Abstract
In this study human motor cortical activity was recorded with a customized micro-ECoG grid during individual finger movements. The quality of the recorded neural signals was characterized in the frequency domain from three different perspectives: (1) coherence between neural signals recorded from different electrodes, (2) modulation of neural signals by finger movement, and (3) accuracy of finger movement decoding. It was found that, for the high frequency band (60–120 Hz), coherence between neighboring micro-ECoG electrodes was 0.3. In addition, the high frequency band showed significant modulation by finger movement both temporally and spatially, and a classification accuracy of 73% (chance level: 20%) was achieved for individual finger movement using neural signals recorded from the micro-ECoG grid. These results suggest that the micro-ECoG grid presented here offers sufficient spatial and temporal resolution for the development of minimally-invasive brain-computer interface applications.
I. Introduction
Brain-computer interface (BCI) technology aims to establish a direct link between the brain and external devices, enabling faster and more intuitive communication and control for individuals with severe motor impairments. One of the major challenges in translating BCI technology from basic research into clinical practice is the development of a minimally invasive technique for obtaining reliable long-term recordings with high spatial and temporal resolution. Electrocorticography (ECoG) records brain activity with intracranial electrodes placed directly on the brain surface. Recent studies have demonstrated that subjects can achieve effective control of cursor movement in a very short period of time using ECoG signals, suggesting ECoG is a promising modality for BCI applications[1][2].
Unlike many previous ECoG studies where cortical activity was recorded with large grids of subdural ECoG electrodes[1][2][3], the current study used a micro-ECoG grid (Fig. 1). By reducing the electrode diameter and inter-electrode distance, we expect that the micro-ECoG grid can record localized neural activity with high specificity and spatial resolution[4]. This study compares neural signals recorded with a standard ECoG grid and a custom micro-ECoG grid. We hypothesize that the coherence of the high frequency band (60–120Hz) recorded by neighboring electrodes of the micro-ECoG grid will be the same or lower than that of the standard ECoG grid, indicating greater signal independence.
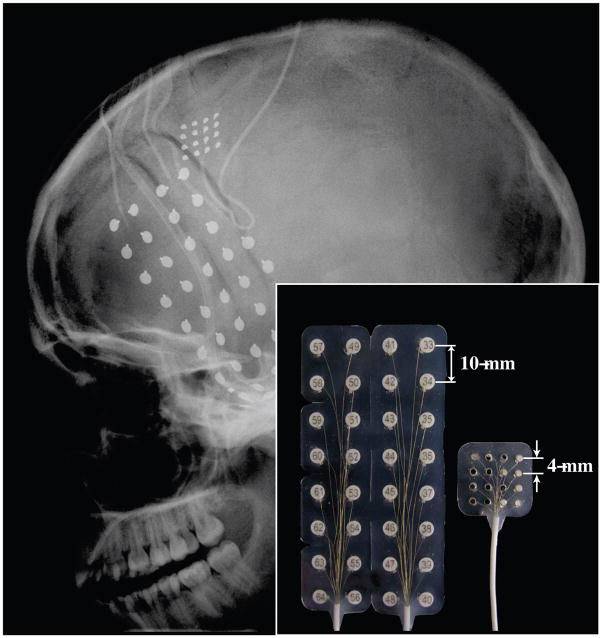
Fig. 1.
Head x-ray (lateral view) showing the locations and sizes of the implanted ECoG electrodes. One micro-ECoG grid (16 contacts), one regular ECoG grid (32 contacts), and two 6-contact regular ECoG strips were implanted. Inset: A side-by-side comparison of the regular ECoG grid and the micro-ECoG grid showing the center-to-center electrode spacing.
Previous studies in non-human primates have shown that individual finger movements can be decoded with a high accuracy (> 80%) from the activity of 20 30 neurons recorded with microelectrodes inserted into the hand area of the motor cortex[5][6]. This study also examines whether micro-ECoG recording can differentiate individual finger movements with accuracies comparable to those of previous single-neuron studies.
II. Materials and Methods
A. Human subjects and behavioral paradigm
This study was approved by the University of Pittsburgh Institutional Review Board and followed all guidelines for human subject research. The subject was a 17-year old right-handed female undergoing monitoring for intractable epilepsy with seizure foci in the left temporal lobe. At the beginning of each session, approximately one minute of baseline data were acquired when the subject relaxed with eyes open. During the finger movement task, the subject was instructed to perform self-paced individual finger movements (finger flexion and extension) for approximately 10 seconds per finger, beginning with the thumb and ending with the little finger. A minimum of 9 repetitions were conducted for each finger movement. The BCI2000 software package was used to prompt the subject on which finger to move[7]. Finger movements were recorded with a 14-sensor 5DT data glove[8].
B. ECoG recording
A standard clinical ECoG grid (Ad-Tech, Corp.) with 32 disc electrodes (3mm in diameter for the contact area, 10mm center-to-center distance) was implanted subdurally over the left temporal lobe and inferior frontal lobe for epilepsy seizure monitoring. In addition, an experimental micro-ECoG grid was implanted subdurally superior-posterior to the standard ECoG grid and anterior to the central sulcus over the motor cortical area. The micro-ECoG grid (Ad-Tech, Corp.) consisted of 16 disc electrodes (contact area diameter 1.5mm, 4mm center-to-center distance) arranged in a 4-by-4 pattern (Fig. 1). Two corner electrodes in the micro-ECoG grid were designated as reference electrodes, and the remaining 14 electrodes were used for neural recording. All ECoG signals were band-pass filtered between 0.1 to 200 Hz and sampled at 1200 Hz using the g.USBamp amplification system (Guger Technologies, OEG) in conjunction with BCI2000.
C. Coherence analysis
Coherence analysis was used to characterize independence between neural signals recorded from different electrodes for both the experimental micro-ECoG grid and the traditional ECoG grid[9]. Coherence analysis was performed using baseline data (i.e. rest periods) for all possible pairs of electrodes in the micro-ECoG grid and for all possible pairs of electrodes in the regular ECoG grid. In addition, coherence was analyzed as a function of distance between electrodes and compared between the micro-ECoG grid and the regular ECoG grid.
D. Finger movement and classification
Individual finger movements were decoded using neural signals recorded from the micro-ECoG grid. The spectral powers of ECoG signals were calculated over the 0–200 Hz frequency band using the maximum entropy method with 3 Hz frequency bins[10]. Classification was performed using spectral power data calculated over periods of individual finger movement. Features were defined as the spectral power within a particular frequency band for a given channel. A total of 938 features (67 frequency bands across 14 channels) were derived from the micro-ECoG grid. A one-way analysis of variance (ANOVA) was used to identify features modulated by finger movement. To control for the multiple comparisons arising from the size of the feature set, the false discovery rate (FDR) method was used to determine the p-value threshold for statistical significance (FDR=0.05)[11]. Significantly modulated features were then used for finger movement classification using two different methods. In the first method, principle component analysis (PCA) was used for further dimensionality reduction, with the number of principle components chosen to maximize classification accuracy. The resultant feature set was then used to classify finger movements using Linear Discriminate Analysis (LDA)[12]. In the second method, classification was performed using multi-class linear support vector machines (SVMs) without PCA-based dimensionality reduction[13]. Leave-one-out cross validation was used in both classification methods.
III. Results
A. Coherence of baseline ECoG data
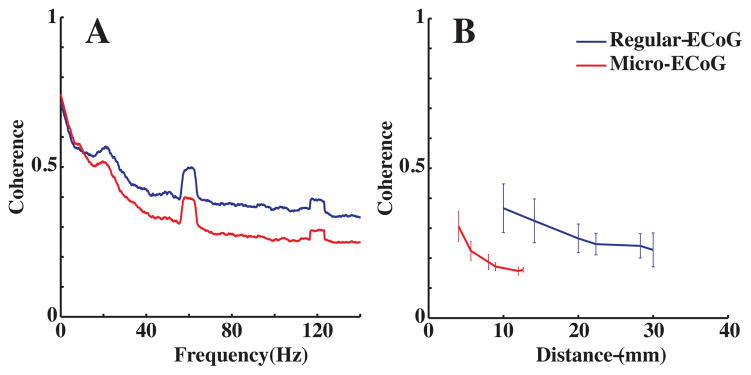
Coherence values between neural signals recorded from all possible pairs of electrodes in the micro-ECoG grid and all possible pairs in the regular ECoG grid were calculated to examine the correlation between those signals in the frequency domain. Fig. 2A compares the coherence values at various frequencies between neighboring electrodes for the micro-ECoG (4mm center-to-center spacing) and the regular ECoG grid (10mm spacing). For both grids, coherence was found to decrease at higher frequencies. The lower coherence in the high frequency band between neighboring electrodes suggests that it may reflect a more localized neuronal activity than the lower frequency bands. Furthermore, between neighboring electrodes, the high frequency band was found to be less coherent for the micro-ECoG grid than for the regular grid. Additionally, coherence of the high frequency (60–120 Hz) band was examined as a function of inter-electrode distance. Fig. 2B shows that the decrease of high frequency band coherence with increasing inter-electrode distance. Furthermore, the high frequency band coherence was found to be significantly smaller for the micro-grid than for the regular grid at comparable distances (approx. 10mm). This suggests that the micro-ECoG grid offers higher spatial resolution for neural recording.
Fig. 2.
Coherence analysis for the micro-ECoG grid (red lines) and the regular grid (blue lines). 2A (left): Averaged coherence between neighboring electrodes. 2B (right): Coherence as a function of distance between electrodes. Error bars are standard deviations. Coherence values were calculated at all possible inter-electrode distances given the geometric configurations of the electrodes on the grids.
B. ECoG signal modulation by finger movement
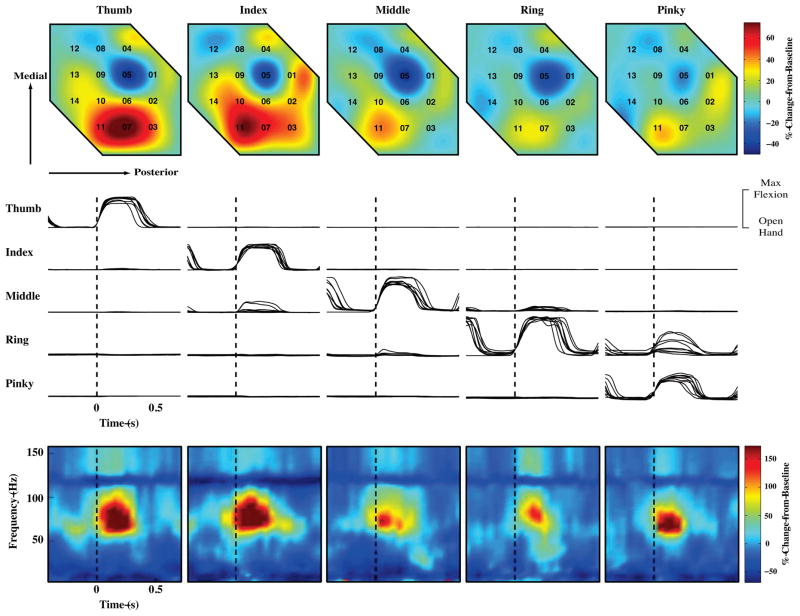
Fig. 3 shows the movement at the five proximal inter-phalangeal (PIP) joints for 9 repetitions of individual finger movements (flexion and extension). The subject was able to move each finger individually with the exception of the little finger, whose movement tended to couple with that of the ring finger. Movement of different fingers elicited distinct patterns of activity on individual electrodes on the micro-grid, most notably in the high frequency (60–120 Hz) range. The bottom row of Fig. 3 shows spectrograms of individual finger movements for channel 11. A clear increase in the high frequency band power is evident during finger movement, with the power varying significantly for different fingers (p<0.05). Furthermore, different finger movement produced distinctive spatial patterns of activity across the micro-grid (Fig. 3, top row). These results indicate that neural signals recorded from the micro-grid carry significant information about finger movement.
Fig. 3.
Modulation of neural signals recorded by the micro-ECoG grid during individual finger movements. The five columns from left to right correspond to instructed thumb, index, middle, ring, and little finger movement, respectively. Top row: Spatial pattern of 60–120 Hz band activity averaged over movement and across all trials for all 14 recording electrodes on the micro-ECoG grid. Numbers indicate electrode locations. Middle: Movements of five fingers (PIP joint angles) from nine repetitions. PIP joints exhibiting the most significant change during this task were chosen for analysis. All joint angle data has been normalized to the angle of thumb PIP joint during maximum thumb flexion. Vertical dotted lines represent onset of finger movement. Bottom row: Spectrograms (spectral power change from baseline as a function of both frequency and time) averaged across all trials for Electrode No. 11. Again, vertical dotted lines represent onset of finger movement.
Decoding analysis was performed to directly test whether finger movement information can be extracted with high accuracy. Among all 938 features, 95 features were found to be significantly modulated by finger movement using the ANOVA analysis with FDR correction. Both multi-class SVM classification without PCA dimensionality reduction as well as LDA classification using eight principal components achieved accuracies of 73% (leave-one-out cross validation, chance level: 20%).
IV. Discussion and Conclusions
The major advantages of micro-ECoG grids are that they can be implanted with a minimally invasive procedure and that they offer higher spatial resolution than the standard clinical ECoG grids for cortical recording. This allows the extraction of control signals with high specificity using a much simpler recording system than single-neuron recording, which will accelerate the development of fully-implantable practical brain-computer interface devices. However, engineers are still searching for the optimal configuration in terms of electrode diameter and inter-electrode distance to achieve reliable neural recording while maximizing information extracted from a micro-ECoG grid. Although electrodes with a small diameter can presumably record very localized cortical activity, it may be difficult for these electrodes to maintain adequate contact with the brain surface for ECoG recordings[14]. Furthermore, while spatial resolution can potentially be increased by reducing the inter-electrode distance, the shunting effect between electrodes may become prominent at these distances, leading to correlated signals across electrodes[14]. Electrodes in the micro-grid used in this study exhibited lower coherence at the high frequency bands (Fig. 2) than that observed in the regular ECoG grid, indicating a significant amount of independence between them for recording cortical activity with high spatial resolution.
Arguably, two random signals that contain little useful information can have very low coherence, as well. We do not believe this is case in the current study. The electrodes in the micro-grid demonstrated modulation by finger movement both temporally and spatially (Fig. 3). Also, high accuracy was achieved for decoding individual finger movement, indicating that the micro-grid used in this study offers high resolution neural recording from a small patch of the cortex.
The pioneering studies by Schieber and his colleagues have demonstrated in non-human primates that there is generally a mixed representation of digits in the hand area of the motor cortex and that finger movement can be decoded from a very confined cortical volume[5][6][15]. This may be the underlying neuronal mechanism for the high accuracy obtained in finger movement decoding. We observed stronger cortical activation by thumb and index finger movement over a group of electrodes on the micro-grid (Fig. 3). This may suggest that, on top of the mixed finger representation, there might be a certain degree of clustering in neurons representing thumb and index finger movement in human motor cortex. It is also possible that more neurons are dedicated to thumb and index finger movement since those two digits contribute significantly to dexterous hand movement.
In summary, this paper describes a micro-ECoG grid that can record human cortical activity with high spatial and temporal resolution. High accuracy was achieved for decoding individual finger movement. This represents the first description of finger movement decoding using micro-ECoG recording in humans. We conclude that micro-ECoG recording is a promising method for obtaining high quality signals for BCI control.
Acknowledgments
This work was supported by the National Science Foundation under Cooperative Agreement EEC-0540865, Grant Number 5 UL1 RR024153 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and a special grant from the Office of the Senior Vice Chancellor for the Health Sciences at University of Pittsburgh. This papers contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp. Additional funding support was provided by NIH grants from the NIBIB (1R01EB007749) and NINDS (1R21NS056136) and grant W81XWH-07-1-0716 from the US Army Medical Research and Material Command.
We would like to thank the participant who kindly made it possible for us to perform this study. We would also like to thank the clinical staff of the epilepsy monitoring unit at the Childrens Hospital of Pittsburgh. We would also like to acknowledge Ms. Patricia Lordeon and Mr. Clinton Young for their engineering and technical support. We thank Dr. Gerwin Schalk for helpful discussions regarding ECoG recording and BCI2000 software.
Contributor Information
Wei Wang, University of Pittsburgh, Pittsburgh, PA.
Alan D Degenhart, University of Pittsburgh, Pittsburgh, PA.
Jennifer L Collinger, University of Pittsburgh, Pittsburgh, PA.
Ramana Vinjamuri, University of Pittsburgh, Pittsburgh, PA.
Gustavo P Sudre, Carnegie Mellon University, Pittsburgh, PA.
P David Adelson, Phoenix Children’s Hospital, Phoenix, AZ.
Deborah L Holder, University of Pittsburgh, Pittsburgh, PA.
Eric C Leuthardt, Washington University in St. Louis, St. Louis, MO.
Daniel W Moran, Washington University in St. Louis, St. Louis, MO.
Michael L Boninger, University of Pittsburgh, Pittsburgh, PA.
Andrew B Schwartz, University of Pittsburgh, Pittsburgh, PA.
Donald J Crammond, University of Pittsburgh, Pittsburgh, PA.
Elizabeth C Tyler-Kabara, University of Pittsburgh, Pittsburgh, PA.
Douglas J Weber, Email: djw50@pitt.edu, Departments of Physical Medicine & Rehabilitation and Bioengineering, University of Pittsburgh, Pittsburgh, PA (412-648-6666).
References
- 1.Leuthardt Eric C, Schalk Gerwin, Wolpaw Jonathan R, Ojemann Jeffrey G, Moran Daniel W. A brain-computer interface using electrocorticographic signals in humans. J Neural Eng. 2004 Jun;1(2):63–71. doi: 10.1088/1741-2560/1/2/001. [DOI] [PubMed] [Google Scholar]
- 2.Schalk G, Miller KJ, Anderson NR, Wilson JA, Smyth MD, Ojemann JG, Moran DW, Wolpaw JR, Leuthardt EC. Two-dimensional movement control using electrocorticographic signals in humans. J Neural Eng. 2008 Mar;5(1):75–84. doi: 10.1088/1741-2560/5/1/008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller Kai J, Leuthardt Eric C, Schalk Gerwin, Rao Rajesh PN, Anderson Nicholas R, Moran Daniel W, Miller John W, Ojemann Jeffrey G. Spectral changes in cortical surface potentials during motor movement. J Neurosci. 2007 Feb;27(9):2424–32. doi: 10.1523/JNEUROSCI.3886-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim J, Wilson JA, Hippensteel J, Hokanson J, Wang W, Smith K, OTTO KJ, Shain W, Webber DJ, Krugner-Higby LA, Moran DW, Williams JC. A cortical microecog platform utilizing thin film polymer electrode arrays. Proceedings of Society for Neuroscience Annual Meeting. Society for Neuroscience; 2008. [Google Scholar]
- 5.Ben Hamed S, Schieber MH, Pouget A. Decoding m1 neurons during multiple finger movements. Journal of Neurophysiology. 2007 Jul;98(1):327–33. doi: 10.1152/jn.00760.2006. [DOI] [PubMed] [Google Scholar]
- 6.Acharya Soumyadipta, Tenore Francesco, Aggarwal Vikram, Etienne-Cummings Ralph, Schieber Marc H, Thakor Nitish V. Decoding individuated finger movements using volume-constrained neuronal ensembles in the m1 hand area. IEEE transactions on neural systems and rehabilitation engineering: a publication of the IEEE Engineering in Medicine and Biology Society. 2008 Feb;16(1):15–23. doi: 10.1109/TNSRE.2007.916269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schalk Gerwin, McFarland Dennis J, Hinterberger Thilo, Birbaumer Niels, Wolpaw Jonathan R. Bci2000: a general-purpose brain-computer interface (bci) system. IEEE transactions on bio-medical engineering. 2004 Jun;51(6):1034–43. doi: 10.1109/TBME.2004.827072. [DOI] [PubMed]
- 8.Vinjamuri Ramana, Sun Mingui, Crammond Donald, Sclabassi Robert, Mao Zhi-Hong. Inherent bimanual postural synergies in hands. Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference; 2008; Jan, 2008. pp. 5093–6. [DOI] [PubMed] [Google Scholar]
- 9.Stocia P, Moses R. Introduction to Spectral Analysis. Upper Saddle River, NJ: Prentice-Hall; 1997. [Google Scholar]
- 10.McFarland D, McCane L, David S, Wolpaw J. Spatial filter selection for eeg-based communication. Electroencephalography and clinical Neurophysiology. 1997 Jan; doi: 10.1016/s0013-4694(97)00022-2. [DOI] [PubMed]
- 11.Genovese Christopher R, Lazar Nicole A, Nichols Thomas. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002 Apr;15(4):870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 12.McLachlan GJ. Discriminant Analysis and Statistical Pattern Recognition. Wiley-Interscience; 2004. [Google Scholar]
- 13.Cristianini N, Shawe-Taylor J. An Introduction to Support Vector Machines and Other Kernel-based Learning Methods. Cambridge University Press; 2000. [Google Scholar]
- 14.Ollikainen JO, Vauhkonen M, Karjalainen PA, Kaipio JP. Effects of electrode properties on eeg measurements and a related inverse problem. Medical engineering & physics. 2000 Oct;22(8):535–45. doi: 10.1016/s1350-4533(00)00070-9. [DOI] [PubMed] [Google Scholar]
- 15.Schieber M, Hibbard L. How somatotopic is the motor cortex hand area? Science. 1993 Jan; doi: 10.1126/science.8332915. [DOI] [PubMed] [Google Scholar]