Abstract
pH regulates many cellular processes and is also an indicator of disease progression. Therefore, pH responsive materials often serve as either tools in the fundamental understanding of cell biology or medicines for disease diagnosis and therapy. While gold nanoparticles have found broad biomedical applications, very few of them exhibit pH dependent interactions with live cells in a native biological environment due to nonspecific serum protein adsorption. Herein, we report that by coating luminescent gold nanoparticles with a natural peptide, glutathione and the simplest stable aminothiol, cysteamine, we enabled the nanoparticles to exhibit not only high resistance to serum protein adsorption but also pH dependent adsorption onto the live cell membrane in the presence of serum proteins. Incorporating this pH dependent membrane adsorption behavior into gold nanoparticles could potentially catalyze new biomedical applications of metal nanoparticles in the fundamental understanding of biological processes as well as disease diagnosis and therapy, where pH changes are involved.
pH is a key parameter for many biological processes1 and also an important indicator for disease progression.2 For example, endocytosis, a process that cells engulf substances, is involved with pH change from neutral to acidic (pH 4.5–6.2)1 Due to Warburg effect, 3 the acidic pH is also characteristic of solid tumors (extracellular pH 6.0–7.0).4 Therefore, pH responsive materials such as organic dye based indicators, cationic polymers and some peptides often serve as either tools in the fundamental understanding of cell biology or medicines for disease diagnosis and therapy.5 Nanoparticles (NPs) often show broad and tunable optical,6 magnetic, 7 electrical, photothermal properties8 and the large surface-to-volume ratio, which allows the integration of different functional groups into one single entity.9 Therefore, nanoparticles showing pH dependent interactions with live cells will provide new multifunctional tools for disease diagnosis and therapy.
While metal NPs hold great promise in bioimaging,10 drug/gene delivery11 and phototherapy,12 their interactions with the cell membrane are generally insensitive to extracellular pH changes in a native biological environment because serum proteins are often adsorbed onto the metal NPs and form a protein “corona”.13 This additional protein corona, rather than surface ligands, governs interactions between NPs and the cell membrane.13–14 Over 3700 proteins in serum/plasma and their dynamic adsorption/desorption with the particles create huge uncertainties in rational manipulation of the NP-cell membrane interactions at different pHs. To address this challenge, by taking advantage of a natural peptide, reduced glutathione, and the simplest stable aminothiol, cysteamine as surface ligands, we created a class of ~3 nm luminescent gold NPs (AuNPs), which have little interactions with serum proteins but exhibit pH dependent adsorption onto the live cell membrane in a biological pH range from mildly acidic to neutral (5.3–7.4). This simple surface chemistry is expected to catalyze new biomedical applications of metal NPs in the fundamental understanding of biological processes as well as disease diagnosis and therapy, where pH changes are involved.
In this work, in order to utilize fluorescence microscopy to probe the interactions between NPs and live cells, we employed luminescent AuNPs, rather than conventional nonluminescent AuNPs, as a model system to investigate how the changes in the surface chemistry of the NPs and the local pH environment influence the NP-cell membrane interaction. Luminescent metal NPs are a class of new metal nanostructures that give intrinsic emission without the need of conjugation with fluorescent dyes.15 Therefore, potential interferences from organic dyes in the studies of surface chemistry effects on the NP-cell interactions can be circumvented.16 Glutathione, an abundant tri-amino acid peptide in nature, was chosen as one surface ligand due to its low affinity to serum proteins and the capability of minimizing nonspecific protein adsorption on the NPs.11a, 17 However, the glutathione-coated luminescent AuNPs (G-AuNPs) have very weak interaction with cells within a pH range from 5.3 to 7.4 (Figure S1 Supporting Information [SI]). To make the NP-cell membrane interaction pH dependent, in addition to glutathione, cationic cysteamine was also introduced into the luminescent AuNPs as the second surface ligand because protonated cysteamine can drive NPs nonspecifically bind to the cell membrane through electrostatic attraction, 18 which was also confirmed by our studies (Figure S2 [SI]).
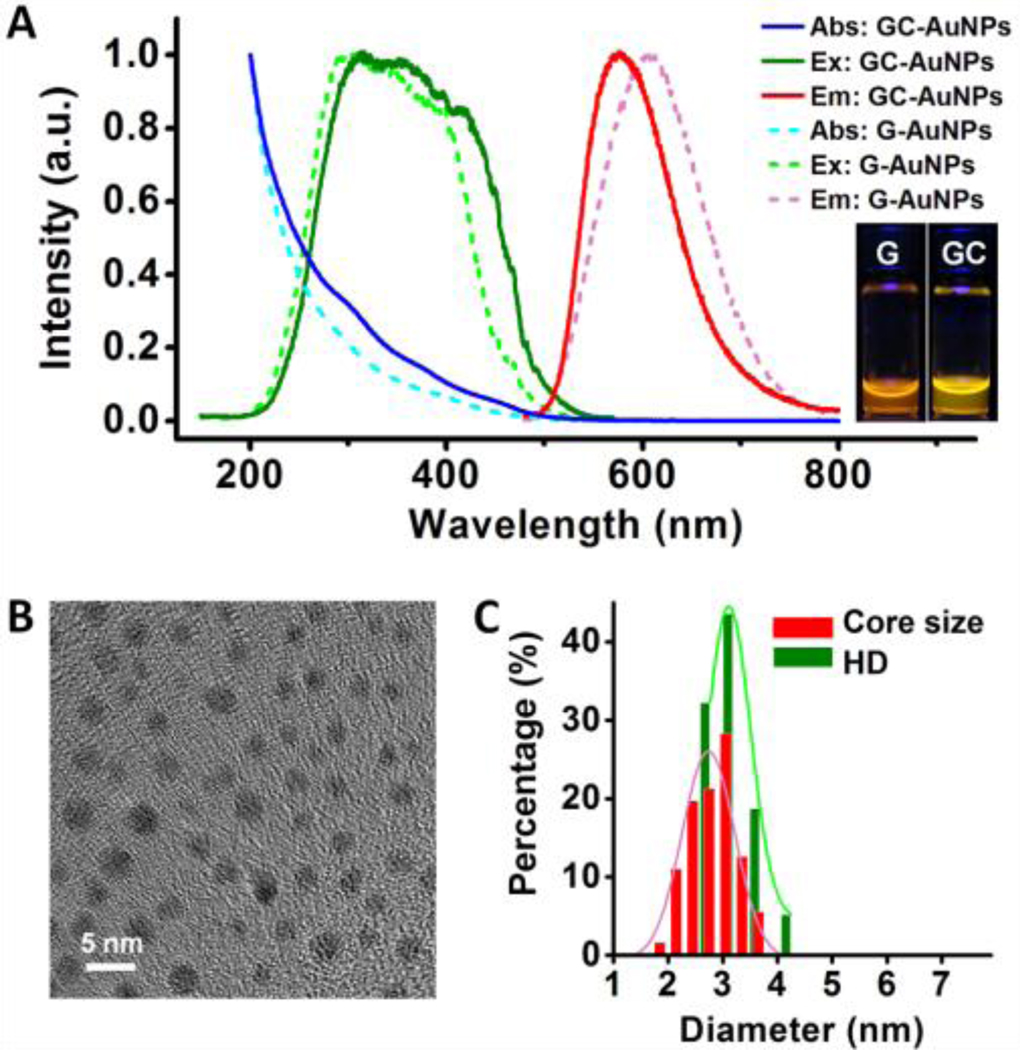
Glutathione and cysteamine-coated luminescent AuNPs (GCAuNPs) were synthesized using a modified method that we used to create G-AuNPs.19 Briefly, fresh aqueous solution (400 µL) containing 12.5 mM glutathione and 12.5 mM cysteamine were added into a 25 mM HAuCl4 aqueous solution (200 µL). Because of the strong Au(I)-S interaction, the two thiolated ligands immediately reacted with gold ions to form Au(I)-glutathione/cysteamine polymers,19 which dissociated into AuNPs naturally coated by glutathione and cysteamine (Scheme S1 and Figure S3 [SI]). The FTIR analysis revealed the ratio of cysteamine to glutathione on the GC-AuNPs was approximately 10 to 7 (Figure S4 [SI]). The GC-AuNPs exhibited intensive yellow emission with a maximum at 575 nm (Figure 1A) and a quantum efficiency of about 9% in a pH range of 4.8–8.0 (Figure S5 [SI]), which is twice higher than that of G-AuNPs. The differences in the adsorption, excitation and emission spectra of G-AuNPs and GC-AuNPs indicated that the introduction of cysteamine actually enhances the emission property of luminescent AuNPs (Figure 1A). The mean core size and hydrodynamic diameter of GC-AuNPs were 2.7±0.5 nm and 3.1±0.4 nm, respectively (Figures 1B, 1C), which are slightly larger than ~2 nm G-AuNPs.
Figure 1.
(A) Absorption, excitation and emission spectra of glutathione-coated luminescent gold nanoparticles (G-AuNPs) and glutathione/cysteamine-coated luminescent gold nanoparticles (GC-AuNPs) in aqueous solution. G-AuNPs: One adsorption shoulder peak at 400 nm, λexmax = 300 nm, λemmax = 605 nm; GC-AuNPs: Three adsorption shoulder peaks at 305, 375 and 450 nm, λexmax = 315 nm, λemmax = 575 nm. Inset: Pictures taken under 365 nm UV excitation. (B) Typical transmission electron microscopy image of GC-AuNPs showing (C) a core size of 2.7±0.5 nm, and dynamic light scattering analysis showing a hydrodynamic diameter (HD) of 3.1±0.4 nm in aqueous solution.
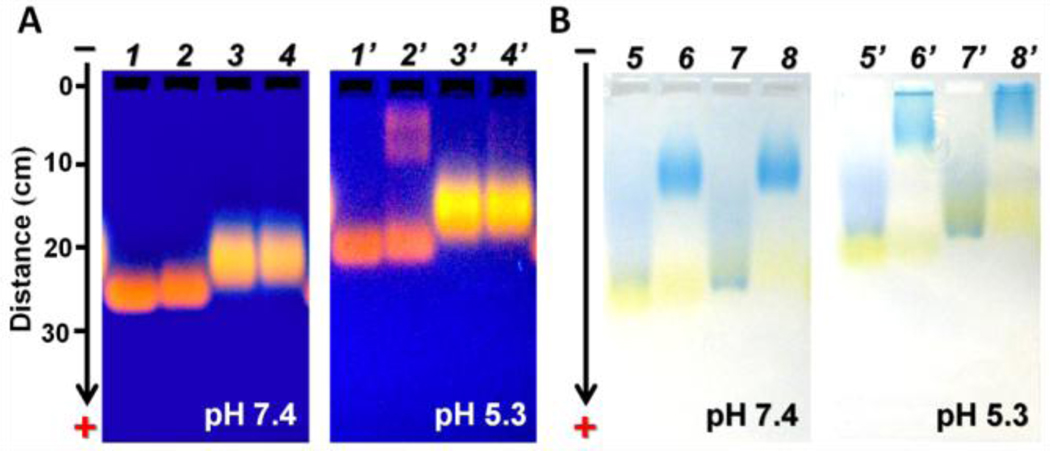
While positively charged cysteamine ligand in mildly acidic environment can drive the AuNPs nonspecifically bind to the cell membrane (Figure S2 [SI]), cationic cysteamine coated-AuNPs became negatively charged in the presence of serum proteins, indicating that serious nonspecific protein adsorption on NPs occurred (Figure S6 [SI]), consistent with previous report.20 Therefore, to assess whether GC-AuNPs coated with both glutathione and cysteamine can still resist protein adsorption, the NPs were incubated in the absence or presence of fetal bovine serum at 37°C for 30 min and then analyzed by agarose gel electrophoresis. GC-AuNPs displayed no changes in the mobility after incubating with fetal bovine serum at pH 7.4 and pH 5.3, suggesting that GC-AuNPs did not aggregate in the serum protein-containing medium and serum proteins were not bound to the particles at neutral and mildly acidic pH values (Figure 2A). In contrast, G-AuNPs started binding to proteins at pH 5.3, revealed by two luminescent bands after fetal bovine serum treatment. After NPs were incubated with fetal bovine serums, additional protein staining by coomassie brilliant blue 250 showed the blue protein band was well separated from the yellow band of GC-AuNPs at both pH 7.4 and pH 5.3 (Figure 2B), further confirming that GC-AuNPs have little interaction with serum proteins in the biological pH range from mildly acidic to neutral.
Figure 2.
Agarose gel electrophoresis of GC-AuNPs with or without incubated with fetal bovine serum (FBS) at pH 7.4 and pH 5.3 respectively. (A) G-AuNPs (well 1, 2, 1’, 2’) and GC-AuNPs (well 3, 4, 3’, 4’) were incubated at pH 7.4 or pH 5.3 in the absence (well 1, 3, 1’, 3’) or presence (well 2, 4, 2’, 4’) of 10% (v/v) FBS at 37 °C for 30 min (pictures taken under 365 nm UV excitation). (B) After G-AuNPs (well 5, 6, 5’, 6’) and GC-AuNPs (7, 8, 7’, 8’) were incubated with or without FBS, serum proteins were stained by coomassie brilliant blue 250 (CBB250). Blue band in well 5, 7, 5’, 7’ (without FBS): Free CBB250; Blue band in well 6, 8, 6’, 8’ (with FBS): CBB250-stained proteins.
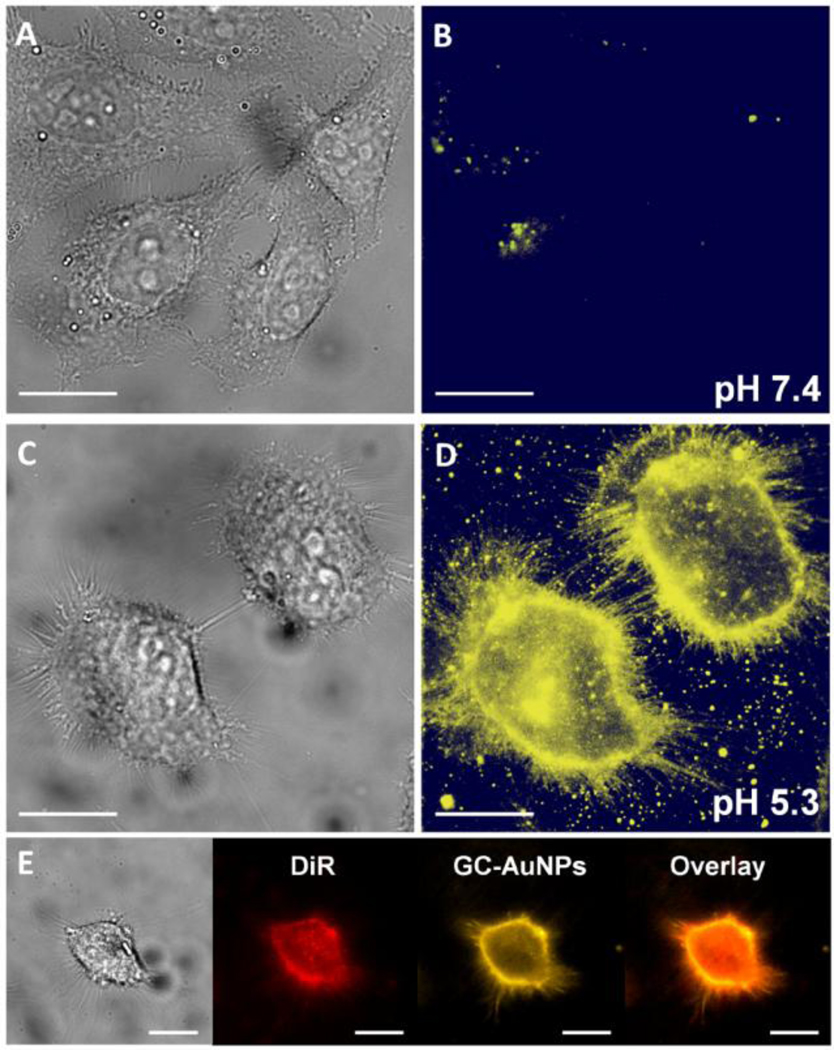
The pH-responsive membrane adsorption of GC-AuNPs in an extracellular pH range from 5.3 to 7.4 was investigated using fluorescence microscopy imaging of live HeLa cells as a model system. HeLa cells were also used in previous studies of pH dependent insertion of a peptide because their membrane remain integral in the range of pH 5.5 to 7.4.21 As is shown in Figures 3A–D and S7, GC-AuNPs had little interaction with cells at pH 7.4 in phosphate buffered saline (PBS). Once the extracellular pH was lowered to pH 6.0 and further dropped to 5.3, luminescence intensity of the cells increased dramatically, suggesting the adsorption of the NPs to the cell membrane was significantly enhanced in a slightly acidic environment. Z stack imaging of cells stained by GC-AuNPs at pH 5.3 indicated that the NPs were not internalized into the cells but bound on the cell surface (Figure S8 [SI]), which was further confirmed by the co-localization of GC-AuNPs with DiR, a phospholipid bilayer membrane dye that can embed in the lipid bilayer (Figure 3E and Scheme S2 [SI]). Due to the membrane adsorption, plasma membrane of filopodia, the slender cytoplasmic projections for cell migration, was also clearly visualized at pH 5.3 using fluorescence microscopy, similar to membrane staining by DiR (Figures 3D, S9 [SI]). Moreover, real-time imaging of live cells revealed that the accumulation of GCAuNPs on the cell membrane reached a maximum within 30 s, implying a fast membrane adsorption of the particles (Figure S10 [SI]). These results clearly indicated that additional surface ligand cysteamine made GC-AuNPs exhibit pH dependent membrane adsorption in the biological pH range.
Figure 3.
pH dependent adsorption of GC-AuNPs on live HeLa cell membrane. Brightfield (A, C) and fluorescence (B, D) images of live HeLa cells incubated with 0.2 mg/mL GC-AuNPs at pH 7.4 (A, B) and 5.3 (C, D) in PBS at 25°C for 10 min respectively (scalar bar, 20 µm). (E) Co-localization of GC-AuNPs with DiR (a phospholipid bilayer membrane dye) on live cell membrane (scalar bar, 20 µm).
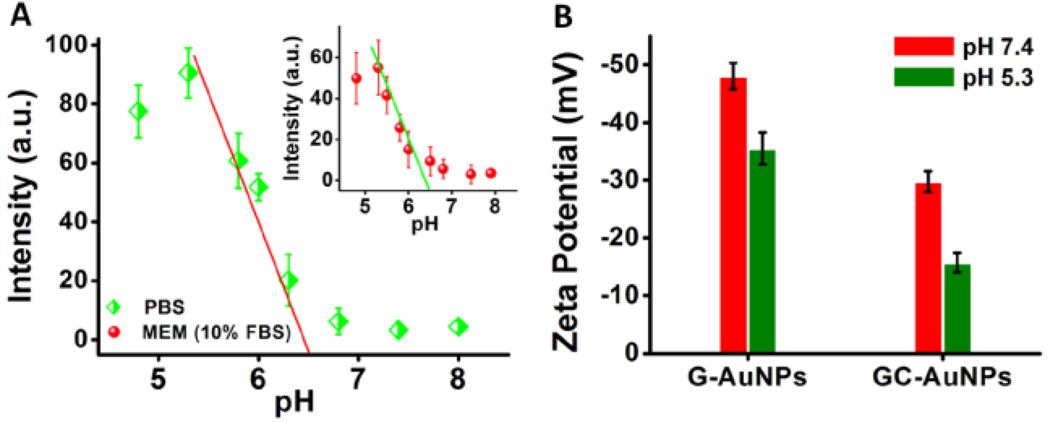
By quantifying the luminescence intensity of the cell membrane after incubated with GC-AuNPs at different pHs in PBS, the adsorption of GC-AuNPs to live cell membrane was found to exponentially increase with the increase of H+ concentration in the pH range of 4.8–8.0 and luminescence intensity of the cell membrane was increased about 28 times once the pH dropped from 7.4 to 5.3 (Figure S11 [SI]). The similar pH dependent membrane adsorption of GC-AuNPs was also observed in serum protein-containing cell culture medium (Figures S12, S13 [SI]). The pH thresholds for the membrane adsorption of GC-AuNPs in the absence or presence of serum proteins are 6.5 and 6.4 respectively (Figure 4A), indicating that serum proteins did not interfere the interaction of GC-AuNPs with the cells. This pH dependent membrane adsorption of GC-AuNPs in the presence of serum proteins is attributed to a collaborative effect of glutathione and cysteamine ligands: glutathione prevents nonspecific protein adsorption while the positively charged cysteamine enables particles to nonspecifically bind to negatively charged cell membrane through electrostatic interactions.
Figure 4.
Luminescence intensities of the cell membrane and zeta potentials of GC-AuNPs at different pHs. (A) Luminescence intensity of the cell membrane incubated with GC-AuNPs at different pH in PBS or in minimum essential medium (MEM) supplemented with 10% (v/v) fetal bovine serum (FBS) (Inset) respectively. (B) Zeta potentials of G-AuNPs and GC-AuNPs at pH 7.4 and pH 5.3 respectively. Results were presented as mean±SD (n = 6).
Interestingly, both gel electrophoresis and zeta potential measurements have confirmed that GC-AuNPs are still negatively charged at pH 7.4 and pH 5.3 (Zeta potential: −29.76±1.81 mV and −15.71±1.71 mV, respectively, Figure 4B). However, the zeta potentials of GC-AuNPs are much lower than those of G-AuNPs at the same pHs (−48.03±2.26 mV and −35.54±2.77 mV at pH 7.4 and pH 5.3 respectively), suggesting that additional cysteamine ligand in GC-AuNPs indeed significantly decreases negative charge of the NPs. Since the cell membrane remains negatively charged at a pH above 5,22 the mildly acidic pH-triggered membrane binding of anionic GC-AuNPs implies that such pH dependent adsorption behavior fundamentally arises from the decrease of global charge repulsion between GC-AuNPs and the cell membrane and the increase of attraction between protonated cysteamine ligand and the cell membrane. At pH 7.4, GC-AuNPs are still highly negatively charged, therefore, strong repulsion between the NPs and the cell membrane prevents the membrane adsorption. When the pH reached a critical point, ~pH 6.5, the threshold for adsorption, the local attraction between cysteamine and the membrane can overcome global electrostatic repulsion, and thus membrane adsorption of anionic GC-AuNPs on negatively charged membrane started being observed.
The observation of negatively charged GC-AuNPs bound to the negatively charged membrane seems against intuition but is consistent with recent molecular dynamic simulation on the nonspecific binding of anionic ~2 nm AuNPs to a negatively charged lipid membrane in a serum free environment23 and the experimental observation that anionic AuNPs with ordered hydrophobic and hydrophilic surface structure can overcome the global repulsion between the NPs and the cell membrane and crossed negatively charged lipid membrane.24 We currently can not determine whether glutathione and cysteamine formed ordered surface structure on the NPs; however, since pure cysteamine-coated AuNPs can nonspecifically bind to the cell membrane (Figure S2 [SI]),25 the driving force for the binding of GC-AuNPs to the cell membrane mainly arise from cysteamine ligand. Using a trypan blue assay,26 we found that the cell membrane remains intact after fusion with the NPs (Figure S14 [SI]), revealing that adsorption of negatively charged NPs to the cell membrane in acidic conditions did not induce the membrane disruption. This observation is distinct from interactions between cationic NPs and the cell membrane, which induced nanopore formation on lipid bilayers but is consistent with previously reported cell-permeable negatively charged AuNPs without destroying membrane integrity.24
In conclusion, by using a natural peptide, glutathione, and a simple aminothiol, cysteamine, as surface ligands, we facilely synthesized a negatively charged luminescent AuNP, which exhibits not only high resistance to nonspecific protein adsorption but also strong pH dependent adsorption to the live cell membrane within a biological pH range (5.3–7.4). This simple surface chemistry strategy offers us a new pathway to manipulate NP-cell membrane interactions in a native biological environment. Considering that pH plays a key role in many cellular processes and is an indicator for a pathological environment, metal NPs with pH dependent membrane adsorption might find new applications in tumor diagnosis and therapy.
Supplementary Material
ACKNOWLEDGMENT
This work was supported in part by the NIH (R21EB009853 to J.Z.) and the start-up fund from the University of Texas at Dallas (J.Z.).
Footnotes
Supporting Information Available: Materials and equipment, synthesis and characterization of luminescent gold nanoparticles, cell culture and microscopy imaging as well as supplementary figures are included in the supporting information. This material is available free of charge via the internet at http://pubs.acs.org.
REFERENCES
- 1.Alberts B, Lewis J, Raff M, Roberts K, Walter P. Molecular biology of the cell. 5th edition. New York: Garland Science; 2008. [Google Scholar]
- 2.Weinberg RA. The biology of cancer. New York: Garland Science; 2007. [Google Scholar]
- 3.Vander Heiden MG, Cantley LC, Thompson CB. Science. 2009;324:1029. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerweck LE, Seetharaman K. Cancer Res. 1996;56:1194. [PubMed] [Google Scholar]
- 5.(a) Roopenian DC, Akilesh S. Nat. Rev. Immunol. 2007;7:715. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]; (b) Gullotti E, Yeo Y. Mol. Pharmaceutics. 2009;6:1041. doi: 10.1021/mp900090z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lee ES, Gao ZG, Bae YH. J. Control. Release. 2008;132:164. doi: 10.1016/j.jconrel.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao XH, Cui YY, Levenson RM, Chung LWK, Nie SM. Nat. Biotechnol. 2004;22:969. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 7.McCarthy JR, Weissleder R. Adv. Drug Deliv. Rev. 2008;60:1241. doi: 10.1016/j.addr.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu M, Chen JY, Li ZY, Au L, Hartland GV, Li XD, Marquez M, Xia YN. Chem. Soc. Rev. 2006;35:1084. doi: 10.1039/b517615h. [DOI] [PubMed] [Google Scholar]
- 9.(a) Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Nat. Biotechnol. 2005;23:1418. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]; (b) Seo D, Park JC, Song H. J. Am. Chem. Soc. 2006;128:14863. doi: 10.1021/ja062892u. [DOI] [PubMed] [Google Scholar]
- 10.(a) Qian XM, Peng XH, Ansari DO, Yin-Goen Q, Chen GZ, Shin DM, Yang L, Young AN, Wang MD, Nie SM. Nat. Biotechnol. 2008;26:83. doi: 10.1038/nbt1377. [DOI] [PubMed] [Google Scholar]; (b) Keren S, Zavaleta C, Cheng Z, de la Zerda A, Gheysens O, Gambhir SS. Proc. Natl. Acad. Sci. 2008;105:5844. doi: 10.1073/pnas.0710575105. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lin AWH, Lewinski NA, West JL, Halas NJ, Drezek RA. J. Biomed. Optics. 2005;10:064035. doi: 10.1117/1.2141825. [DOI] [PubMed] [Google Scholar]
- 11.(a) Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean AKR, Han MS, Mirkin CA. Science. 2006;312:1027. doi: 10.1126/science.1125559. [DOI] [PubMed] [Google Scholar]; (b) Kim C, Agasti SS, Zhu ZJ, Isaacs L, Rotello VM. Nat. Chem. 2010;2:962. doi: 10.1038/nchem.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Daniel MC, Astruc D. Chem. Rev. 2004;104:293. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]; (b) Rosi NL, Mirkin CA. Chem. Rev. 2005;105:1547. doi: 10.1021/cr030067f. [DOI] [PubMed] [Google Scholar]; (c) Jain PK, Huang XH, El-Sayed IH, El-Sayed MA. Acc. Chem. Res. 2008;41:1578. doi: 10.1021/ar7002804. [DOI] [PubMed] [Google Scholar]
- 13.(a) Lynch I, Dawson KA. Nano Today. 2008;3:40. [Google Scholar]; (b) Röcker C, Zhang F, Parak WJ, Nienhaus GU. Nat. Nanotechnol. 2009;4:577. doi: 10.1038/nnano.2009.195. [DOI] [PubMed] [Google Scholar]
- 14.(a) Vincent A, Babu S, Heckert E, Dowding J, Hirst SM, Inerbaev TM, Self WT, Reilly CM, Masunov AE, Rahman TS, Seal S. Acs Nano. 2009;3:1203. doi: 10.1021/nn9000148. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Patil S, Sandberg A, Heckert E, Self W, Seal S. Biomaterials. 2007;28:4600. doi: 10.1016/j.biomaterials.2007.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Maiorano G, Sabella S, Sorce B, Brunetti V, Malvindi MA, Cingolani R, Pompa PP. Acs Nano. 2010;4:7481. doi: 10.1021/nn101557e. [DOI] [PubMed] [Google Scholar]
- 15.(a) Zheng J, Petty JT, Dickson RM. J. Am. Chem. Soc. 2003;125:7780. doi: 10.1021/ja035473v. [DOI] [PubMed] [Google Scholar]; (b) Zheng J, Nicovich PR, Dickson RM. Annu. Rev. Phys. Chem. 2007;58:409. doi: 10.1146/annurev.physchem.58.032806.104546. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Richards CI, Hsiang JC, Senapati D, Patel S, Yu JH, Vosch T, Dickson RM. J. Am. Chem. Soc. 2009;131:4619. doi: 10.1021/ja809785s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Xie JP, Zheng YG, Ying JY. J. Am. Chem. Soc. 2009;131:888. doi: 10.1021/ja806804u. [DOI] [PubMed] [Google Scholar]; (e) Wei WT, Lu YZ, Chen W, Chen SW. J. Am. Chem. Soc. 2011;133:2060. doi: 10.1021/ja109303z. [DOI] [PubMed] [Google Scholar]
- 16.Xia T, Rome L, Nel A. Nat. Mater. 2008;7:519. doi: 10.1038/nmat2213. [DOI] [PubMed] [Google Scholar]
- 17.Zhou C, Long M, Qin YP, Sun XK, Zheng J. Angew. Chem. Int. Ed. 2011;50:3168. doi: 10.1002/anie.201007321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leroueil PR, Berry SA, Duthie K, Han G, Rotello VM, McNerny DQ, Baker JR, Orr BG, Holl MMB. Nano Lett. 2008;8:420. doi: 10.1021/nl0722929. [DOI] [PubMed] [Google Scholar]
- 19.Zhou C, Sun C, Yu MX, Qin YP, Wang JG, Kim M, Zheng J. J. Phys. Chem. C. 2010;114:7727. doi: 10.1021/jp9122584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Ipe BI, Bawendi MG, Frangioni JV. Nat. Biotechnol. 2007;25:1165. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reshetnyak YK, Andreev OA, Lehnert U, Engelman DM. Proc. Natl. Acad. Sci. 2006;103:6460. doi: 10.1073/pnas.0601463103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y, Raphael RM. Biophys. J. 2007;92:2451. doi: 10.1529/biophysj.106.096362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin JQ, Zhang HW, Chen Z, Zheng YG. Acs Nano. 2010;4:5421. doi: 10.1021/nn1010792. [DOI] [PubMed] [Google Scholar]
- 24.Verma A, Uzun O, Hu YH, Hu Y, Han HS, Watson N, Chen SL, Irvine DJ, Stellacci F. Nat. Mater. 2008;7:588. doi: 10.1038/nmat2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kong T, Zeng J, Wang XP, Yang XY, Yang J, McQuarrie S, McEwan A, Roa W, Chen J, Xing JZ. Small. 2008;4:1537. doi: 10.1002/smll.200700794. [DOI] [PubMed] [Google Scholar]
- 26.Celis JE, Carter N, Hunter T, Simons K, Small JV, Shotton D. Cell Biology (Third Edition): a laboratory handbook. Elsevier Academic Press; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.