Abstract
BACKGROUND:
Clarithromycin resistance has decreased the eradication rates of Helicobacter pylori.
AIMS:
To determine whether a 10-day course of sequential therapy (ST) is more effective at eradicating H pylori infection than triple therapy (TT) in the first or second line, and to assess side effects and compliance with therapy.
METHODS:
One hundred sixty treatment-naive and 40 non-treatment-naive patients who were positive for H pylori infection by 13C-urea breath test or endoscopy were enrolled. Eighty of 160 patients underwent TT, while 80 of 160 underwent ST with omeprazole (20 mg) plus amoxicillin (1 g) twice/day for five days, followed by omeprazole (20 mg) with tinidazole (500 mg) twice/day and clarithromycin (500 mg) twice/day for five consecutive days. H pylori eradication was evaluated by 13C-urea breath test no sooner than four weeks after the end of treatment.
RESULTS:
Eradication was achieved in 59 of 80 treatment-naive patients treated with TT (74%), in 74 of 80 patients treated with ST (93%), and in 38 of 40 non-treatment-naive patients (95%). Eradication rates in treatment-naive patients with ST were statistically significantly higher than TT (92.5% versus 73.7%; P=0.0015; OR 4.39 [95% CI 1.66 to 11.58]). Mild adverse effects were reported for both regimens.
CONCLUSIONS:
ST appears to be a well-tolerated, promising therapy; however, randomized controlled trials with larger and more diverse sample populations are needed before it can be recommended as a first-line treatment.
Keywords: Helicobacter pylori, Sequential therapy, Standard therapy
Abstract
HISTORIQUE :
La résistance à la clarithromycine a réduit l’éradication des taux de Helicobacter pylori.
OBJECTIFS :
Déterminer si un traitement séquentiel (TS) de dix jours est plus efficace pour éradiquer l’infection à H pylori que la trithérapie (TT) en première et deuxième ligne, et évaluer les effets secondaires et l’adhésion au traitement.
MÉTHODOLOGIE :
Cent soixante patients naïfs de traitement et 40 patients non naïfs de traitement positifs à l’infection à H pylori, d’après le test respiratoire à l’urée marquée au 13C ou l’endoscopie, ont participé à l’étude. Quatre-vingts des 160 patients ont subi une TT, et les 80 autres ont subi un TS à l’oméprazole (20 mg) associé à l’amoxicilline (1 g) deux fois par jour pendant cinq jours, suivi de l’oméprazole (20 mg) associé au tinidazole (500 mg) deux fois par jour et de clarithromycine (500 mg) deux fois par jour pendant cinq jours consécutifs. Les chercheurs ont évalué l’éradication de l’infection à H pylori non moins de quatre semaines après la fin du traitement.
RÉSULTATS :
Les chercheurs ont observé une éradication chez 59 des 80 patients naïfs de traitement soignés par TT (74 %), chez 74 des 80 patients soignés par TS (93 %), et chez 38 des 40 patients non naïfs de traitement (95 %). Les taux d’éradication des patients naïfs de traitement soignés par TS étaient plus élevés, de manière statistiquement significative, que ceux soignés par TT (92,5 % par rapport à 73,7 %; P=0,0015; RRR 4,39 [95 % IC 1,66 à 11,58]). Des effets indésirables légers ont été déclarés pour les deux posologies.
CONCLUSIONS :
Le TS semble une thérapie bien tolérée et prometteuse, mais des essais aléatoires et contrôlés auprès d’échantillons de population plus vastes et plus diversifiés s’imposent avant qu’on puisse le recommander comme traitement de première ligne.
Helicobacter pylori is the principal cause of peptic ulcer disease and gastric cancer (1). Eradication of H pylori has been shown to prevent the recurrence of peptic ulcer disease, to reverse gastric atrophy – a precursor of gastric cancer – and to cure some localized low-grade gastric lymphomas (1). Therefore, the treatment of H pylori infection is of global significance. The success rate of eradication therapy for H pylori is decreasing. Recent studies conducted in the United States (US) and Europe (1–3) have shown that the eradication rate has decreased to below 85%, which is the threshold recommended by most consensus guidelines. Resistance rates for commonly used antimicrobial agents in current treatment regimens are increasing and represent an important cause of treatment failures (4). A recent review (1) summarized the resistance rates for commonly used antimicrobial agents and showed that clarithromycin resistance is a major problem in most developed countries, with a few notable exceptions such as regions of Scandinavia. However, antimicrobial resistance is not the only explanation for decreasing eradication rates – lack of adherence to treatment is a well-recognized factor in failed eradication and the occurrence of side effects (5).
Current guidelines recommend triple therapy with proton pump inhibitors (PPIs) and two antibiotics as the principal treatment to be used worldwide; however, it is generally accepted that new strategies are required (3,6). A 10-day course of sequential therapy (ST) is a novel therapeutic regimen that has shown promising results in controlled trials involving both adults and children (7).
The aim of the present study was to evaluate the efficacy of a 10-day course of ST as first-line and rescue therapies.
The primary outcome measure of the study was the eradication rate of H pylori infection and to determine the efficacy of sequential treatment against clarithromycin-resistant strains of H pylori. The secondary outcome measures were to assess adherence to therapy and to determine the frequency of self-reported side effects of the 10-day course of ST.
METHODS
Patients
The present analysis was an open-label, single-centre study. Between January 2008 and December 2009, 200 patients with urea breath test (UBT)-positive or endoscopy-proven H pylori infection were enrolled in the study. One hundred sixty of these patients never received treatment for H pylori infection (group A: first-line therapy), while 40 had previously undergone H pylori eradication treatment by standard seven-day first-line therapy (group B: second-line therapy) with PPIs plus amoxicillin (1 g) and clarithromycin (500 mg) twice/day for seven days.
Eighty patients from group A and all patients from group B underwent a 10-day course of ST consisting of omeprazole (20 mg) plus amoxicillin (1 g), twice/day for five days, followed by omeprazole (20 mg) with tinidazole (500 mg) twice/day, and clarithromycin (500 mg) twice/day for five consecutive days (Figure 1). The other 80 patients from group A received standard triple therapy consisting of omeprazole (20 mg) or pantoprazole (40 mg) plus amoxicillin (1 g) and clarithromycin (500 mg) twice/day for seven days. After the patients completed the 10-day ST and the standard therapy, PPI therapy was discontinued in all patients.
Figure 1).
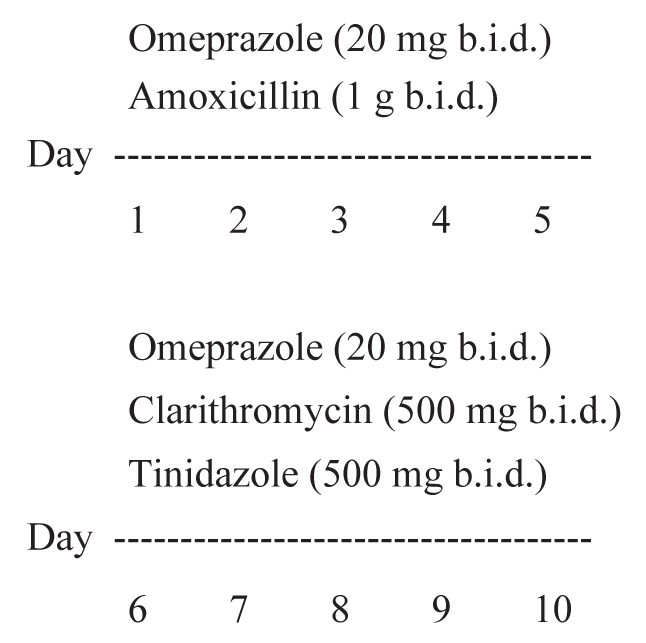
Sequential therapy. b.i.d Twice/day
Exclusion criteria were as follows: chronic use of PPIs or H2-receptor antagonists; use of antibiotics in the previous two weeks; concomitant anticoagulant or nonsteroidal anti-inflammatory drug use; Zollinger-Ellison syndrome; known allergy to the prescribed antibiotics; pregnant or breastfeeding women; severe or unstable cardiovascular, pulmonary or endocrine disease; clinically significant renal or hepatic disease or dysfunction; hematological disorders; any other clinically significant medical condition that could increase risk of side effects; and malignant disease of any kind during the previous five years. Patients with Barrett’s esophagus and high-grade dysplasia were also excluded, as were patients with severe psychiatric or neurological disorders.
The baseline demographic and clinical characteristics of the patients are shown in Table 1. The modality of diagnosis and type of treatment are shown in Table 2.
TABLE 1.
Patient characteristics (n=200)
| Variable | Men (n=105) | Women (n=95) |
|---|---|---|
| Age, years (mean) | 47.9 | 50.7 |
| Nonulcer dyspepsia | 107 (61.1) | 68 (38.8) |
| Duodenal ulcer | 12 (63.1) | 7 (39.6) |
| Gastric ulcer | 4 (66.4) | 2 (33.6) |
Data presented as n (%) unless otherwise indicated
TABLE 2.
Diagnosis and treatment type
| Variable |
Diagnostic test |
Treatment |
||
|---|---|---|---|---|
| Endoscopy (n=75) | 13C-UBT (n=125) | Classic triple therapy | 10-day sequential therapy | |
| Nonulcer dyspepsia | 50 | 175 | 68 | 107 |
| Duodenal ulcer | 19 | 0 | 10 | 9 |
| Gastric ulcer | 6 | 0 | 2 | 4 |
Data presented as n. 13C-UBT Carbon-13 urea breath test
Confirmation of eradication: 13C-UBT
After an overnight fast, breath samples from each patient were collected in aluminized plastic bags for nondispersive isotope-selective infrared spectrometry analysis 30 min after ingestion of 75 mg of carbon-13 (13C) urea dissolved in 150 mL of 0.033 M citric acid (Cortex Italia, Italy). This dose of urea was used because it was shown to be as reliable as higher doses (8), and because citric acid is the optimal test drink for the 13C-UBT (9). Breath samples were analyzed by infrared spectroscopy (IRIS Breath Analyser [Wagner Analysen-Technik, Germany]). The operators of the infrared spectrometer were unaware of patient H pylori status and the results of other tests. A baseline change of greater than 3.5 units/mL indicated H pylori infection (10).
Participants were classified as either negative (uninfected) or positive (infected). Indeterminate results were repeated for analysis and, if the same result was obtained, it was considered to be negative. The infection was considered to have been successfully eradicated when results were negative.
Follow-up procedures
Treatment compliance and side effects:
Patients were asked to return at the end of therapy to assess compliance and side effects. Open-ended questions regarding side effects were specific to 10-day ST of H pylori infection. Compliance was defined as consumption of more than 90% of the prescribed drugs. Side effects were assessed by patient self-report during a personal interview; no questionnaires were used.
Causality was assessed by the temporal relationship between symptoms and the start of therapy. Patients were asked to report any adverse events during the treatment period.
Statistical analysis
Data were categorically analyzed using the χ2 test and Fisher’s exact test. P<0.05 was considered to be statitically significant. The study was conducted according to good clinical practice guidelines and the principles of the Declaration of Helsinki (11–12). Informed consent was obtained from all enrolled patients.
RESULTS
Patients
A total of 200 patients were enrolled in the study, which included 105 men (52.5%) and 95 women (47.5%) with a mean (± SD) age of 49.48±13.65 years (range 20 to 77 years), all of whom completed the study. Forty patients who initially enrolled in the study were lost to follow-up and, consequently, were not included in the analysis.
Among the 200 patients, 175 (87.5%) were diagnosed as nonulcer dyspeptics, whereas 19 (9.5%) were diagnosed with duodenal ulcer and six (3.0%) with non-neoplastic gastric ulcer based on endoscopic and histological findings. Multiple biopsy samples were obtained from patients with duodenal ulcers.
Fifty of 75 patients diagnosed with nonulcer dyspepsia (28.5%) underwent endoscopy and, in all, multiple antral biopsy samples were obtained for histological examination.
Eradication of H pylori infection
The 13C-UBT test was conducted no sooner than four weeks after discontinuing the treatment, and all 200 patients were followed up.
‘Standard’ seven-day triple therapy:
Twenty-one of 80 treatment-naive patients (26.3%) were positive; the remaining 59 (73.7%) tested negative with the 13C-UBT.
10-day ST:
Only six of 80 treatment-naive patients (7.5%) were positive; the remaining 74 (92.5%) tested negative with the 13C-UBT.
Thirty eight of 40 group B patients (95%) tested negative with the 13C-UBTs at the end of the treatment.
The eradication rate achieved in group A patients for 10-day ST (74 of 80) was statistically significantly higher than that for ‘standard’ triple therapy (59 of 80) (P=0.0015; OR 4.39 [95% CI 1.66 to 11.58]) (Table 3). All 200 patients (100%) successfully completed both therapies.
TABLE 3.
Helicobacter pylori eradication rate
| Variable |
Therapy regimen |
Patients, n (%) |
||
|---|---|---|---|---|
| Classic triple | 10-day sequential | Naive | Non-naive | |
| Negative 13C-urea breath test (successful treatment), n | 59 | 112 | 74 (92.5) | 38 (95.0) |
| Positive 13C-urea breath test (treatment failure), n | 21 | 8 | 6 (7.5) | 2 (5) |
Adverse events
Both treatments were well tolerated. No patient discontinued treatment. A total of 14 (17.5%) patients who received standard triple therapy, and 12 (15.0%) patients who received ST, reported minor side effects (17.5% versus 15.0%; P=0.668). The most frequent side effects in both groups were nausea, bloating and mild diarrhea (Table 4).
TABLE 4.
Patient self-report of adverse events during therapy
| Adverse event | Classic triple therapy (n=14) | 10-day sequential therapy (n=12) |
|---|---|---|
| Nausea | 5 | 3 |
| Bloating | 3 | 2 |
| Diarrhea | 3 | 3 |
| Epigastric pain | 0 | 1 |
| Glossitis | 0 | 1 |
| Headache | 1 | 1 |
| Vomiting | 1 | 1 |
| Heartburn | 1 | 0 |
Data presented as n
DISCUSSION
The goal of H pylori eradication therapy is the effective elimination of the infection in all treated patients. Currently, recommended first-line treatments for H pylori infection fail in a significant proportion of patients for several reasons such as bacterial resistance, poor compliance, treatment-related factors including the number and dose of medications used in combination, dosing frequency, treatment duration and patient-related factors (1–13).
Triple therapy with a PPI, clarithromycin and either amoxicillin or metronidazole, or tinidazole is the standard treatment regimen to cure H pylori infection among primary care physicians and gastroenterologists in the US and Europe (14–16). Two recent double-blind, US multicentre studies (5,17) found disappointingly low eradication rates with standard triple therapy. In one study (5), 75.6% of 402 patients and, in another (17), 77.2% of 307 patients were cured of H pylori infection by means of a modified intention-to-treat analysis following a 10-day triple regimen.
Low eradication rates with standard triple therapy have also been reported in Europe, Australia and Asia (18).
The prevalence of H pylori infection varies among countries. In developing countries, the infection is acquired in early childhood at high rates, with 70% to 90% of people infected by 20 years of age (4,19).
The Third Maastricht Consensus Report agreed that effective treatment for H pylori should achieve an intention-to-treat eradication rate of greater than 80% (3); however, in clinical practice, eradication rates are lower than 80% for many standard treatment regimens.
Eradication rates show regional variation within and between countries. Alternative antibiotics, based on local resistance rates, may improve eradication rates. Clarithromycin resistance has a greater impact on treatment efficacy (19). For example, in Thailand, the rates of clarithromycin resistance increased from 19% in 2002 to 23% in 2004, and has resulted in a deterioration of efficacy of the standard seven-day triple therapy from 92.5% to 56% (20,21).
ST is a novel, recently developed therapeutic approach based on a different combination of available antibiotics. It is the only therapeutic regimen that is proven to be superior to seven to 10 days of triple therapy in large, multicentre randomized trials.
Vaira et al (22) showed that the eradication rate achieved with the ST regimen was significantly greater than that obtained with the standard treatment in the intention-to-treat analysis (89% versus 77%; P=0.0134; difference 12% [95% CI 3% to 20%]), the modified intention-to-treat analysis (91% versus 78%; P=0.0022; difference 13% [95% CI 5% to 21%]) and the per-protocol analysis (93% versus 79%; P=0.0013; difference 14% [95% CI 6% to 21%]). ST was significantly more effective in patients with clarithromycin-resistant strains (89% versus 29%; P=0.0034). The incidence of major and minor side effects did not differ between therapy groups (17% in both groups). The recent meta-analysis by Jafri et al (23) showed that the crude rates of H pylori eradication in 10 randomized controlled trials involving 2747 patients were 93.4% (95% CI 91.3% to 95.5%) for ST (n=1363), and 76.9% (95% CI 71.0% to 82.8%) for standard triple therapy (n=1384) (RR reduction 71% [95% CI 64% to 77%]; absolute risk reduction 16.5% [95% CI 14% to 19%]). The median rates of adherence were 97.4% (range 90.0% to 98.9%) for ST and 96.8% (range 93.0% to 100%) for standard therapy.
ST appeared to be superior in prespecified sensitivity (subgroup) analyses stratified according to trial quality, smoking status, diagnosis, resistance to clarithromycin, imidazoles or both, duration of triple therapy and method of diagnosis.
The systematic review and meta-analysis of randomized controlled trials involving adults and children by Gatta et al (24) in a comprehensive study of more than 3200 patients confirmed that the OR for eradication of H pylori with ST compared with classic triple therapy (CTT) was 2.99 (95% CI 2.47 to 3.62), yielding a number needed to treat (NNT) of 6 (95% CI 5 to 7), favouring ST. The OR for eradication with ST compared with 10-day CTT was 2.92 (95% CI 1.95 to 4.38), yielding an NNT of eight (95% CI 6 to 12), favouring ST. In patients with clarithromycin resistance, the OR for eradication with ST was 10.21 (95% CI 3.01 to 34.58) compared with CTT; however, the numbers studied were small. Three RCTs enrolled 260 children and adolescents, and the OR for eradication was 1.98 (95 % CI 0.96 to 4.07). Similarly, a more recent meta-analysis by Tong et al (25) identified 11 randomized trials, including eight full-text articles and three abstracts. Pooled analysis demonstrated the clear superiority of ST over the seven-day triple regimen (RR 1.23 [95% CI 1.19 to 1.27]), and over the 10-day triple regimen (RR 1.16 [95% CI 1.10 to 1.23]).
The precise mechanism explaining the success of ST is unknown; however, bacteria can develop efflux channels for clarithromycin, which rapidly transfer the drug out of the cell, thereby preventing the antibiotic from binding to ribosomes (4). As amoxicillin acts on the bacterial cell wall and weakens it, the initial phase of treatment may prevent the development of efflux channels, which in turn, may improve the efficacy of clarithromycin in the second phase of treatment (4). In ST, the first five days of amoxicillin and PPI treatment undoubtedly results in an 8- to 10-log reduction of H pylori and even its eradication in at least 50% of patients. At this stage, the second part of the regimen (clarithromycin and tinidazole) acts to eradicate a relatively small residual population of viable organisms. The weakness of clarithromycin therapy is that random mutations in the 23S ribosome gene of H pylori can prevent binding of the antibiotic, rendering it ineffective. By reducing the H pylori population before it is exposed to clarithromycin, such mutations are statistically much less likely to occur. Similarly, nitroimidazoles (metronidazole/tinidazole) become ineffective when a random mutation inactivates the rdxA gene – the antibiotic is no longer metabolized to its bacteria-toxic form (26). Once again, the higher efficacy of ST may be related to the larger number of antibiotics (three drugs; two dissimilar agents, ie, clarithromycin and tinidazole), or its combination, to which the organism is exposed, or to the use of imidazole, which is not part of the standard triple drug regimen.
Our study demonstrated an eradication rate of 92.5% for 10-day ST (74 of 80 patients), which was statistically significantly higher than that for ‘standard’ triple therapy and a very high success rate of eradication (95%) in the failed treatment group. The precise reason for the success of ST is not known; however, it may be due to the following factors: inclusion of patients who had a very high H pylori infectivity rate (especially in the non-treatment-naive patients of group B); the choice of exclusion criteria; and/or the fact that the subjects were all compliant and completed the therapy regimen to which they were assigned. However, our study had limitations. The nature of the study design did not allow us to draw conclusions as to whether the improved effect with ST was due to sequential administration or to the additional antibiotic (tinidazole) that is not part of the standard regimen.
Finally, the duration of therapy must be addressed. Seven days is a very short timeframe; however, 10 days appears to be optimal with respect to patient adherence and cure rate, and treatment never needs to exceed 14 days. Therefore, ST of five days plus five days appeared to be a wise choice, and was supported with good clinical data.
CONCLUSIONS
The results of our study showed that ST is superior to standard triple therapy for the eradication of H pylori infection both as a first-line and second-line treatment (eradication rates of 92.5% and 95%, respectively; P<0.05). The present study also confirmed that triple therapy, which is the current standard treatment, has low eradication rates. Both treatments are well tolerated and have similar rates of side effects. The fact that the cost of the ST regimen is similar to that of the standard regimen makes it an attractive alternative to triple therapy.
ST appears to be a promising therapy; however, additional randomized controlled trials with larger and more diverse sample populations are needed before it can be definitely recommended as a first-line treatment.
REFERENCES
- 1.Vakil N, Megraud F. Eradication treatment for Helicobacter pylori. Gastroenterology. 2007;133:985–1001. doi: 10.1053/j.gastro.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Fuccio L, Minardi M, Zagari R, et al. Meta-analysis of first-line proton pump inhibitor-based triple therapy for Helicobacter pylori eradication. Ann Intern Med. 2007;147:553–62. doi: 10.7326/0003-4819-147-8-200710160-00008. [DOI] [PubMed] [Google Scholar]
- 3.Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection: The Maastricht III Consensus Report. Gut. 2007;56:772–81. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Francesco V, Margiotta M, Zullo A, et al. Clarithromycin-resistant genotypes and eradication of Helicobacter pylori. Ann Intern Med. 2006;144:94–100. doi: 10.7326/0003-4819-144-2-200601170-00006. [DOI] [PubMed] [Google Scholar]
- 5.Vakil N, Lanza F, Schwartz H, et al. Seven-day therapy for Helicobacter pylori in the United States. Aliment Pharmacol Ther. 2004;20:99–107. doi: 10.1111/j.1365-2036.2004.02029.x. [DOI] [PubMed] [Google Scholar]
- 6.Cianci R, Montalto M, Pandolfi F, et al. Third-line rescue therapy for Helicobacter pylori infection. World J Gastroenterol. 2006;12:2313–9. doi: 10.3748/wjg.v12.i15.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zullo A, De Francesco V, Hassan C, et al. The sequential therapy regimen for Helicobacter pylori eradication: A pooled-data analysis. Gut. 2007;56:1353–7. doi: 10.1136/gut.2007.125658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eggers RH, Kulp A, Tegeler R, et al. A methodological analysis of the 13C-urea breath test for detection of Helicobacter pylori infections: High sensitivity and specificity within 30 min using 75 mg of 13C-urea. Eur J Gastroenterol Hepatol. 1990;2:437–44. [Google Scholar]
- 9.Dominguez-Muñoz JE, Leodolter A, Sauerbruch T, et al. A citric acid solution is an optimal test drink in the 13C-urea breath test for the diagnosis of Helicobacter pylori infection. Gut. 1997;40:459–62. doi: 10.1136/gut.40.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Logan RPH, Dill S, Bauer FE, et al. The European 13C-urea breath test for the detection of Helicobacter pylori. Eur J Gastroenterol Hepatol. 1991;3:915–8. [Google Scholar]
- 11.European Agency for the Evaluation of Medicinal Products, International Conference on Harmonisation – World Health Organization . Guideline for Good Clinical Practice. ICH Topic E6. Canary Wharf, UK: EMEA; 2002. < http://www.emea.europa.eu/pdfs/human/ich/013595en.pdf> (Accessed on January 21, 2008). [Google Scholar]
- 12.Salako SE. The Declaration of Helsinki 2000: Ethical principles and the dignity of difference. Med Law. 2006;25:341–54. [PubMed] [Google Scholar]
- 13.Cutler AF, Schubert TT. Patient factors affecting H. pylori eradication with triple therapy. Am J Gastroenterol. 1993;88:505–9. [PubMed] [Google Scholar]
- 14.Sharma VK, Howden CW. A national survey of primary care physicians’ perceptions and practices related to Helicobater pylori infection. J Clin Gastroenterol. 2004;38:326–31. doi: 10.1097/00004836-200404000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Maconi G, Tosetti C, Miroglio G, et al. Management of Helicobacter pylori-related gastrointestinal diseases by general practitioners in Italy. Aliment Pharmacol Ther. 1999;13:1499–504. doi: 10.1046/j.1365-2036.1999.00648.x. [DOI] [PubMed] [Google Scholar]
- 16.Shirin H, Birkenfeld S, Shevah O, et al. Application of Maastricht 2-2000 guidelines for the management of Helicobacter pylori among specialists and primary care physicians in Israel: Are we missing the malignant potential of Helicobacter pylori? J Clin Gastroenterol. 2004;38:322–5. doi: 10.1097/00004836-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Laine L, Fennerty MB, Osato M, et al. Esomeprazole-based Helicobacter pylori eradication therapy and the effect of antibiotic resistance: Results of three US multicenters, double-blind trials. Am J Gastroenterol. 2000;95:3393–8. doi: 10.1111/j.1572-0241.2000.03349.x. [DOI] [PubMed] [Google Scholar]
- 18.Laheij RJ, Van Rossum LGM, Jansen JB, et al. Evaluation of treatment regimens to cure Helicobacter pylori infection. A meta-analysis. Aliment Pharmacol Ther. 1999;13:857–64. doi: 10.1046/j.1365-2036.1999.00542.x. [DOI] [PubMed] [Google Scholar]
- 19.Fischbach L, Evans EL. Meta-analysis: Effect of antibiotic resistance on the efficacy of triple and quadruple first-line therapy for Helicobacter pylori. Aliment Pharmacol Ther. 2007;1:343–57. doi: 10.1111/j.1365-2036.2007.03386.x. [DOI] [PubMed] [Google Scholar]
- 20.Tangmankongworakoon N, Mahachai V, Thong-Ngam D, et al. Pattern of drug resistant Helicobacter pylori in dyspeptic patients in Thailand. J Med Assoc Thai. 2003;86(Suppl 2):S439–44. [PubMed] [Google Scholar]
- 21.Mahachai V, Thong-Ngam D, Noophun P, et al. Efficacy of clarithromycin-based triple therapy for treating Helicobacter pylori in Thai non-ulcer dyspeptic patients with clarithromycin resistant strains. J Med Assoc Thai. 2006;89(Suppl 3):S74–8. [PubMed] [Google Scholar]
- 22.Vaira D, Zullo A, Vakil N, et al. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: A randomized trial. Ann Intern Med. 2007;146:556–63. doi: 10.7326/0003-4819-146-8-200704170-00006. [DOI] [PubMed] [Google Scholar]
- 23.Jafri NS, Hornung CA, Howden CW. Meta-analysis: Sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008;148:923–31. doi: 10.7326/0003-4819-148-12-200806170-00226. [DOI] [PubMed] [Google Scholar]
- 24.Gatta L, Vakil N, Leandro G, et al. Sequential therapy or triple therapy for Helicobacter pylori: Systematic review and meta-analysis of randomized controlled trials in adult and children. Am J Gastroenterol. 2009;104:3069–79. doi: 10.1038/ajg.2009.555. [DOI] [PubMed] [Google Scholar]
- 25.Tong JL, Ran ZH, Shen J, Xiao SD. Sequential therapy vs. standard triple therapies for Helicobacter pylori infection: A meta-analysis. J Clin Pharm Ther. 2009;34:41–53. doi: 10.1111/j.1365-2710.2008.00969.x. [DOI] [PubMed] [Google Scholar]
- 26.Sisson G, Goodwin A, Raudonikiene A, et al. Enzymes associated with reductive activation and action of nitazoxanide, nitrofurans, and metronidazole in Helicobacter pylori. Antimicrob Agents Chemother. 2002;46:2116–23. doi: 10.1128/AAC.46.7.2116-2123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


