the same analysis was repeated for three shorter age ranges using sliding and partially overlapping time windows. The time windows spanned the following age ranges: 73–97 months (74 subjects), 86–110 months (71 subjects), and 99–129 months (53 subjects).
4.5. Analysis of volumetric differences in subcortical gray matter and cerebral white matter associated with age
The volume of each subcortical structure was calculated by the FreeSurfer program as described in the previous section. A subset of those structures was selected to investigate volumetric differences associated with age. Total brain volume was expected to strongly correlate with the size of each structure; therefore, intracranial volume (ICV) was used as a nuisance variable. We verified that ICV was not significantly associated with child age r()=0.098, p=0.137.
For this analysis, the volume of each of the subcortical structure in native space was entered into a linear regression analysis in which age in months and ICV were entered as independent variables.
Table 3.
Race/ethnic distribution distribution of the study cohort. The total number of children in each racial category was given together with their percentage in the study population. Note that the percentages are rounded to the nearest integer.
| Child race | Total number (%) |
|---|---|
| White (non-Hispanic) | 43 (34%) |
| Hispanic | 49 (38%) |
| Black | 7 (6%) |
| Asian | 10 (8%) |
| Multi-ethnic | 17 (13%) |
Acknowledgments
This research was supported by NIH R01 HD050662 to EPD and NIH R01 HD048947 to CAS. The authors wish to thank the families who participated in this project. The assistance of Megan Blair, Natalie Hernandez, Christina Canino, and Susan Jackman is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akbarian S, Sucher NJ, Bradley D, Tafazzoli A, Trinh D, Hetrick WP, Potkin SG, Sandman CA, Bunney WE, Jr, Jones EG. Selective alterations in gene expression for NMDA receptor subunits in prefrontal cortex of schizophrenics. J Neurosci. 1996;16:19–30. doi: 10.1523/JNEUROSCI.16-01-00019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neuroscience and Biobehavioral Reviews. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Bhojraj TS, Prasad KM, Eack SM, Francis AN, Miewald JM, Montrose DM, et al. Gray matter loss in young relatives at risk for schizophrenia: Relation with prodromal psychopathology. NeuroImage. doi: 10.1016/j.neuroimage.2010.04.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288(14):1740–8. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Jr, Kennedy DN, Richelme C, Rademacher J, Filipek PA. The human brain age 7–11 years: a volumetric analysis based on magnetic resonance images. Cereb Cortex. 1996;6(5):726–36. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J Cogn Neurosci. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Damsa C, Kosel M, Moussally J. Current status of brain imaging in anxiety disorders. Current opinion in psychiatry. 2009;22:96–110. doi: 10.1097/YCO.0b013e328319bd10. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Mora F. Neurotransmitters and prefrontal cortex-limbic system interactions: implications for plasticity and psychiatric disorders. J Neural Transm. 2009;116:941–952. doi: 10.1007/s00702-009-0243-8. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999a;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999b;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004a;23(Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004b;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Garrett A, Penniman L, Epstein JN, Casey BJ, Hinshaw SP, Glover G, et al. Neuroanatomical abnormalities in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2008;47:1321–1328. doi: 10.1097/CHI.0b013e318185d285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze E, Vermetten E, Bremner JD. MR-based in vivo hippocampal volumetrics: 2. Findings in neuropsychiatric disorders. Mol Psychiatry. 2005;10:160–184. doi: 10.1038/sj.mp.4001579. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Lalonde FM, Celano MJ, White SL, Wallace GL, Lee NR, Lenroot RK. Anatomical Brain Magnetic Resonance Imaging of Typically Developing Children and Adolescents. JAACP. 2009;48:465–70. doi: 10.1097/CHI.0b013e31819f2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature neuroscience. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Lin W, Prastawa MW, Looney CB, Vetsa YS, Knickmeyer RC, Evans DD, Smith JK, Hamer RM, Lieberman JA, Gerig G. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J Neurosci. 2007;27:1255–1260. doi: 10.1523/JNEUROSCI.3339-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, III, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. PNAS. 2004;101:8174–79. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Nugent TF, Herman DH, Ordonez A, Greenstein D, Hayashi KM, Clasen L, Toga AW, Giedd JN, Rapoport JL, Thompson Paul M. Dynamic Mapping of Normal Human Hippocampal Development. Hippocampus. 2006;16:664–672. doi: 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Busa E, Pacheco J, Albert M, Killiany R, Maguire P, Rosas D, Makris N, Dale A, Dickerson B, Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A. Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through neuregulin-1-ErbB4 and DISC1. Trends Neurosci. 2009;32:485–495. doi: 10.1016/j.tins.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, Kennedy D, Schmitt F, Brown G, Macfall J, Fischl B, Dale A. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30:436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Kates WR, Burnette CP, Eliez S, Strunge LA, Kaplan D, Landa R, et al. Neuroanatomic variation in monozygotic twin pairs discordant for the narrow phenotype for autism. The American journal of psychiatry. 2004;161:539–546. doi: 10.1176/appi.ajp.161.3.539. [DOI] [PubMed] [Google Scholar]
- Kates WR, Frederikse M, Mostofsky SH, Folley BS, Cooper K, Mazur-Hopkins P, et al. MRI parcellation of the frontal lobe in boys with attention deficit hyperactivity disorder or Tourette syndrome. Psychiatry research. 2002;116:63–81. doi: 10.1016/s0925-4927(02)00066-5. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, Hamer RM, Lin W, Gerig G, Gilmore JH. A structural MRI study of human brain development from birth to 2 years. J Neurosci. 2008 Nov 19;28(47):12176–82. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res. 2009;201:239–243. doi: 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SC, van der Kouwe AJ, Salat DH, Dale AM, Fischl B. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Kyriakopoulos M, Frangou S. Recent diffusion tensor imaging findings in early stages of schizophrenia. Current opinion in psychiatry. 2009;22:168–176. doi: 10.1097/YCO.0b013e328325aa23. [DOI] [PubMed] [Google Scholar]
- Moulton EA, Keaser ML, Gullapalli RP, Maitra R, Greenspan JD. Sex differences in the cerebral BOLD signal response to painful heat stimuli. Am J Physiol Regul Integr Comp Physiol. 2006;291:R257–R267. doi: 10.1152/ajpregu.00084.2006. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Mitchell SR, Reiss AL, Tatusko DH, Ikuta I, Kazmerski DB, Botti JA, et al. Neuroanatomic alterations and social and communication deficits in monozygotic twins discordant for autism disorder. The American journal of psychiatry. 2009;166:917–925. doi: 10.1176/appi.ajp.2009.08101538. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Segonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 2007;26:518–529. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- Shaw P, Rabin C. New insights into attention-deficit/hyperactivity disorder using structural neuroimaging. Current psychiatry reports. 2009;11:393–398. doi: 10.1007/s11920-009-0059-0. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage. 1999;9:587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal Mapping of Cortical Thickness and Brain Growth in Normal Children. J Neuroscience. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeszko PR, Ardekani BA, Ashtari M, Kumra S, Robinson DG, Sevy S, et al. White matter abnormalities in first-episode schizophrenia or schizoaffective disorder: a diffusion tensor imaging study. The American journal of psychiatry. 2005;162:602–605. doi: 10.1176/appi.ajp.162.3.602. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Vidal C, Giedd JN, Gochman P, Blumenthal J, Nicolson R, Toga AW, Rapoport JL. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci USA. 2001;98:11650–5. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven JS, De Cock P, Lagae L, Sunaert S. Neuroimaging of autism. Neuroradiology. 2009 doi: 10.1007/s00234-009-0583-y. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- White T, Cullen K, Rohrer LM, Karatekin C, Luciana M, Schmidt M, et al. Limbic structures and networks in children and adolescents with schizophrenia. Schizophrenia bulletin. 2008;34:18–29. doi: 10.1093/schbul/sbm110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Krageloh-Mann I, Holland SK. Global and local development of gray and white matter volume in normal children and adolescents. Exp Brain Res. 2007;178:296–307. doi: 10.1007/s00221-006-0732-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witthaus H, Mendes U, Brune M, Ozgurdal S, Bohner G, Gudlowski Y, et al. Hippocampal subdivision and amygdalar volumes in patients in an at-risk mental state for schizophrenia. J Psychiatry Neurosci. 35:33–40. doi: 10.1503/jpn.090013. [DOI] [PMC free article] [PubMed] [Google Scholar]