Abstract
The primary function of epithelia is to provide a barrier between the extracellular environment and the interior of the body. Efficient epithelial repair mechanisms are therefore crucial for homeostasis. The epithelial wound-healing process involves highly regulated morphogenetic changes of epithelial cells that are driven by dynamic changes of the cytoskeleton. P21-activated kinases are serine/threonine kinases that have emerged as important regulators of the cytoskeleton. These kinases, which are activated downsteam of the Rho GTPases Rac and cd42, were initially mostly implicated in the regulation of cell migration. More recently, however, these kinases were shown to have many additional functions that are relevant to the regulation of epithelial wound healing. Here, we provide an overview of the morphogenetic changes of epithelial cells during wound healing and the many functions of p21-activated kinases in these processes.
Keywords: Epithelial morphogenesis, Wound healing, Rho GTPases, p21-activated kinase, Cell adhesion, Cell migration
1. Introduction
Epithelial cells are organized in sheets of adherent cells that are polarized, meaning that they have distinct apical and basolateral surfaces. An important function of polarized epithelial cells is to form barriers between distinct physiological environments (Nelson, 2003; O’Brien et al., 2002; Zegers et al., 2003b). Examples of such barriers are the skin and the luminal surfaces of internal organs such as the respiratory, gastrointestinal and uritogenitary tracts, as well as the mammary, prostate and other exocrine glands. The functions of epithelia rely entirely on the ability of epithelial cells to form a polarized monolayer. It is therefore essential that epithelia have efficient mechanisms to repair injuries induced by trauma, surgery, inflammation and toxic or ischemic insults. In general, epithelial repair can be divided into a start and a stop phase (Jacinto et al., 2001). In the start phase, cells adjacent to injured areas partially dedifferentiate and migrate into the site of injury. These cells migrate by extending protrusions and lamellipodia into the wound, while pulling along cells located further back from the wound edge. In large wounds, cell proliferation is stimulated to replace lost or damaged cells. In the stop phase, newly formed cell-cell contacts block cell migration and proliferation.
In this review I will discuss intracellular signaling pathways that control the diverse morphogenetic changes that epithelial cells undergo during the different stages of epithelial wound healing. In vivo wound healing is a complex and highly regulated process that involves a wide range of extracellular signals and epithelial cells, stromal and inflammatory cells. Interestingly, the signaling pathways that control epithelial behavior during injury repair appear to be highly conserved and bear strong resemblance with related behaviors that are observed during embryonic development. One of the conserved features for epithelial morphological changes is the crucial role of the cytoskeleton. Rho GTPases in particular, are ubiquitous intracellular signaling intermediates critical for cytoskeletal regulation and wound healing in a wide variety of models. In this review I will first discuss general aspects of epithelial morphogenesis during wound healing. Next, I will focus on the role of one particular effector family of Rho GTPases, the p21-activated kinases, and their role in epithelial cell behavior during wound healing.
2. Biology of Wound Healing in Different Model Systems
Epithelial wound healing in vivo has been most widely studied in the context of the skin (Martin, 1997; Singer and Clark, 1999). Cutaneous wounds in adult tissue are temporarily repaired by the formation of a fibrin-rich blood clot to which platelets bind. The clot acts as a reservoir for growth factors and cytokines secreted by platelets and damaged keratinocytes, such as platelet-derived growth factor (PDGF), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), the transforming growth factor α (TGF-α), and members of the transforming growth factor β (TGF-β) family. These factors attract neutrophils and fibroblasts, which also secrete growth factors and proteases such as matrix metalloproteases (MMPs). Upon secretion, MMPs degrade specific components of the extracellular matrix, thereby allowing matrix remodeling, while at the same time releasing additional growth factors that had been linked to the extracellular matrix. This complex mix of these growth factors generated in the stroma of wounded tissue will also induce angiogenesis, by stimulating endothelial cells to proliferate and form new capillaries into the wound stroma.
A prime objective of wound healing is to restore epithelial function by inducing the epithelial cells to undergo sheet movements in which migration, proliferation and cell adhesion processes are highly coordinated. The released growth factors and proteases in the wound stroma profoundly affect the epithelial cells at the wound edge and induce phenotypical changes that resemble an epithelial-mesenchymal transition (EMT, see below). As a result, the interaction of epithelial cells with the underlying basement membrane and neighboring cells is reduced and cells migrate as a sheet over the provisional wound matrix. This provisional matrix forms under the clot and contains fibronectin, vitronectin and other matrix molecules. These morphological changes and rapid stimulation of epithelial cell migration is generally followed by sharp increase in cell proliferation to replace lost cells (Martin, 1997; Singer and Clark, 1999).
The initial events at the “start phase” of wounding, which includes the inflammatory, angiogenic, migratory and mitogenic responses, need to be inhibited at the “stop phase” of wound closure (Jacinto et al., 2001). Presently, the downregulation of these different responses remains poorly understood. In epithelial cells, “contact inhibition,” a mechanism that inhibits cell motility and proliferation upon reaching high density and/or establishment of cell-cell contacts (Abercrombie, 1979; Fagotto and Gumbiner, 1996; Middleton, 1972; Stoker and Rubin, 1967) is likely to be involved, but the molecular mechanisms underlying contact inhibition are still largely unknown.
The basic wounding response in adult mammalian epithelia other then the skin appears to be generally similar to cutaneous wound healing. Some differences, however, exist dependent on the nature of the insult, the specific tissue involved, or the developmental stage of the organism. As has become clear from in vivo model systems, wound healing is a complex process that involves many different cell types and cellular behaviors. In addition, it generates a complex mixture of secreted growth factors, proteases and matrix molecules, which in turn will act on a wide array of membrane receptors and adhesion molecules. For this reason, the signaling pathways that underlie epithelial cell behavior at the cellular level have been difficult to decipher in mammalian in vivo models. For this reason, investigators have used a variety of alternative in vivo and in vitro model systems to study the mechanisms that control epithelial repair.
2.1. Developmental models
The forward movement and fusion of epithelial sheets that occur during wound healing is not unique to wound repair, but is in fact a common phenomenon in many other morphogenetic processes, in particular during development. Examples are eyelid closure, in which fetal mouse eyelids move toward the center of the eye and tightly fuse which each other, only to open again 2 weeks after birth (Harris and McLeod, 1982). During late Drosophila embryogenesis, retraction of the germ band results in an epithelial hole, which is closed by lateral sheets of epithelia which move towards each other and fuse at the dorsal midline in a process called dorsal closure (Harden, 2002; Martin and Parkhurst, 2004). Embryonic tissue movements similar to Drosophila dorsal closure occur in the worm C. elegans, where the epidermis spreads from the dorsal surface of the C. elegans embryo, until the epithelial sheets encloses the embryo and seal at the ventral midline (Ding et al., 2004). As the molecular mechanism that drive these tissue movements appear to be largely conserved (Jacinto et al., 2001; Martin and Parkhurst, 2004), aspects of epithelial sheet migration during embryonic development can serve as a model for epithelial wound healing. Not all aspects of wound healing are recapitulated during development, as these models for instance lack inflammatory responses. Nevertheless, the study of epithelial sheet movements during development has provided many insights into the regulation of sheet migration.
2.2. Scrape wound healing
One of the simplest models systems for epithelial repair in vitro are scrape wound healing assays, in which closure of a monolayer is analyzed following the removal of a few rows of cells from a confluent monolayer of cells grown in culture dishes. Although this approach is obviously reductionistic, the advantages of these scrape wound models are the ease of experimental manipulation and the fact that such model systems only comprise epithelial cells. Thus, scrape wound healing assays allows investigators to study the intrinsic epithelial response to wounding in the absence of the complex mix of factors contributed by the stroma, and allows an analysis of the role of the individual components of this mix. In fact, most of our current knowledge on both the intracellular signaling pathways and the molecular machinery required for epithelial sheet migration during wound healing has been elucidated using scrape wound healing assays. Even though not all regulatory factors identified in scrape wound healing assays appear to be crucial in vivo (DiPersio, 2007), the mechanisms that drive wound healing in scrape wound healing assays have been found to be recapitulated to a remarkable extent in the different in vivo and developmental models (Van Aelst and Symons, 2002). This suggests that the epithelial wound healing response is driven by robust and conserved signaling pathways.
2.3. Wound healing and cancer
Based on the similarities in both histology and signaling process that promote tumor progression, tumors have been proposed to behave as “wounds that never heal” (Dvorak, 1986). This notion has been further supported by recent genomic analyses comparing carcinoma cells and cells engaging in or mimicking a wounding response (Chang et al., 2004; Iyer et al., 1999; Pedersen et al., 2003). Data from those studies not only demonstrate significant overlap between transcriptional profiles of both cell types, but also indicate that increased similarities correlate with a tendency of tumor cells to metastasize (Chang et al., 2004). Thus, the extensive analysis of cellular behaviors of cancer cells, in particular those concerning regulation of motility and epithelial dedifferentiation, is highly relevant for understanding epithelial wound healing. Clearly, the opposite is equally true, in that understanding the epithelial wound healing response may yield novel insights in cancer progression.
2.4. Epithelial plasticity during wound healing
As discussed above, epithelial wound healing is accompanied by dramatic cell shape changes of wound edge cells. In intact epithelia, epithelial cells have apical-basolateral polarization and their lateral membranes tightly interact through specialized structures such as tight junctions and E-cadherin-based adherens junctions. At the basal side, cells interact with basement membrane (a specialized form of the extracellular matrix) through adhesion receptors like integrins. Several of these epithelial characteristics will largely disappear at sites of injury. Cells will lose apical-basolateral polarization and tight cell-cell and cell-matrix interactions will be down-regulated, weakened or altered. These morphological changes recapitulate aspects of epithelial-mesenchymal transition (EMT). EMT is a process mainly found during embryonic development in which epithelial cells lose their epithelial characteristics and acquire a mesenchymal phenotype, allowing cells to migrate and invade the stroma. EMT is characterized by a down-regulation of epithelial-specific proteins, such as E-cadherin and the acquisition of mesenchymal-specific proteins like vimentin (Grunert et al., 2003; Hay and Zuk, 1995). The opposite process, mesenchymal-epithelial transition (MET), in which mesenchymal cells revert to cells with an epithelial phenotype, also exist and is instrumental for kidney development (Hay and Zuk, 1995).
The term EMT has recently also been used to describe many types of epithelial plasticity. As a consequence, the initial stages of wound healing and sealing of epithelial sheets in the final stages of wound repair is sometimes suggested to represent EMT and MET, respectively. EMT in the strict sense however, is characterized by the ability of individual cells to leave the epithelial monolayer entirely, which does not occur during wound healing. Moreover, EMT is mainly regulated by transcriptional programs through transcriptional regulators such as Snail family proteins (Thiery and Sleeman, 2006) and Twist (Yang et al., 2004), and it is currently unclear to what extent these transcription factors play a role in normal epithelial wound healing. At least in in vitro scrape wound healing assays, wound healing can occur in the absence of protein synthesis (Altan and Fenteany, 2004), which would argue against a crucial role of transcriptional regulation, at least during some stages of epithelial wound healing. On the other hand, stromal growth factors such as TGF-β, which are released during wound healing and play important roles during the process, are well known to induce EMT. Furthermore, wounded epithelial cells are more susceptible to TGF-β-induced EMT (Masszi et al., 2004), and EMT has been implicated in pathological wound healing processes such as kidney fibrosis in chronically injured kidney epithelia (Boutet et al., 2006; Zeisberg et al., 2003). Therefore, it is possible that wound healing shares the same signaling events with the initial stages of EMT, but that does normally not progress to a complete EMT.
3. Rho GTPases and Epithelial Morphogenesis During Wound Healing
3.1. Steps in wound healing
Epithelial wound healing critically depends on the ability of cells to migrate. Cell migration can be regarded as a cyclical process in which the following distinct steps are distinguished (Ridley et al., 2003):
Polarization. In response to migration-inducing factors, cells polarize and form protrusions towards the direction of migration. These protrusions can be lamellipodia, which are large and sheet-like and driven by formation of actin meshworks, or filopodia, which are spike-like and driven by actin bundles.
Traction at the leading edge. The polarized protrusions at the leading edge are stabilized by adhesion to the ECM though transmembrane adhesion receptors which link to the actin cytoskeleton. The formation of such focal contacts allows the cell to generate traction force at the leading edge that the cell uses to move forward.
Retraction at the trailing edge. Focal contacts at the trailing edge will disassemble and the tail of the cell will retract. These steps in cell migration are found in wide variety of cells and although they have been mainly characterized in single cells, epithelial sheet migration during wound healing appears to proceed in a similar fashion, with many cells acting in concert (Farooqui and Fenteany, 2005).
3.2. Rho GTPases
Local rearrangements of the cytoskeleton drive the specific cell morphological changes that accompany cell migration. The small GTPases of the Rho family are crucial regulators of the actin cytoskeleton and it is therefore not surprising that these molecules are involved in the distinct steps of cell migration. With regard to epithelial wound healing, the role of Rho GTPase is not limited to regulation of migration, as these GTPases have also been implicated in many other aspects of this process, such as the regulation of cell-cell adhesion, apical-basolateral cell polarization and cell cycle control. Many excellent reviews on the role of Rho GTPases in these processes are available (Hall, 1998; Jaffe and Hall, 2005; Jaffer and Chernoff, 2004; Kaibuchi et al., 1999; Marshall, 1999; Schmidt and Hall, 2002; Schmitz et al., 2000; Settleman, 2000; Van Aelst and Symons, 2002). Rho GTPases act as switches between extracellular signals and intracellular effector molecules. Rho GTPases can be activated by activated growth factor receptors or in response to cell-cell or cell-matrix adhesion. With regards to the latter types of activation, they participate in bidirectional signaling with both cadherins (Kaibuchi et al., 1999) and cell-matrix receptors like integrins (Keely et al., 1998), meaning that they are not only activated through these adhesion receptors, but they also regulate their adhesive functions. Rho GTPases are activated via guanine nucleotide exchange factors (GEFs), which replace the GDP bound to the GTPase with GTP. Upon activation, Rho-GTPases activate different effector molecules, thereby stimulating signaling cascades that regulate a variety of cellular processes. Currently, over 20 Rho GTPase members have been identified in mammalian cells (Jaffe and Hall, 2005). Most research however has focused on the prototypical family Rho GTPase family members Rac1, RhoA and cdc42.
Rac, Rho and cdc42 all have been implicated in regulation of wound healing and sheet migration. Although their respective roles in these processes are to some extent cell type and tissue-specific, the roles of Rac1 appear the most widely conserved. In Drosophila, loss of function or inhibition of Rac leads to defects in dorsal closure, likely by an inhibition of lamellipodia and filopodia and inhibition of actin-myosin contractility (Hakeda-Suzuki et al., 2002; Harden et al., 1995, 1999; Woolner et al., 2005). In vitro scrape wound healing in the epithelial Madin-Darby canine kidney (MDCK) monolayers is blocked when dominant-negative Rac1 is microinjected in the first three rows of cells at the wound edge, whereas dominant-negative RhoA or cdc42 essentially have no effect (Fenteany et al., 2000). Studies in bronchial epithelial cells demonstrated similar requirement for Rac1, but in these cells wound healing also depends on RhoA (Desai et al., 2004). Together, these studies indicate a crucial role for Rac in wound healing. This was recently confirmed in vivo, as inhibition or deletion of Rac1 in mouse skin was shown to inhibit incisional epidermal wound healing (Tscharntke et al., 2007). The mechanism responsible for these wound healing defects likely involves the intrinsic inhibition of keratinocyte migration and proliferation (Castilho et al., 2007; Tscharntke et al., 2007), but may also involve the depletion of follicular stem cells (Benitah et al., 2005). Other in vivo studies however demonstrated that Rac1 deletion only inhibits hair follicle development by follicular stem cell depletion, but does not affect epidermal development and maintenance (Castilho et al., 2007; Chrostek et al., 2006).
4. P21-Activated Kinases
4.1. Background of P21-activated kinases
Rho GTPases, such as Rac, mediate their biological functions by activating effector molecules, which are mostly, but not always, kinases that initiate cellular behaviors by phosphorylating downstream substrates which may initiate signaling cascades. In order to understand the role of Rac in the regulation of wound healing and sheet migration, it is therefore crucial to identify and characterize the specific Rac effector molecules.
P21-activated kinases (Paks) were the first identified binding partners of GTP-bound Rac and cdc42 (Manser et al., 1994) and are among the best characterized of the many Rho GTPase effector molecules currently known. Indeed, Paks were named after this characteristic, as the p21 in their name stands for Rac and cdc42, which, as all Rho GTPases, have a molecular weight around 21 kDa. Initial reports showed that Paks specifically interact with GTP-bound forms of Rac1 and cdc42, but not with the GDP-bound versions of these protein (Bagrodia et al., 1995; Knaus et al., 1995; Manser et al., 1994; Martin et al., 1995). More recently, several additional small GTPases of the Rac and cdc42 subfamilies (Bustelo et al., 2007) were found to activate Pak, including Rac2 (Knaus et al., 1998), Rac3 (Mira et al., 2000), Chp (Aronheim et al., 1998;Weisz Hubsman et al., 2007), TC10 (Neudauer et al., 1998) andWrch-1 (Tao et al., 2001). Paks are not activated by Rho A-G or by Ras superfamily members (Bokoch, 2003).
To date, six Pak family members have been identified. The human Pak1 (rat αPak), human Pak2 (rat γPak) and human Pak3 (rat βPak) are now classified as conventional, group I or group A Paks. In addition, there are the nonconventional, group II or group B Paks, which are named Pak4, Pak5 (sometimes described as Pak7) and Pak6 (Bokoch, 2003; Dan et al., 2001; Hofmann et al., 2004; Jaffer and Chernoff, 2002; Zhao and Manser, 2005). The structure and regulation of group II Paks differs significantly from the group I Paks and a detailed understanding of their roles is only beginning to emerge. For these reasons, this review will focus on Pak1–3.
4.2. Structure
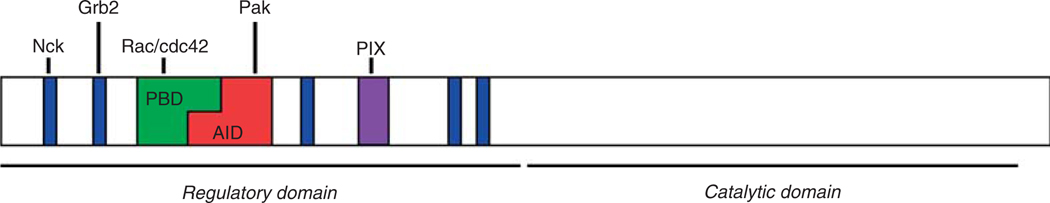
Pak1–3 share several conserved characteristic features. As shown in Figure 6.1, their general structure comprises a regulatory N-terminal domain and a catalytic C-terminal domain. The N-terminal domain contains a p21-binding domain (PBD), which interacts with the active, GTP-bound forms of Rac and cdc42. Partially overlapping with the PBD is an autoinhibitory domain (AID). A large part of the combined PBD and AID comprises the so-called “inhibitory switch domain” (Lei et al., 2000), which is crucial for the activation of Pak. The N-terminus furthermore contains several proline-rich domains with canonical PxxP SH3 binding domains (five in Pak1, two in Pak2 and four in Pak3) and a conserved nontypical proline-rich PxP SH3 domain which binds the GEFs of the PIX/Cool family (Bokoch, 2003; Jaffer and Chernoff, 2002). The serine/threonine kinase domain of Pak is at the C-terminus of the protein and is at least 93% identical in Pak1, 2 and 3 (Jaffer and Chernoff, 2002).
Figure 6.1.
Structural features of Pak kinases. Image represents the domain structure of Pak1. Pak1-3 contain an N-terminal regulatory domain and a C-terminal serine-threonine kinase domain. Several domains within the regulatory domain are indicated: The dashed areas represent the canonical proline-rich, SH3-binding domains. The adaptor proteins Nck and Grb2 bind to the first and second proline-rich domain, respectively. In black, the non-canonical, proline-rich PIX-binding domain is indicated. In dark-grey, the p21-binding domain (PBD) is indicated, which binds GTP-bound forms of Rac and cdc42. Partially overlapping with the PBD is the autoinhibitory domain (AID) in light-grey, which inhibits Pak activity in trans by binding to the catalytic domain of another Pak molecule.
4.3. Mechanism of activation
4.3.1. Activation by Rho GTPases
The mechanism of Pak activation has been analyzed at the molecular level in Pak1, for which a crystal structure of both the inactive and active kinase domain has been resolved (Lei et al., 2000; Lei et al., 2005). Based on the crystal structure, it was concluded that Pak1 exists as a dimer in a trans-autoinhibitory conformation in which the inhibitory switch domain of one Pak1 molecule inhibits the catalytic domain of the other. It is believed that Pak1 exist in this form both in solution and in unstimulated cells (Buchwald et al., 2001; Lei et al., 2000; Parrini et al., 2002). Binding of GTP-bound Rac or cdc42 induces a series of conformational changes, which results in a disruption of the dimer and ends with the kinase domain in a stable catalytically active conformation. Central to Pak1 activation is the phosphorylation of the Thr423 residue in the activation loop of Pak1. Thr423 is exposed upon Rac/cdc42 binding and its phosphorylation allows for kinase activation and stabilization of the active conformation. It furthermore allows for autophosphorylation of several other sites, which also contribute to kinase activation (Chong et al., 2001; Frost et al., 1998; Hoffman and Cerione, 2000; Lei et al., 2000, 2005; Tu and Wigler, 1999; Zenke et al., 1999; Zhao et al., 1998). Though Thr423 phosphorylation in solution can occur through autophosphorylation (Parrini et al., 2002), in cells it may be mediated by 3-phosphoinositide–dependent kinase 1 (PDK1), perhaps through a mechanism that depends on the membrane lipid sphingosine (Bokoch et al., 1998; King et al., 2000a,b).
4.3.2. Rho GTPase–independent activation
Even though Paks are considered bona fide downstream targets of active Rac/cdc42, several Rac/cdc42-independent activation mechanisms have been reported as well. Initial studies that characterized Pak activity in vitro, had shown that proteolytic cleavage of Pak, which removes its N-terminus, yields a highly active Pak in solution (Benner et al., 1995; Roig and Traugh, 2001). Interestingly, proteolytic cleavage was demonstrated to be a physiological mechanism of Pak activation during apoptosis, when Pak2 is cleaved by caspase-3 (Rudel and Bokoch, 1997; Walter et al., 1998).
Pak can be recruited to the plasma membrane by several different mechanisms, which activates the kinase by a process that is not fully understood. Membrane recruitment of Pak1, either through binding to the adaptor protein Nck (Lu et al., 1997), or experimentally induced by introduction of a C-terminal isoprenylation sequence (Daniels et al., 1998) activates Pak1 kinase activity, possibly through a sphingosine- and PDK1-dependent phosphorylation of Pak1. Phosphorylation and activation of Paks by the kinase Akt, either downstream of Ras (Sun et al., 2000; Tang et al., 2000) or downstream of the heterotrimeric G-protein β/γ subunits (Menard and Mattingly, 2004) has been reported as well. These Pak phosphorylations by PKD1 or Akt occur in the presence of dominant-negative mutants of Rac1 and cdc42, suggesting that they are independent of these GTPases. However, since the activating phosphorylations occur at sites at the catalytic domain that are masked by the inhibitory switch domains in inactive Pak1 dimers, the question remains how these residues are accessible to PKD1 or Akt. As will be discussed in detail later, Paks can also be recruited to the membrane by an interaction with the Pak-interacting exchange factor (PIX). Pak binds directly to PIX, which in turn tightly interacts with the G protein-coupled receptor kinase-interacting target (GIT1). This Pak-PIX-GIT complex accumulates at focal adhesions in migrating cells where the (indirect) interaction of GIT1 with Pak can activate Pak1. As this activation also occurs in the presence of dominant-negative Rac1 or cdc42 or in a Pak1 mutant that cannot bind active cdc42, this activation appears to be independent of Rho GTPases (Loo et al., 2004). In mitotic cells, GIT1 can target Pak1 to the centrosome, which results in Pak1 activation at this site (Zhao et al., 2005). Interestingly, when Pak1 is targeted to the centrosome by the addition of a centrosomal targeting domain, Pak1 is activated at the centrosome as well, suggesting that targeting to the centrosome is sufficient to drive Pak activation.
The molecular mechanisms underlying GTPase-independent Pak activation is still unclear. At this point, it cannot be excluded that initial disruption of the Pak1 dimers in the studies mentioned above is mediated by a Rac/cdc42 subfamily GTPase other than Rac1 or cdc42 (Lu and Mayer, 1999) and that Pak phosphorylation by PKD1/Akt or other yet unidentified kinases cooperate in Pak1 activation. A role for sphingosine in making the activation loop accessible to phosphorylations by other kinases is possible as well (Zenke et al., 1999). Alternatively, “dimer breathing” has been proposed, in which the kinase domain is temporarily released from the inhibitory switch domain, thus allowing activation in a Rho-GTPase independent manner (Loo et al., 2004).
4.4. Inactivation
As in all signaling pathways, it is important for cellular homeostasis that activating signals are counteracted by inactivating signals. Consistent with this notion are findings that the activity of Pak is tightly regulated: Pak activation in response to stimuli peaks within 15 seconds and returns to base levels after 3 minutes (Huang et al., 1998). The initial Pak-activating signals, i.e., GTP-bound Rho-GTPases are rapidly inactivated through the action of GTPase activating proteins (GAPs). The inactivation of Rho GTPases is however unlikely to deactivate Pak, since autophosphorylated Pak has a decreased affinity for the GTP-bound forms, and GTP-bound Rho GTPases are thought to be released from Pak upon its activation (Manser et al., 1994). Indeed, it was shown that separate signals activate and deactivate Pak (Huang et al., 1998). Several proteins have been implicated in the negative regulation of Pak. Two serine/threonine phosphatases of the PP2C family, POPX1 and POPX2, directly interact with PIX and form a heterotrimeric complex with PIX and Paks. POPX1 and POPX2 dephosphorylate and downregulate Pak activity, most likely by dephosphorylating the Thr423 residue (Koh et al., 2002). The phosphorylation and activation of Pak may also target it for degradation by the proteosome. Interestingly, this process is mediated by the small GTPases Chp or cdc42 (Weisz Hubsman et al., 2007). Since these GTPases also activate Pak1, these findings suggesting a dual role for Chp and cdc42 as both activators and as negative feedback regulators of Pak1. A third mechanism of negative regulation of Pak can be accomplished by blocking its activation, which can be mediated by a number of proteins. Caveolin (Kang et al., 2006), nischarin (Alahari et al., 2004), CRIPak (Talukder et al., 2006) and hPIP (Xia et al., 2001) all bind to Pak and prevent the activation of Pak by GTP-bound Rac/cdc42. Also, even though one study implicated G protein β/γ subunits upstream of Pak activation (Menard and Mattingly, 2004), G protein β/γ subunits have also been implicated in Pak inhibition (Wang et al., 1999). Currently, it is unknown how the activity and expression of any of the Pak inhibitors is regulated. Clearly, this knowledge is required to fully understand the roles of Pak in regulation of wound healing and other processes.
5. Pak Activation During Wound Healing and Epithelial Sheet Migration
5.1. Background
Since Rac is crucial for wound repair, it seems likely that Pak kinases are important regulators of this process. Indeed, several lines of evidence have implicated Pak1 in the regulation of developmental epithelial sheet movements or wound healing. During Drosophila dorsal closure, the Pak family member Dpak accumulates in the leading edge cells (Harden et al., 1996) where it is required for the integrity of the actin cytoskeleton and for epithelial sealing (Conder et al., 2004). Even though loss-of-function Dpak mutants survive, they are sterile (Hing et al., 1999) and have various defects in the follicular epithelium that covers the egg chamber, including a loss of apical-basolateral polarity (Conder et al., 2007). In C. elegans embryos, the Pak homologue CePak is highly expressed at hypodermal cell boundaries and regulates embryonic body elongation by controlling an actin-dependent process called hypodermal fusion (Chen et al., 1996). Pak1 may also have a role in epithelial morphogenesis during mammalian embryonic development since high levels of a phosphorylated form of Pak1 have been found in developing epithelial organs such as the lung, kidney, intestine and skin (Zhong et al., 2003). Furthermore, many in vitro studies in mammalian cells have demonstrated a role for Pak in cell migration during scrape wound healing or in cell migration of single fibroblasts or epithelial cells. Together, these studies support the hypothesis that Paks play important roles in the regulation of the cytoskeleton. (Bokoch, 2003).
One of the clues that Pak1 is involved the regulation of the actin cytoskeleton and cell motility came from observations that a constitutively active form of Pak1, the phosphomimetic Pak1-T423E, induces lamellipodia and increases migration in 3T3 fibroblasts (Sells et al., 1997). Later studies showed that silencing Pak1 expression with small interference RNA (siRNA) inhibits fibroblast migration (Rhee and Grinnell, 2006). Consistent with a role in regulating actin polymerization at the leading edge, Pak1 distributes from the cytosol to the cortical actin in lamellipodia in v-Src–transformed fibroblasts or in normal cells during wound healing or after PDGF stimulation (Dharmawardhane et al., 1997). In breast cancer cells, Pak1 relocalizes to the leading edge of motile cells and promotes invasiveness in response to heregulin treatment (Adam et al., 2000). Finally, numerous other studies have demonstrated that Paks also localize to focal contacts, which stabilize the protrusions at the leading edge (Brown et al., 2002; Frost et al., 1998; Manser et al., 1997; Obermeier et al., 1998; Sells et al., 1997, 2000; Stofega et al., 2004; Zegers et al., 2003a). As will be discussed in a later section, this localization likely reflects their regulatory role in the formation and turnover of focal adhesions.
5.2. Activation of Pak by wounding-associated signals
Growth factors and cytokines that are released during epithelial wound healing are able to activate Pak. Numerous studies have reported stimulation of Pak by PDGF in many different cell types, likely by a PDGF-induced activation of Rac (Dharmawardhane et al., 1997; Sells et al., 2000; Yoshii et al., 1999). At least one study reported that Pak1 activation by PDGF relies on βPIX (Lee et al., 2001). PDGF may also activate Pak1 through transactivation of the EGF receptor by the active PDGF receptor (He et al., 2001). Direct stimulation of EGF receptor family receptors with heregulin (Adam et al., 1998) or EGF (Galisteo et al., 1996) also activates Pak1. In addition, other growth factors, including hepatocyte growth factor (Royal et al., 2000) and VEGF (Stoletov et al., 2001) have been reported to activate Pak in epithelial and endothelial cells respectively. Even though several different growth factors can activate Pak, the downstream signaling response appears to some extent dependent on the specific growth factor. For instance, stimulation of HeLa and NIH-3T3 cells with either PDGF or EGF leads to activation of Pak1 and Pak2, but only PDGF stimulation links Pak kinases to extracellular-regulated kinase (ERK) activation (Beeser et al., 2005). The strong correlation of Pak signaling downstream of PDGF signaling is of particular importance for wound healing from a clinical standpoint. PDGF-BB, a recombinant form of PDGF comprising PDGF β-chain homodimers, is currently the only FDA-approved growth factor in clinical use to accelerate wound healing (Harrison-Balestra et al., 2002; Papanas and Maltezos, 2007). Understanding the roles of Pak downstream of PDGF-signaling is therefore highly relevant for the development of future options for the treatment of wounds.
Interestingly, activation of Paks upon in vitro scrape wounding does not rely on the addition of exogenous growth factors, and it currently unclear which wounding-induced signals are responsible for Pak activation. It is possible that factors released from damaged cells are involved. As an alternative, shear stress, which activates integrin signaling, or alterations or absence of cell-cell and cell-matrix contacts may be involved as well. Integrin-mediated signaling is well known to activate Rho-GTPases and β1-integrin mediated attachment to extracellular matrix is required for GTP-bound Rac to interact with Pak1 and to activate the kinase (Chaudhary et al., 2000; del Pozo et al., 2000; Howe, 2001; Price et al., 1998). Activation of Pak1 is specific for some matrix-integrin interactions. For instance, Pak1 activity is induced by shear stress in endothelial cells plated on fibronectin, but not in cells plated on Matrigel basement membrane or on collagen I (Orr et al., 2007). Furthermore, cell attachment to laminin-332 through α3β1 integrin activates Pak1, whereas α2β1 integrin-mediated attachment to collagen I by did not influence Pak1 activation (Zhou and Kramer, 2005).With respect to these two latter studies, it is relevant to note that fibronectin is an important component of the wound provisional matrix (Martin, 1997; Singer and Clark, 1999), whereas laminin-332 is an important regulator of wound healing which synthesis is induced by both shear stress (Avvisato et al., 2007) and upon epithelial injury in vitro and in vivo (Mak et al., 2006; Schneider et al., 2007). Taken together, it seems likely that integrin signaling is an important factor in wounding-induced Pak1 activation.
5.3. Kinase-independent functions and Pak-interacting proteins
Presently, over 40 different kinase substrates of activated Paks have been identified (Bokoch, 2003; Kumar et al., 2006). Pak substrates comprise a diverse group of proteins, many of which have been implicated in the regulation of the cytoskeleton. Pak effectors also include several transcriptional regulators and signaling proteins involved in regulation of cell proliferation and cell death. As will discussed below, many of the Paks’ functions in wound healing-related processes depend on its functional catalytic domain and involve phosphorylation of specific substrates. In addition, Pak has functions that do not rely on its catalytic activity. Numerous studies have demonstrated that at least some of the Paks’ morphological effects, such as stimulation of cell motility or the formation of actin-based structures like lamellipodia, invadapodia and podosomes are kinase-independent (Furmaniak-Kazmierczak et al., 2007; Manser et al., 1997; Sells et al., 1997, 1999; Webb et al., 2005; Zegers et al., 2003a). In addition, kinase-independent transcriptional regulation by Pak has been reported (Hullinger et al., 2001).
The kinase-independent effects of Pak depend on SH3-domain–containing proteins that bind to one of the several PxxP-containing motifs (in which x is any amino acid) within the Pak N-terminus (Frost et al., 1998). Two of such proteins are the SH3/SH2-domain–containing proteins adaptor proteins Nck and Grb2, which link activated receptor tyrosine kinases to intracellular signaling molecules through their SH2 and SH3 domains respectively. Several growth factor receptors that play important roles in in vivo wound healing interact with Pak1 through these adaptor proteins. Nck interacts with the first PxxP motif of Pak1 and links it to tyrosine-phosphorylated PDGF or EGF receptors or to activated integrins, thereby recruiting the kinase to the plasma membrane (Bokoch et al., 1996; Galisteo et al., 1996; Howe, 2001; Lu et al., 1997). In the keratinocyte cell line HaCaT, Grb2 recruits Pak1 to the plasma membrane by coupling it to the activated EGF receptor. When the interaction of Pak1 with Grb2 is inhibited with an inhibitory SH3-containing peptide, EGF-mediated lamellipodia extension is blocked, indicating a crucial role for Pak1 in this process (Puto et al., 2003). Using a similar approach, it was shown that the Pak-Nck interaction is important for endothelial cell migration and angiogenesis (Kiosses et al., 2002). Though membrane recruitment by adaptor proteins has generally been implicated in Pak activation (Galisteo et al., 1996; Lu et al., 1997), it is possible that the kinase-independent effects of Pak are mediated by Pak acting as a scaffold. As Nck and Grb2 bind exclusively to the first and second PxxP motif of Pak1 (Bokoch et al., 1996; Galisteo et al., 1996; Puto et al., 2003), respectively, and PIX binds to a third central proline-rich domain in Pak (Manser et al., 1998), it is possible that Pak integrates different signaling pathways during wound healing. Consistent with this notion are data from several studies that demonstrated the formation and membrane recruitment of Nck-Pak- PIX–containing protein complexes in response to either PDGF-stimulation (Yoshii et al., 1999), or as a result of cell-matrix adhesion and integrin signaling (Brown et al., 2005; Zhao et al., 2000a). Furthermore, Nck and Grb2 likely indirectly associate with PIX via GIT, as both the Nck and Grb2 SH2 domains directly bind to GIT when GIT is tyrosine-phosphorylated (Brown et al., 2005).
5.4. The PIX-GIT complex
Of all the Pak-interacting proteins, PIX and its binding partner GIT have been studied most extensively and appear to be crucial for many of the Paks’ functions. αPIX and βPIX (Pak-interacting exchange factor) and the identical p85cool-1 and p85cool-2 were first identified as Pak-binding proteins and interact with a specific proline-rich domain in Paks through a SH3 domain (Bagrodia et al., 1998; Manser et al., 1998). Based on the presence of tandem DH/PH (Dbl homology/Pleckstin homology) domains, a conserved characteristic of Rho-GTPase GEF proteins, the PIX proteins were predicted to exhibit GEF activity. However, while GEF activity of αPIX towards cdc42 and Rac could be readily demonstrated (Feng et al., 2002), it is still uncertain to what extent βPIX exhibits GEF activity towards these GTPases. In vitro GEF assays have indicated that βPIX contains an autoinhibitory domain, and GEF activity towards cdc42 could only be demonstrated upon deletion of this domain (Feng et al., 2002). Data from some studies suggest that phosphorylation of βPIX by Pak2 or protein kinase A can relieve βPIX from its autoinhibitory state and allows it to act as a GEF in vitro (Chahdi et al., 2005; Shin et al., 2002, 2004), but evidence for such a mechanism at the molecular level is still lacking. Resolving this question has been further complicated by findings that αPIX and βPIX can both homo- and heterodimerize and that specificity of GEF activity of αPIX depends on its dimerization state (Feng et al., 2002, 2004). Currently, it is unclear whether its ability to activate Rac and/or cdc42 is the main role of PIX proteins. As many Pak functions depend on its ability of Pak to interact with PIX, it is possible that one of PIX functions is to recruit Pak to specific intracellular sites. Furthermore, PIX appears to facilitate the formation of large oligomeric complexes that function in the regulation of focal adhesions (see V.A-2). Indeed, PIX tightly binds to the highly homologuos family of G protein-coupled receptor interacting target (GIT) proteins, which comprises GIT1 (or Cat1/p95-APP1), GIT2 (or Cat2) and p95PKL (or p95-APP2) (Bagrodia et al., 1999; Di Cesare et al., 2000; Paris et al., 2003; Premont et al., 1998, 2000; Turner et al., 1999). GITs are multidomain proteins. At their N-terminus, they contain an ARF-GAP domain, which exhibits activity towards several different small GTPases of the ARF family. They furthermore contain a Spa2-homology motif, which is required for its interaction with PIX proteins, a coiled-coil motif that mediates homo- and heterodimerization of GIT1 and GIT2 and a C-terminal paxillin-binding site, which binds the focal adhesion protein paxillin.
In many migrating cells, Pak is recruited to focal contacts through a complex that forms through the sequential interactions with PIX, GIT and paxillin (Bagrodia et al., 1998, 1999; Manser et al., 1998; Turner et al., 1999; Zhao et al., 2000b). This complex plays important roles in cytoskeletal dynamics and cell motility. The dynamic assembly and disassembly of the complex, which we will call here Pak-PIX-GIT complexes, is highly regulated. The functions of Pak-PIX-GIT complexes are still not completely understood, but they appear to be involved in many aspects of epithelial wound healing, as will be discussed in the following sections.
6. Regulation of Wound Healing Downstream of Pak
Although many studies have implicated Pak in the regulation of epithelial wound healing and sheet migration, knowledge about the molecular mechanisms by which Pak controls these processes is only beginning to emerge. As discussed earlier, wound healing occurs via distinct steps, and depends on many different interconnected signaling pathways. Here, an attempt is made to review the specific molecular mechanisms by which Pak regulates these distinct steps.
6.1. Cell motility and sheet migration
6.1.1. Cell polarization
One of the first processes during wound healing is the polarization of the actin cytoskeleton. In response to an extracellular migration signal, cells polarize and extend protrusions such as lamellipodia or filopodia in the direction of migration. Migratory cell polarization involves numerous interconnected signaling pathways and both positive and negative feedback loops that involve integrins, growth factor receptors, Rho GTPases and phosphoinositide-mediated signaling. Pak kinases regulate cell polarity in many different organisms. In yeast, the Pak homologs Ste20 and Cla4p are required for polarized actin assembly during bud formation and cytokinesis (Eby et al., 1998;Holly and Blumer, 1999), while Pak induces actin polarization during directed cell migration of Dictyostelium and Entemoebe amoebas. In mammalian organisms, Paks regulates polarized actin rearrangements during many different cellular processes, including cell migration and polarized actin assembly that occur at the immunological synapse and during neurogenesis (Bokoch, 2003). Together, these findings suggest a rather direct role of Pak at the level of the cytoskeleton.
6.1.1.1. Lamellipodia extension
Protrusion of lamellipodia involves the formation of a newly assembled actin meshwork. This is mediated by the Arp2/3 complex, which binds to the side or tip of an existing actin filament and nucleates and branches new filaments at the leading edge (Pollard and Borisy, 2003). Pak1 phosphorylates the p41-Arc subunit of the Arp2/3 complex, and phosphorylation of p41-Arc regulates its association with the Arp2/3 complex at actin nucleation sites at the leading edge of the cells (Vadlamudi et al., 2004). As a non-phosphorylatable mutant of p41-Arc slows cell migration in breast cancer epithelial cells, these data indicate a functional role for Pak1 in Arp2/3-regulated actin branching and lamellipodia extension (Vadlamudi et al., 2004). However, Pak1 may also inhibit the Arp2/3 complex by phosphorylating caldesmon, which increases the ability of caldesmon to compete with the Arp2/3 complex for actin binding (Morita et al., 2007). It remains to be determined whether these differences reflect cell type-dependent differences or that they may reflect different levels of regulation. Clearly, the activity of the Arp2/3 complex in actin branching needs to be spatially restricted to the leading edge of migrating cells, but it is currently unclear if the these apparent opposite roles of Pak are involved in the spatial restriction of the active Arp2/3 complex.
In addition to its proposed role in actin nucleation by acting on the Arp2/3 complex, Pak may stabilize actin filaments. The actin depolymerizing factor/cofilin destabilizes actin filaments by severing actin filaments and by actin depolymerization. LIM kinase is activated by Pak1, and, upon activation, phosphorylates and inactivates cofilin, thus promoting actin filament stability (Edwards et al., 1999). The same study showed that a kinase-inactive LIM kinase abolishesmany of Pak1-induced cytoskeletal changes and membrane ruffling. This suggests that Pak1 stabilizes lamellipodia by a mechanism that involves LIM kinase-mediated inactivation of cofilin. Caldesmon and tropomyosin are two actin-filament stabilizing proteins (Gunning et al., 2005; Hai and Gu, 2006) that have also been implicated in Pak-mediated stabilization of actin. In breast cancer epithelial cells, the kinase-dead Pak1-K299R stabilizes F-actin filaments by causing an increased association of tropomyosin and caldesmon with actin stress fibers (Adam et al., 2000). Furthermore, Pak induces caldesmon phosphorylation in Rous sarcoma-transformed fibroblasts (Morita et al., 2007) and in response to wounding in CHO cells (Eppinga et al., 2006). In the latter cells, wound healing is impaired in cells that express either the Pak-phosphomimetic or a nonphosphorylatable form of caldesmon (Eppinga et al., 2006).
6.1.1.2. Regulation of microtubules
The polarization of the cortical actin at the leading edge during cell migration is accompanied by reorganization of the microtubule cytoskeleton. Though most attention has been focused on the dynamics of the actin cytoskeleton, recent work provided evidence that directional migration depends on microtubules as well, and that both components of the cytoskeleton are in fact tightly integrated during cell migration (Siegrist and Doe, 2007; Watanabe et al., 2005). Microtubules nucleate from their minus ends, which are generally located at the microtubule organizing center (MTOC). At their plus ends, they undergo phases of growth and shrinkage, known as dynamic instability. Plus ends can be captured at specific targets, often associated with the actin cytoskeleton, which prevents shrinkage and stabilizes the microtubules. In migrating cells, microtubule plus ends are selectively stabilized at the leading edge, where they can interact with the cortical actin. In addition, the MTOC usually reorients towards to direction of migration. Though reorganization of microtubules is likely not required for the protrusion of leading edge and migration per se, it is thought be essential for the positioning of the leading edge and persistent directional movement by stabilizing cell polarization of the migrating cell. Similar to the regulation of actin dynamics, Rho GTPases play important roles in the reorganization of microtubules during cell migration. Though a detailed understanding of the cross-talk between microtubules and Rho GTPases is only beginning to emerge and is reviewed in detail elsewhere (Fukata et al., 2003; Raftopoulou and Hall, 2004; Siegrist and Doe, 2007; Small et al., 2002; Small and Kaverina, 2003; Watanabe et al., 2005), several lines of evidence support a role for Pak family kinases in the regulation of microtubules.
One of the mechanisms by which Pak regulates microtubules is by phosphorylating stathmin. Stathmin, also called oncoprotein 18 (Op18), binds α/β-tubulin dimers, thereby preventing tubulin polymerization and causing catastrophe, the rapid shrinkage of microtubule plus ends (Cassimeris, 2002). Microtubule destabilization by stathmin is inhibited by its phosphorylation on Ser16, which prevents binding of stathmin to tubulin. Several studies demonstrated that Pak1 is required for this phosphorylation (Daub et al., 2001;Wittmann et al., 2003, 2004). A later study showed that stathmin is a direct substrate of Pak1 in vitro, and that its phosphorylation on Ser16 by Pak1 results in a decreased ability of stathmin to inhibit tubulin polymerization in an in vitro assay (Wittmann et al., 2004). In vivo however, additional factors appear to be involved in stathmin phosphorylation (Wittmann et al., 2004), which would be consistent with reports that Pak1 is required but not sufficient for Rac1-mediated stimulation of microtubule growth at the leading edge of migrating cells (Wittmann et al., 2003).
Both Pak1 (Zenke et al., 2004) and Pak4 (Callow et al., 2005) phosphorylate GEF-H1. GEF-H1 is a GEF for Rho whose activity is suppressed by binding to microtubules. As GEF-H1 can bind both actin and tubulin, it may locally integrate regulation of the actin and microtubule cytoskeleton by a spatial control of Rho activation (Krendel et al., 2002). Phosphorylation of GEF-H1 by Pak4 causes its release from microtubules in NIH 3T3 cells, which co-incided with a dissolution of stress fibers (Callow et al., 2005). In contrast, phosphorylation of GEF-H1 by Pak1 on an analogous Ser residue did not affect the association of GEF-H1 with microtubules in a study using HeLa cells. Rather, this study showed that Pak1-mediated GEF-H1 phosphorylation results in binding of the scaffold protein 14-3-3 to GEF-H1, thereby recruiting 14-3-3 to microtubules, which could potentially affect GEF-H1 function (Zenke et al., 2004).
In addition to the potential roles of Pak in microtubule stabilization, Pak has also been implicated in the regulation of centrosomes and the centrosomal MTOC during mitosis. Pak1 is targeted to the MTOC of mitotic cells, which leads to its activation, as revealed by immunofluorescent staining of an antibody specific for rat Pak1 phosphorylated at Thr422 (Zhao et al., 2005). Furthermore, inducible overexpression of an active analogous human Pak1 phosphomimetic (Pak1-T423E) in epithelial breast cancer cells induces mitotic spindle abnormalities such as multiple spindles (Vadlamudi et al., 2000). The aberrant spindles may be due to phosphorylation of tubulin co-factor B by Pak1, as overexpression of tubulin co-factor B, but not expression of forms that cannot be phosphorylated by Pak1, gives rise to a similar phenotype (Vadlamudi et al., 2005). Alternatively, the phenotype may be mediated through Aurora A. Aurora A is a kinase that has been implicated in centrosome maturation and centrosomal microtubule assembly (Brittle and Ohkura, 2005), and was recently shown to be activated by Pak1 at the centrosome (Zhao et al., 2005). Finally, Pak1, but not Pak2 or Pak3 (Thiel et al., 2002), can be phosphorylated on T212 via p35/cdk5 kinase in neuronal cells (Nikolic et al., 1998), or by cyclinB1/cdc2 in mitotic fibroblasts and other cells (Banerjee et al., 2002; Thiel et al., 2002). This phosphorylation, which does not affects Pak1 activity (Thiel et al., 2002), targets Pak1 to the MTOC, where it has been implicated in microtubule destabilization during mitosis (Banerjee et al., 2002).
Pak’s effect on microtubule stability and its association with the MTOC would suggest important roles of the kinase on microtubule organization during cell migration. However, to date stathmin is the only Pak substrate directly implicated in the regulation of directional motility (Wittmann et al., 2003). It is currently unclear to what extent other Pak substrates are involved in the control of microtubule polarization and MTOC reorientation during migration and wound healing. Recent studies in fibroblasts and astrocytes have indicated that polarized microtubule stabilization is initiated by localized activation of Rac and Cdc42 at the leading edge but does not involve Pak. Instead, upon activation, Cdc42 mediates the reorientation of the MTOC through a pathway that involves the Cdc42 effector PAR6, which forms a complex with PAR3 and the atypical PKC-zeta (Cau and Hall, 2005; Etienne-Manneville and Hall, 2001). Formation of polarized actin-based protrusions in response to active Cdc42 on the other hand, was reported to be regulated independently of PAR3/PAR6/PKC-zeta, through a pathway that did depend on a Pak1-dependent recruitment of βPIX at the leading edge (Cau and Hall, 2005). The Pak-βPIX complex may then facilitate downstream activation of Rac and/or cdc42, which in turn may control spatial actin reorganization through downstream effectors, which may include Pak. It is possible that these data are cell type-dependent, considering the diverse functions of Pak in regulating microtubule stabilization in other cells. Also, as will discussed below, Pak may be involved in the polarization of microtubules through its interaction with βPIX. Furthermore, Pak can directly regulate atypical PKC-zeta in prostate carcinoma cells where PKC-zeta constitutively associates with Pak1 and is phosphorylated in a Pak1-dependent manner (Even-Faitelson and Ravid, 2006). Thus, these findings may suggest that PKC-zeta and Pak can integrate Cdc42 signaling to microtubules and filaments respectively.
Finally, it is relevant to note that all studies discussed above were done in either non-epithelial cells or in epithelial cells that lacked apico-basolateral polarity. In such cells microtubules radiate out from a perinuclear MTOC, which is often, but not always, oriented towards the leading edge (Salaycik et al., 2005). In contrast, in polarized epithelial cells (i.e., cells with apical-basolateral polarization), microtubules do not radiate from a centrosomal MTOC. Rather, they are organized in parallel arrays, in which the minus ends are associated with the apical membrane, and the plus ends extend towards the basal surface (Fukata et al., 2003; Luders and Stearns, 2007). This organization must undergo significant changes upon wounding since epithelial cells at wound edges exhibit a radial organization similar to that observed in non-polarized cells. How the transitions in microtubule organization during wound healing are regulated is unclear, and it would be important to know if the downstream effectors of Pak are involved in this process. In that respect, it is interesting to note that MARK2/Par-1 induces a change from a parallel organization to an organization in which microtubules nucleate from a single MTOC in the MDCK cell line (Cohen et al., 2004). In neuronal cells, MARK2/Par-1 destabilizes microtubules by phosphorylating tau, causing its dissociation from microtubules (Nishimura et al., 2004). Pak5 binds MARK2/Par-1 and when both molecules are overexpressed in CHO cells, Pak5 counteracts the function of MARK2/Par-1, thereby stabilizing microtubules. Though it is tempting to speculate that Pak may be involved in regulating parallel or radial microtubule organizations, the kidney-derived MDCK are unlikely to express Pak5 (Dan et al., 2002), and it remains to be established if other Pak forms are involved in this process.
6.1.1.3. The Pak-PIX-GIT complex in cell polarization
PIX-GIT – containing complexes may regulate cell polarization by recruiting other cell polarity protein complexes. Three major protein complexes that localize at apical cell junctions and control epithelial polarization were initially identified in Drosophila and C. elegans. The general function and key components of these complexes are highly conserved in different vertebrate and invertebrate organisms. The Par3/Par6/aPKC complex is recruited to cadherin-based junctions and appears to initiate formation of the apical membrane. Maintenance of “apical identity” of the apical membrane is mediated by the Crb/Stardust complex, which antagonizes the function of the Lgl/Dlg/Scrib complex. This Lgl/Dlg/Scrib complex is proposed to generate and maintain basolateral identity by counteracting Par3/Par6/aPKC function (Nelson, 2003). Although these three complexes have been mostly implicated in apical-basolateral polarization, it has become increasingly clear that they also function in other types of cell polarization. For instance, the Par3/Par6/aPKC and the Lgl/Dlg/Scrib complexes engage in bidirectional signaling with Rho GTPases and have been implicated in regulation of cell polarization during migration (Humbert et al., 2006).
Mass spectrometry analysis of proteins that co-immunoprecipitate with Scrib in mammary epithelial cells identified βPIX (and associated GIT1) as a main binding partner of Scrib (Audebert et al., 2004). Recent studies indicate that Scrib plays a crucial role in directional motility and epithelial wound healing by a mechanism that depends on βPIX. Previously it was found that loss of the Drosophila forms of Scrib and Dlg results in defects of dorsal closure (Bilder et al., 2000), while mice that carry Scrib mutations exhibit defects in embryonic fusion events such as eyelid- and neural tube closure (Murdoch et al., 2003; Zarbalis et al., 2004). These observed effects on sheet migration suggest a potential role of these proteins in wound healing. Indeed, expression of mutant mammalian Scrib was recently shown to inhibit epidermal wound healing in an in vivo mouse model (Dow et al., 2007). It appears that deregulation of migratory polarity underlies these defects. In scrape wound healing assays, βPIX and Scrib are recruited to the leading edge in mammary epithelial cells (Dow et al., 2007) and astrocytes (Osmani et al., 2006). Knockdown of Scrib expression significantly interferes with migratory polarization of these cells; it blocks recruitment of βPIX, cdc42 (Osmani et al., 2006) and Rac (Dow et al., 2007) to lamellipodia and results in a loss of polarized actin and microtubule organization and directional motility. In astrocytes, it also inhibits wounding-induced activation of cdc42 (Osmani et al., 2006). Interestingly, whereas knockdown of Scrib abolishes sheet migration in mammary epithelial cells, it does not affect general rates of cell motility when the cells are subconfluent (Dow et al., 2007), suggesting functional cross-talk with cell-cell adhesions. Subsequent experiments suggested that the phenotype of Scrib knockdown cells depends on the interaction of Scrib with βPIX. This conclusion was based on findings that knockdown of βPIX or expression of βPIX mutants that lack the Scrib binding motif or the DH domain (required for GEF function) phenocopied the Scrib knockdown phenotype (Osmani et al., 2006). Taken together, these data are consistent with a model in which Scrib and βPIX-GIT1 complexes recruit and regulate the activation of Rac and cdc42 at the leading edge of migrating cells. The active Rac and cdc42 in turn, may then induce cytoskeletal rearrangements and lamellipodia formation by activating effector proteins, including, quite likely, Pak kinases.
It must be noted that some of Scrib’s effects on cell migration may be context or cell-type dependent and/or appear to mediated by alternative mechanisms. For instance, while knockdown of Scrib results in a loss of directional migration in MDCK cells, it increases overall motility in these cells (Qin et al., 2005). In these cells, however, increased motility appeared to be caused by a destabilization of adherens junctions, which occurs independently of βPIX function. Thus, it is possible that Scrib-containing complexes with different compositions and/or distinct intracellular localizations have different and perhaps even opposite functions in directional migration. Such distinct functions have already been demonstrated for GIT1-containing complexes, which, depending on intracellular localization and molecular composition either promote or inhibit lamellipodia formation. Thus, while Pak1-PIX-GIT1–containing complexes, in association with paxillin, stimulate motility and protrusion of lamellipodia, likely by promoting activation of Rac at the leading edge of the cell, GIT1 inhibits Rac activation at the trailing edge when it is associated with α4 integrin through paxillin (Nishiya et al., 2005). As a consequence, GIT1 promotes cell polarization by mediating opposite effects at the leading and trailing edge of the cell. On a related note, even though the different GIT family members appear to interact equally well with paxillin and PIX, they may have distinct roles, as it was recently shown that GIT2, but not GIT1 represses motility in nontransformed mammary epithelial cells (Frank et al., 2006).
6.1.2. Stabilization of cell protrusions and the dynamic regulation of focal contacts
To promote cell migration, protrusions at the leading edge must be stabilized and anchored to the underlying extracellular matrix. The main proteins that mediate this process are integrins; heterodimeric matrix receptors that bind to different components of the extracellular matrix. At the inside of the cell, integrins link to the cytoskeleton. The connection of the extracellular matrix to the actin cytoskeleton allows the cells to exert traction forces, which are required to pull the cell forward. In addition, integrins are important signaling molecules that transmit intracellular signals upon binding to the extracellular matrix (“outside in signaling”), while their function is also being regulated by intracellular signals (“inside out signaling”). Key regulators of bidirectional integrin signaling are Rho GTPases (Schmitz et al., 2000;Wozniak et al., 2004; Yu et al., 2005). The formation and regulation of integrin-based adhesion sites is not completely understood. Upon adhesion, integrins are activated and cluster in focal complexes, in which many different multidomain proteins, including paxillin, interact and ultimately link to the actin cytoskeleton. Different types of integrin clusters exist: Focal complexes are relatively small, are found at the cell periphery, form by a mechanism that depends on Rac activity and exhibit high turnover rates (Ballestrem et al., 2001; Hall, 1998; Zaidel-Bar et al., 2003). Focal complexes are also thought to be the precursors of focal adhesions (Hall, 1998), which are larger, more stationary complexes that generally localize more distally and form in Rho-dependent manner. As differences between the two different integrin-based contact sites are not always obvious, I will use the term focal contact to refer to either complex.
Focal contacts need to turn over to allow the cell to move forward and highly migratory cells tend to have many smaller focal contacts that turn over rapidly. Numerous studies have shown that Pak1-PIX-GIT–containing complexes are targeted to focal contacts and have implicated the complex in the dynamic regulation of these adhesion sites. The precise targeting mechanisms and functions of these proteins at focal contacts is still not entirely clear as apparently conflicting evidence have been reported. As mentioned earlier, Paks are activated in response to integrin-mediated adhesion (Chaudhary et al., 2000; del Pozo et al., 2000; Howe, 2001; Orr et al., 2007; Price et al., 1998; Zhou and Kramer, 2005). Activated Pak1 mutants localize to focal contacts (Kiosses et al., 1999; Sells et al., 1997; Stofega et al., 2004) and endogenous Pak1 is recruited to focal contacts upon its activation by PDGF or VEGF (Dharmawardhane et al., 1997; Sells et al., 2000; Stoletov et al., 2001) or following expression of active Rac1 or cdc42 mutants (Manser et al., 1997), although this latter study did not find that active Pak mutants localized to focal contacts (Manser et al., 1997). Conversely, many studies demonstrated that inactive Pak accumulates in focal adhesions. Thus, inhibition of Pak function by expression of the Pak-autoinhibitory domain or by expression of kinase-dead Pak results in recruitment of Pak in focal adhesions (Kiosses et al., 1999; Royal et al., 2000; Zegers et al., 2003a; Zhao et al., 2000a). How Pak is initially targeted to focal contacts is also matter of some debate. Pak can be recruited to focal contacts by both Nck (Kiosses et al., 1999; Zhao et al., 2000a) and PIX (Manser et al., 1998; Zegers et al., 2003a). PIX-dependent recruitment is likely mediated by sequential interactions of paxillin, GIT family proteins and PIX, in which GIT serves as a linker between paxillin and PIX (Brown et al., 2002, 2005; Manabe Ri et al., 2002; Turner et al., 1999; Zhao et al., 2000b).
The precise function of the complex at focal contacts is still under investigation, but appears to be multifaceted. Overexpression of active Pak mutants leads to disassembly of focal contacts in some systems (Manser et al., 1997; Sells et al., 1997), but was not observed in endothelial or epithelial cells (Kiosses et al., 1999; Zegers et al., 2003a). In fact, Pak activity and recruitment to focal contacts is required for formation of these structures in VEGF-stimulated endothelial cells (Stoletov et al., 2001). Conversely, an increase of the number of large focal adhesions upon inhibition of Pak function has been widely reported in many cell types (Kiosses et al., 1999; Royal et al., 2000; Zegers et al., 2003a; Zhao et al., 2000a) (Fig. 6.2). In summary, although most studies are consistent with the hypothesis that active Pak promotes focal contact turnover, there is no straightforward correlation between the recruitment of Pak and its binding partners PIX and GIT to focal contacts, the formation of these structures, and the effect on cell motility. This may not be surprising, as motility depends on a tightly regulated balance of focal contact formation and breakdown. The functional effects of a disruption of this balance will likely depend on the spatial and molecular context of the complex. In that respect, it is relevant to note that the Pak-PIX-GIT complex is subject to different intermolecular interactions and posttranslational modifications. For instance, phosphorylation of Pak1 on Ser21 by Akt decreases the interaction of Pak1 with Nck, which leads to the release of Pak1 from focal contacts and an increase in cell motility (Zhou et al., 2003). Autophosphorylation of Pak1 also decreases its affinity for PIX and Nck binding (Manser et al., 1997; Zhao et al., 2000a), and induces disassembly of focal contacts and retraction of peripheral membrane, which suggests a potential inhibitory effect on migration. Finally, Pak1 phosphorylates paxillin on Ser273 (Nayal et al., 2006) and Ser709 (Webb et al., 2006), which increases the affinity of paxillin for GIT and promotes cell protrusion (Webb et al., 2006). Pak-mediated phosphorylation of paxillin also induces formation of small highly dynamic focal contacts that promote cell motility by a mechanism that depends on Pak-PIX and PIX-GIT interactions (Nayal et al., 2006).
Figure 6.2.
Dominant-negative Pak1 accumulates in focal contacts in scrape-wounded epithelial cells. Image represents MDCK cells, which express wild type or a dominant-negative (Pak1-K299R, kinase-dead) under control of a tetracycline-regulatable promoter (Zegers et al., 2003a).Monolayers of MDCK cells expressing these HA-tagged forms of Pak1 were scrape wounded. The next day, cells were fixed and stained using antibodies against the HA-tag and the focal contact marker vinculin. Note that wild type Pak1 localized to focal contacts to a limited extent, and localization is restricted to very peripheral focal contacts. In contrast, kinase-dead Pak1 accumulates at focal contacts and is found both peripheral and more distally within lamellipodia.
Taken together, the data appear to be consistent with the hypothesis that Pak-PIX-GIT complexes may promote formation of focal complexes by a mechanism that relies on the local, PIX-mediated activation of Rac at the leading edge. This mechanism may not require Pak activation, or perhaps relies on a partial activation, but could be mainly mediated by recruiting PIX to the leading edge. Full activation, mediated by the resulting local Rac activation, may subsequently lead to full Pak activation, which results in a release of Pak from focal contacts through its diminished affinity for PIX and Nck. Furthermore, activation of Pak will induce the degradation of focal contacts. How this latter process is regulated is still an open question. It is possible that degradation occurs through proteolytic cleavage by calpain, which is recruited to focal contacts by interacting with αPIX (Rosenberger et al., 2005). Also, as will discussed below, Pak can regulate myosin activity, which may be involved in adhesion disassembly (Crowley and Horwitz, 1995). Finally, microtubules have recently also emerged as regulators of focal adhesions (Palazzo and Gundersen, 2002). Hence, Pak-mediated focal adhesion disassembly may also be regulated indirectly by through Pak’s diverse effects on microtubules.
6.1.3. Generation of traction forces
The traction forces required to move cells forward are generated by the interaction of the non-muscle myosin II with actin filaments. Crucial to actin-myosin contractility is the phosphorylation of myosin II, which regulates both its association with actin and its motor activity. The phosphorylation of the regulatory myosin light chain (MLC) is controlled by myosin light chain kinase (MLCK). This kinase needs to be non-phosphorylated to be active, and phosphorylation of MLCK negatively inhibits the activity of the kinase. The role of Pak in the regulation of actin-myosin contractility has been somewhat controversial. One study provided evidence that Pak phosphorylates MLCK, thereby promoting dephosphorylation of MLC, thus potentially decreasing actin-myosin contractility (Sanders et al., 1999). Others however, showed that active Pak1 mutants lead to phosphorylation of MLC (Kiosses et al., 1999; Sells et al., 1999), and that MLC is a direct substrate of Pak1 (Bokoch, 2003).
In addition to MLC phosphorylation, themyosin heavy chain (MHC) can be phosphorylated as well. The function of MHC phosphorylation in actin-myosin contractility is somewhat unclear, but at least for non-muscle myosin II-B, it may promote myosin filament assembly (Even-Faitelson and Ravid, 2006; van Leeuwen et al., 1999). Bradykinin-induced Rac activation results in MHC phosphorylation in PC12 cells, which is inhibited by dominant-negative Pak1. However, as active Pak1 mutants does not increase MHC phosphorylation, MHC may not be a direct substrate of Pak1 (van Leeuwen et al., 1999). In that respect, it was recently shown that Pak can mediate MHC phosphorylation through atypical PKC-zeta in the metastatic prostate carcinoma cell line TSU-pr1. In these cells, EGF stimulation drives the formation of protein complex containing Pak1, the atypical PKC-zeta, and the MHC of myosin II-B. Pak1 induces phosphorylation of PKC-zeta, and, upon stimulation with EGF, PKC-zeta phosphorylates myosin II-B directly, leading to slower filament assembly of myosin II-B (Even-Faitelson and Ravid, 2006). Since PKC-zeta has a clear role in directional cell migration (Cau and Hall, 2005; Etienne-Manneville and Hall, 2001), it would be of considerable interest to know how this interaction is regulated.
6.2. Regulation of cell proliferation by Pak, PIX and GIT
6.2.1. Positive regulation of mitogenic signaling
Although the repair of minor wounds and other epithelial injuries relies on epithelial sheet migration and can occur independently of cell proliferation, healing of larger wounds is accompanied and critically depends on proliferation to replace lost cells (Mammen and Matthews, 2003; Martin, 1997; Singer and Clark, 1999). There is increasing evidence that Paks, in addition to their well-established roles in migration, play significant roles in the regulation of cell proliferation. As discussed previously, Paks are activated downstream of several mitogenic growth factors and interact with the EGF and PDGF receptors through adaptor proteins. Furthermore, Paks play important roles in growth factor-induced effects on cell migration.
The canonical Raf→MEK→ERK pathway is well known for regulating cell proliferation in response to adhesion or growth factors, and appears to be regulated by Pak on several different levels. Pak is required for ERK activation and transformation by Ras (Tang et al., 1997), and both Raf-1 and MEK1 (King et al., 1998; Li et al., 2001; Slack-Davis et al., 2003; Sun et al., 2000) are believed to be direct substrates of Pak. ERK is activated upon scrape wounding in many cell types, and at least in some epithelial cells, the wounding-induced activation depends on an upstream activation of Src (Matsubayashi et al., 2004). Several groups showed that activated ERK localizes to focal contacts in fibroblasts and poorly differentiated epithelial cells (Fincham et al., 2000; Slack-Davis et al., 2003; Yin et al., 2005). Recently, it has become evident that Pak-PIX-GIT–containing complexes play a crucial role in recruiting and activating ERK at these sites. For instance, cell matrix-adhesion sequentially activates FAK, Src and Pak1. Active Pak1, in turn, phosphorylates MEK1 at S298, which primes MEK for its activation and allows for subsequent MEK and ERK activation (summary: adhesion→FAK→Src→Pak1→p-S298-MEK1→p-MEK1(S218/S222, active)→p-ERK (T202,Y204, active) (Eblen et al., 2004; Slack-Davis et al., 2003). Moreover, GIT1, when phosphorylated by Src, is required for recruitment of ERK to focal adhesions and can bind both MEK1 and ERK2. Then, acting as a scaffold for MEK and ERK, GIT1 mediates sustained ERK activation at focal adhesions (Yin et al., 2004, 2005). Finally, Pak1 interacts with the MEK-ERK scaffold MP1, and this interaction is required for Pak1-mediated ERK activation (Pullikuth et al., 2005). The signaling pathway at focal contacts mentioned above and summarized here for simplicity as Src→GIT1/Pak1→MEK→ERK, is under control of several negative feedback steps. Specifically, ERK phosphorylates MEK on T292, which blocks the ability of Pak to activate MEK, and thus subsequent ERK activation. ERK also phosphorylates Pak1 on T212, which attenuates ERK signaling as well (Sundberg-Smith et al., 2005). On the other hand, ERK-dependent phosphorylation of βPIX and activation of Pak2 was also reported (Shin et al., 2002). Interestingly, available studies suggest that this type of Pak1-mediated ERK activation is particularly important in signaling downstream of cell-matrix adhesion. Although the same pathway has been reported to be activated in response to growth factors, the response seems specific for some, but not all growth factors that are known to activate ERK and may therefore be of lesser importance. Also, even though several groups have shown that Pak1 phosphorylates Raf-1 on S338, the most recent studies show that Raf is, at least in some systems, not required for Pak-mediated ERK activation (Beeser et al., 2005 and discussion therein).
Mitogenic signaling by growth factors is under tight control. One of the negative regulators of EGF signaling is ubiquitin ligase Cbl, which binds and mono-ubiquitinates the activated EGF receptor (EGFR), thereby targeting it for endocytosis and degradation in the lysosome (Dikic, 2003). Recently, a series of studies showed that βPIX inhibits Cbl-mediated EGFR down-regulation, thereby prolonging EGF signaling. EGF stimulation of cells induces a Src and Fak-dependent phosphorylation of βPIX on Tyr442, which stimulates its GEF activity toward Cdc42 (Feng et al., 2006), likely by releasing autoinhibitory constraints (Feng et al., 2002; Peterson and Chernoff, 2006). Upon phosphorylation, βPIX forms a complex with both activated Cdc42 and Cbl, thereby sequestering Cbl away from the EGFR, leading to an inhibition of EGFR endocytosis and degradation. As a result, EGFR-coupled signaling, such as the activation of ERK is sustained (Schmidt et al., 2006). Furthermore, these studies have provided evidence for an essential role of βPIX for cellular transformation and deregulated cell growth induced by either v-Src or Cdc42 (Feng et al., 2006; Wu et al., 2003). Though the effects on epithelial wound healing was not specifically addressed in these studies, others showed that either silencing of Cdc42 or overexpression of a Cbl mutant that cannot bind βPIX inhibits cell proliferation and wound closure in scrape wound healing assays in EGFR-overexpressing breast cancer cells (Hirsch et al., 2006). Based on these observations, a role for βPIX in EGF-stimulated wound healing seems likely.
6.2.2. Regulation of the cell cycle
Many of the extracellular signals that regulate cell division are interpreted during the G1 phase of the cell cycle, which precedes DNA replication (S phase). Cyclin-dependent kinases are the major kinases that drive the cell cycle and are activated by cyclins. D-type cyclins, such as cyclin D1, control cyclin-dependent kinase 4 and are major regulators of cell cycle progression through the G1 phase. The expression of cyclin D1 is controlled by both mitogens and signaling pathways downstream of integrins. Studies with constitutive and dominant-negative Rho GTPases have shown that Rho GTPases are essential for progression through G1 (Olson et al., 1995). Rho GTPases appear to regulate the cell cycle through several different mechanism, which are not yet completely understood (Coleman et al., 2004). Several studies have indicated that Paks can induce cyclin D1. Pak is required for Ras-induced cyclin D1 expression (Nheu et al., 2004) and in normal and transformed epithelial cell lines, overexpression of wild-type and constitutively active Pak1 stimulates cyclin D1 promoter activity as measured by in vitro reporter assays. Furthermore, overexpression of active Pak induces an increase of cyclin D1 protein levels and accumulation of cyclin D1 in the nucleus (Balasenthil et al., 2004), whereas inhibition of Pak by siRNA-mediated knockdown, expression of the Pak1 autoinhibitory domain, or by a pharmacological inhibitor decreased cyclin D1 expression. Interestingly, cyclin D1 expression is also inhibited by a peptide that inhibits Pak-PIX interaction (Nheu et al., 2004), which may suggest a role for PIX in this process. On the other hand, another study demonstrated an inhibitory effect of Pak1 on cyclin D1 expression and cell cycle progression. The mechanism underlying this inhibition, which can be induced by overexpression of either wild-type or kinase-dead Pak1, is still unclear but is mediated by the domain that comprises the Pak-autoinhibitory domain (Thullberg et al., 2007). Surprisingly, the mechanism does not involve an inhibition of Pak kinase activity, since the same study showed that an inactive autoinhibitory domain elicits the same effect and that active Pak cannot rescue the defect. This suggests that inhibition of proliferation by the Pak autoinhibitory domain is mediated by a yet unidentified function of this domain in the control of cell proliferation.
Pak phosphorylation on Thr212 is regulated in a cell cycle-dependent manner and markedly increases in mitotic cells (Banerjee et al., 2002; Li et al., 2002; Thiel et al., 2002). While it is unclear how this phosphorylation affects Pak activity, it is suggested to promote its association with centrosomes, as a Thr212-phosphomimic Pak peptide is targeted to centrosomes (Banerjee et al., 2002). Active endogenous Pak also localizes to centrosomes during metaphase where it phosphorylates the centrosomal Aurora A kinase (Li et al., 2002; Zhao et al., 2005). Finally, overexpression of an active Pak1 mutant interferes with normal spindle formation (Vadlamudi et al., 2000). Taken together, these data point to a regulatory role of Pak in spindle formation in mitotic cells.
6.2.3. Contact inhibition and Pak signaling at cell-cell contacts
The roles of Pak in wound healing I have discussed in this review suggest that Pak functions primarily in the “start phase” of wound healing, i.e., the promotion of cell migration and proliferation upon initial wounding. However, in order to properly close a wound, this initial phase is temporary, and is followed by a “stop phase” (Jacinto et al., 2001), in which cell migration and proliferation is inhibited and the epithelial cells regain their apico-basolateral polarization. Contact inhibition, the ability of cells to cease cell migration and proliferation upon the establishment of cell-cell contacts, is thought to be in important factor in the final stage of wound healing (Jacinto et al., 2001). However, even though it has been known for over 50 years that cells can undergo contact inhibition, the mechanism underlying this process are still poorly understood (Abercrombie, 1979; Middleton, 1972; Stoker and Rubin, 1967). Most likely, signaling pathways that induce contact inhibition in epithelial cells are initiated by the E-cadherin-based adherens junctions, which form when adjacent cells come into contact with each other (Fagotto and Gumbiner, 1996). Rho GTPases are well known regulators of cell-cell junctional integrity and involved in many signaling pathways that are activated in response to cell-cell adhesion (Jaffer and Chernoff, 2004). Thus, Rho GTPases may be important signaling intermediates in the regulation of contact inhibition. Several studies have indicated that Pak-PIX-GIT complexes are involved in adherens junction-related signaling. In epithelial and endothelial cell, Pak, PIX and GIT can be recruited to cell-cell contacts (Audebert et al., 2004; Orr et al., 2007; Stockton et al., 2007; Zegers et al., 2003a) but the precise functions of the complex at cell-cell contacts is still unclear and may be cell-type dependent. In endothelial cells, shear stress induces recruitment of endogenous active Pak to cell junctions, where it promotes vascular permeability (Orr et al., 2007; Stockton et al., 2007). Others however proposed that activation of Pak and junctional recruitment of Pak, PIX and GIT in response to oxidized phospholipids enhances the barrier function of endothelial cells (Birukova et al., 2007a,b).
Pak may negatively regulate contact inhibition by inhibiting the tumor suppressor protein Merlin. Merlin has high homology to members of the ERM (Ezrin-radixin-moesin) family of actin linker proteins, which link the cytoskeleton to the plasma membrane. When active, Merlin is in a closed conformation and acts as a growth suppressor by inducing contact inhibition through mechanisms that are not well understood (Okada et al., 2007). Pak phosphorylates Merlin on Ser518, which inactivates the protein and enables cell proliferation (Kissil et al., 2002; Xiao et al., 2002, 2005). Interestingly, Pak and Merlin engage in bidirectional signaling, as Merlin also inhibit Pak function and inhibits the recruitment and activation of Rac and Pak at the plasma membrane, which may be part of the mechanism by which Merlin regulates contact inhibition (Kissil et al., 2003; Okada et al., 2005; Shaw et al., 2001). Indeed, in endothelial cells, Pak1 activity reduces when cells reach confluency, and expression of an active membrane-targeted form of Pak1 is sufficient to release cells from contact inhibition of growth (Okada et al., 2005).
On the other hand, Pak may also promote contact inhibition, since localization of Pak-PIX-GIT complexes at cell-cell contacts is required for the establishment of contact inhibition upon wound closure in epithelial cells (Zegers et al., 2003a). Using a model system of scrape-wounded MDCK cells, it was shown that the complex localizes to focal contacts in cells at wound edges, but is dramatically retargeted to areas of cell-cell contacts upon establishment of cell-cell contacts and wound closure. Inhibition of endogenous Pak1 blocks the ability of cells to undergo contact inhibition of proliferation by causing an accumulation of Pak1 and βPIX at focal contacts, which results in an inhibition of their recruitment to lateral membranes. Interestingly, although these cells are unable to undergo contact inhibition of proliferation, they still form adherens junctions and are able to polarize (Zegers et al., 2003a). This suggests that Pak-PIX-GIT complex may act as a sensor of extracellular environment, and acts as a signaling intermediate downstream of integrin in wounded epithelial cells, but downstream of E-cadherin upon wound closure. Such a dual role would be consistent with the apparent opposite roles of Rac, which is both necessary for cell migration (see Section 3.2) and for the establishment of adherens junctions (Van Aelst and Symons, 2002).
7. Concluding Remarks
While it is obvious that Paks play important roles in the control of cellular behaviors that accompany and drive epithelial wound healing and sheet migration, many questions remain. An important outstanding issue is the spatiotemporal regulation of Pak function. This type of regulation likely entails both its spatiotemporal activation as well as the formation of distinct Pak-containing protein complexes. Clearly, many processes during wound healing need to be locally controlled, and a process of cell motility and cell polarization often depends on opposite behaviors at the leading versus the trailing edge of cells. As is obvious from the reviewed literature, a common theme of wounding-associated roles of Pak that are reviewed here are the often opposing effects that Pak appears to have in different cell types or under slightly different experimental conditions. One possible explanation of such findings is that most studies rely on approaches that modify Pak functions uniformly within the cell, and, it that light it may not be surprising that, depending on the context, opposite results can be obtained. A related question is how Pak is inactivated. While our knowledge of signals upstream of Pak is fairly extensive, our understanding of Pak inhibitors is scarce. Although several Pak inhibitory proteins, such as for instance the Pak phosphatase POPX, have been identified, insight into the regulation of these inhibitors is almost entirely lacking. Studies that specifically address Pak’s activation and deactivation in space and time, will undoubtedly lead to significant new insights in Pak biology and our understanding of epithelial wound healing.
ACKNOWLEDGMENTS
I thank Martin ter Beest for critical comments on the manuscript. The work in my laboratory is funded by the NIH (GM076363) and the Concern Foundation.
REFERENCES
- Abercrombie M. Contact inhibition and malignancy. Nature. 1979;281:259–262. doi: 10.1038/281259a0. [DOI] [PubMed] [Google Scholar]
- Adam L, Vadlamudi R, Kondapaka SB, Chernoff J, Mendelsohn J, Kumar R. Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase. J. Biol. Chem. 1998;273:28238–28246. doi: 10.1074/jbc.273.43.28238. [DOI] [PubMed] [Google Scholar]
- Adam L, Vadlamudi R, Mandal M, Chernoff J, Kumar R. Regulation of microfilament reorganization and invasiveness of breast cancer cells by kinase dead p21-activated kinase-1. J. Biol. Chem. 2000;275:12041–12050. doi: 10.1074/jbc.275.16.12041. [DOI] [PubMed] [Google Scholar]
- Alahari SK, Reddig PJ, Juliano RL. The integrin-binding protein Nischarin regulates cell migration by inhibiting Pak. EMBO J. 2004;23:2777–2788. doi: 10.1038/sj.emboj.7600291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altan ZM, Fenteany G. c-Jun N-terminal kinase regulates lamellipodial protrusion and cell sheet migration during epithelial wound closure by a gene expression-independent mechanism. Biochem. Biophys. Res. Commun. 2004;322:56–67. doi: 10.1016/j.bbrc.2004.07.079. [DOI] [PubMed] [Google Scholar]
- Aronheim A, Broder YC, Cohen A, Fritsch A, Belisle B, Abo A. Chp, a homologue of the GTPase Cdc42Hs, activates the JNK pathway and is implicated in reorganizing the actin cytoskeleton. Curr. Biol. 1998;8:1125–1128. doi: 10.1016/s0960-9822(98)70468-3. [DOI] [PubMed] [Google Scholar]
- Audebert S, Navarro C, Nourry C, Chasserot-Golaz S, Lecine P, Bellaiche Y, Dupont JL, Premont RT, Sempere C, Strub JM, Van Dorsselaer A, Vitale N, et al. Mammalian Scribble forms a tight complex with the betaPIX exchange factor. Curr. Biol. 2004;14:987–995. doi: 10.1016/j.cub.2004.05.051. [DOI] [PubMed] [Google Scholar]
- Avvisato CL, Yang X, Shah S, Hoxter B, Li W, Gaynor R, Pestell R, Tozeren A, Byers SW. Mechanical force modulates global gene expression and beta-catenin signaling in colon cancer cells. J. Cell. Sci. 2007;120:2672–2682. doi: 10.1242/jcs.03476. [DOI] [PubMed] [Google Scholar]
- Bagrodia S, Bailey D, Lenard Z, Hart M, Guan JL, Premont RT, Taylor SJ, Cerione RA. A tyrosine-phosphorylated protein that binds to an important regulatory region on the cool family of p21-activated kinase-binding proteins. J. Biol. Chem. 1999;274:22393–22400. doi: 10.1074/jbc.274.32.22393. [DOI] [PubMed] [Google Scholar]
- Bagrodia S, Taylor S, Creasy C, Chernoff J, Cerione R. Identification of a mouse p21Cdc42/Rac activated kinase. J. Biol. Chem. 1995;270:22731–22737. doi: 10.1074/jbc.270.39.22731. [DOI] [PubMed] [Google Scholar]
- Bagrodia S, Taylor SJ, Jordon KA, Van Aelst L, Cerione RA. A novel regulator of p21-activated kinases. J. Biol. Chem. 1998;273:23633–23636. doi: 10.1074/jbc.273.37.23633. [DOI] [PubMed] [Google Scholar]
- Balasenthil S, Sahin AA, Barnes CJ, Wang RA, Pestell RG, Vadlamudi RK, Kumar R. p21-activated kinase-1 signaling mediates cyclin D1 expression in mammary epithelial and cancer cells. J. Biol. Chem. 2004;279:1422–1428. doi: 10.1074/jbc.M309937200. [DOI] [PubMed] [Google Scholar]
- Ballestrem C, Hinz B, Imhof BA, Wehrle-Haller B. Marching at the front and dragging behind: Differential alphaVbeta3-integrin turnover regulates focal adhesion behavior. J. Cell Biol. 2001;155:1319–1332. doi: 10.1083/jcb.200107107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee M, Worth D, Prowse DM, Nikolic M. Pak1 phosphorylation on t212 affects microtubules in cells undergoing mitosis. Curr. Biol. 2002;12:1233–1239. doi: 10.1016/s0960-9822(02)00956-9. [DOI] [PubMed] [Google Scholar]
- Beeser A, Jaffer ZM, Hofmann C, Chernoff J. Role of group A p21-activated kinases in activation of extracellular-regulated kinase by growth factors. J. Biol. Chem. 2005;280:36609–36615. doi: 10.1074/jbc.M502306200. [DOI] [PubMed] [Google Scholar]
- Benitah SA, Frye M, Glogauer M, Watt FM. Stem cell depletion through epidermal deletion of Rac1. Science. 2005;309:933–935. doi: 10.1126/science.1113579. [DOI] [PubMed] [Google Scholar]
- Benner GE, Dennis PB, Masaracchia RA. Activation of an S6/H4 kinase (Pak 65) from human placenta by intramolecular and intermolecular autophosphorylation. J. Biol. Chem. 1995;270:21121–21128. doi: 10.1074/jbc.270.36.21121. [DOI] [PubMed] [Google Scholar]
- Bilder D, Li M, Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 2000;289:113–116. doi: 10.1126/science.289.5476.113. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Malyukova I, Mikaelyan A, Fu P, Birukov KG. Tiam1 and betaPIX mediate Rac-dependent endothelial barrier protective response to oxidized phospholipids. J. Cell. Physiol. 2007a;211:608–617. doi: 10.1002/jcp.20966. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Malyukova I, Poroyko V, Birukov KG. Paxillin-beta-catenin interactions are involved in Rac/Cdc42-mediated endothelial barrier-protective response to oxidized phospholipids. Am. J. Physiol. Lung Cell Mol. Physiol. 2007b;293:L199–L211. doi: 10.1152/ajplung.00020.2007. [DOI] [PubMed] [Google Scholar]
- Bokoch G, Reilly A, Daniels R, King C, Olivera A, Spiegel S, Knaus U. A GTPase-independent mechanism of p21-activated kinase activation. Regulation By sphingosine and other biologically active lipids. J. Biol. Chem. 1998;273:8137–8144. doi: 10.1074/jbc.273.14.8137. [DOI] [PubMed] [Google Scholar]
- Bokoch GM. Biology of the p21-activated kinases. Annu. Rev. Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- Bokoch GM, Wang Y, Bohl BP, Sells MA, Quilliam LA, Knaus UG. Interaction of the Nck adapter protein with p21-activated kinase (PAK1) J. Biol. Chem. 1996;271:25746–25749. doi: 10.1074/jbc.271.42.25746. [DOI] [PubMed] [Google Scholar]
- Boutet A, De Frutos CA, Maxwell PH, Mayol MJ, Romero J, Nieto MA. Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. EMBO J. 2006;25:5603–5613. doi: 10.1038/sj.emboj.7601421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittle AL, Ohkura H. Centrosome maturation: Aurora lights the way to the poles. Curr. Biol. 2005;15:R880–R882. doi: 10.1016/j.cub.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Brown MC, Cary LA, Jamieson JS, Cooper JA, Turner CE. Src and FAK kinases cooperate to phosphorylate paxillin kinase linker, stimulate its focal adhesion localization, and regulate cell spreading and protrusiveness. Mol. Biol. Cell. 2005;16:4316–4328. doi: 10.1091/mbc.E05-02-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, West KA, Turner CE. Paxillin-dependent paxillin kinase linker and p21-activated kinase localization to focal adhesions involves a multistep activation pathway. Mol. Biol. Cell. 2002;13:1550–1565. doi: 10.1091/mbc.02-02-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwald G, Hostinova E, Rudolph MG, Kraemer A, Sickmann A, Meyer HE, Scheffzek K, Wittinghofer A. Conformational switch and role of phosphorylation in Pak activation. Mol. Cell Biol. 2001;21:5179–5189. doi: 10.1128/MCB.21.15.5179-5189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays. 2007;29:356–370. doi: 10.1002/bies.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callow MG, Zozulya S, Gishizky ML, Jallal B, Smeal T. PAK4 mediates morphological changes through the regulation of GEF-H1. J. Cell. Sci. 2005;118:1861–1872. doi: 10.1242/jcs.02313. [DOI] [PubMed] [Google Scholar]
- Cassimeris L. The oncoprotein 18/stathmin family of microtubule destabilizers. Curr. Opin. Cell. Biol. 2002;14:18–24. doi: 10.1016/s0955-0674(01)00289-7. [DOI] [PubMed] [Google Scholar]
- Castilho RM, Squarize CH, Patel V, Millar SE, Zheng Y, Molinolo A, Gutkind JS. Requirement of Rac1 distinguishes follicular from interfollicular epithelial stem cells. Oncogene. 2007;26:5078–5085. doi: 10.1038/sj.onc.1210322. [DOI] [PubMed] [Google Scholar]
- Cau J, Hall A. Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways. J. Cell. Sci. 2005;118:2579–2587. doi: 10.1242/jcs.02385. [DOI] [PubMed] [Google Scholar]
- Chahdi A, Miller B, Sorokin A. Endothelin 1 induces beta 1Pix translocation and Cdc42 activation via protein kinase A-dependent pathway. J. Biol. Chem. 2005;280:578–584. doi: 10.1074/jbc.M411130200. [DOI] [PubMed] [Google Scholar]
- Chang HY, Sneddon JB, Alizadeh AA, Sood R, West RB, Montgomery K, Chi JT, van de Rijn M, Botstein D, Brown PO. Gene expression signature of fibroblast serum response predicts human cancer progression: Similarities between tumors and wounds. PLoS Biol. 2004;2:E7. doi: 10.1371/journal.pbio.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary A, King WG, Mattaliano MD, Frost JA, Diaz B, Morrison DK, Cobb MH, Marshall MS, Brugge JS. Phosphatidylinositol 3-kinase regulates Raf1 through Pak phosphorylation of serine 338. Curr. Biol. 2000;10:551–554. doi: 10.1016/s0960-9822(00)00475-9. [DOI] [PubMed] [Google Scholar]
- Chen W, Chen S, Yap SF, Lim L. The Caenorhabditis elegans p21-activated kinase (CePAK) colocalizes with CeRac1 and CDC42Ce at hypodermal cell boundaries during embryo elongation. J. Biol. Chem. 1996;271:26362–26368. doi: 10.1074/jbc.271.42.26362. [DOI] [PubMed] [Google Scholar]
- Chong C, Tan L, Lim L, Manser E. The mechanism of Pak activation. Autophosphorylation events in both regulatory and kinase domains control activity. J. Biol. Chem. 2001;276:17347–17353. doi: 10.1074/jbc.M009316200. [DOI] [PubMed] [Google Scholar]
- Chrostek A, Wu X, Quondamatteo F, Hu R, Sanecka A, Niemann C, Langbein L, Haase I, Brakebusch C. Rac1 is crucial for hair follicle integrity but is not essential for maintenance of the epidermis. Mol. Cell Biol. 2006;26:6957–6970. doi: 10.1128/MCB.00075-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D, Brennwald PJ, Rodriguez-Boulan E, Musch A. Mammalian PAR-1 determines epithelial lumen polarity by organizing the microtubule cytoskeleton. J. Cell Biol. 2004;164:717–727. doi: 10.1083/jcb.200308104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman ML, Marshall CJ, Olson MF. RAS and RHO GTPases in G1-phase cell-cycle regulation. Nat. Rev. Mol. Cell Biol. 2004;5:355–366. doi: 10.1038/nrm1365. [DOI] [PubMed] [Google Scholar]
- Conder R, Yu H, Ricos M, Hing H, Chia W, Lim L, Harden N. dPak is required for integrity of the leading edge cytoskeleton during Drosophila dorsal closure but does not signal through the JNK cascade. Dev. Biol. 2004;276:378–390. doi: 10.1016/j.ydbio.2004.08.044. [DOI] [PubMed] [Google Scholar]
- Conder R, Yu H, Zahedi B, Harden N. The serine/threonine kinase dPak is required for polarized assembly of F-actin bundles and apical-basal polarity in the Drosophila follicular epithelium. Dev. Biol. 2007;305:470–482. doi: 10.1016/j.ydbio.2007.02.034. [DOI] [PubMed] [Google Scholar]
- Crowley E, Horwitz AF. Tyrosine phosphorylation and cytoskeletal tension regulate the release of fibroblast adhesions. J. Cell Biol. 1995;131:525–537. doi: 10.1083/jcb.131.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan C, Nath N, Liberto M, Minden A. PAK5, a new brain-specific kinase, promotes neurite outgrowth in N1E-115 cells. Mol. Cell Biol. 2002;22:567–577. doi: 10.1128/MCB.22.2.567-577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan I, Watanabe NM, Kusumi A. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 2001;11:220–230. doi: 10.1016/s0962-8924(01)01980-8. [DOI] [PubMed] [Google Scholar]
- Daniels R, Hall P, Bokoch G. Membrane targeting of p21-activated kinase 1 (PAK1) induces neurite outgrowth from PC12 cells. EMBO J. 1998;17:754–764. doi: 10.1093/emboj/17.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daub H, Gevaert K, Vandekerckhove J, Sobel A, Hall A. Rac/Cdc42 and p65PAK regulate the microtubule-destabilizing protein stathmin through phosphorylation at serine 16. J. Biol. Chem. 2001;276:1677–1680. doi: 10.1074/jbc.C000635200. [DOI] [PubMed] [Google Scholar]
- del Pozo MA, Price LS, Alderson NB, Ren XD, Schwartz MA. Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector Pak. EMBO J. 2000;19:2008–2014. doi: 10.1093/emboj/19.9.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai LP, Aryal AM, Ceacareanu B, Hassid A, Waters CM. RhoA and Rac1 are both required for efficient wound closure of airway epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;287:L1134–L1144. doi: 10.1152/ajplung.00022.2004. [DOI] [PubMed] [Google Scholar]
- Dharmawardhane S, Sanders L, Martin S, Daniels R, Bokoch G. Localization of p21-activated kinase 1 (PAK1) to pinocytic vesicles and cortical actin structures in stimulated cells. J. Cell Biol. 1997;138:1265–1278. doi: 10.1083/jcb.138.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cesare A, Paris S, Albertinazzi C, Dariozzi S, Andersen J, Mann M, Longhi R, de Curtis I. p95-APP1 links membrane transport to Rac-mediated reorganization of actin. Nat. Cell Biol. 2000;2:521–530. doi: 10.1038/35019561. [DOI] [PubMed] [Google Scholar]
- Dikic I. Mechanisms controlling EGF receptor endocytosis and degradation. Biochem. Soc. Trans. 2003;31:1178–1181. doi: 10.1042/bst0311178. [DOI] [PubMed] [Google Scholar]
- Ding M, Woo WM, Chisholm AD. The cytoskeleton and epidermal morphogenesis in C. elegans. Exp. Cell Res. 2004;301:84–90. doi: 10.1016/j.yexcr.2004.08.017. [DOI] [PubMed] [Google Scholar]
- DiPersio CM. Double duty for Rac1 in epidermal wound healing. Sci. STKE. 2007;2007:pe33. doi: 10.1126/stke.3912007pe33. [DOI] [PubMed] [Google Scholar]
- Dow LE, Kauffman JS, Caddy J, Peterson AS, Jane SM, Russell SM, Humbert PO. The tumour-suppressor Scribble dictates cell polarity during directed epithelial migration: Regulation of Rho GTPase recruitment to the leading edge. Oncogene. 2007;26:2272–2282. doi: 10.1038/sj.onc.1210016. [DOI] [PubMed] [Google Scholar]
- Dvorak HF. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Eblen ST, Slack-Davis JK, Tarcsafalvi A, Parsons JT, Weber MJ, Catling AD. Mitogen-activated protein kinase feedback phosphorylation regulates MEK1 complex formation and activation during cellular adhesion. Mol. Cell Biol. 2004;24:2308–2317. doi: 10.1128/MCB.24.6.2308-2317.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eby JJ, Holly SP, van Drogen F, Grishin AV, Peter M, Drubin DG, Blumer KJ. Actin cytoskeleton organization regulated by the Pak family of protein kinases. Curr. Biol. 1998;8:967–970. doi: 10.1016/s0960-9822(98)00398-4. [DOI] [PubMed] [Google Scholar]
- Edwards DC, Sanders LC, Bokoch GM, Gill GN. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics [see comments] Nat. Cell Biol. 1999;1:253–259. doi: 10.1038/12963. [DOI] [PubMed] [Google Scholar]
- Eppinga RD, Li Y, Lin JL, Mak AS, Lin JJ. Requirement of reversible caldesmon phosphorylation at P21-activated kinase-responsive sites for lamellipodia extensions during cell migration. Cell Motil. Cytoskeleton. 2006;63:543–562. doi: 10.1002/cm.20144. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- Even-Faitelson L, Ravid S. PAK1 and aPKCzeta regulate myosin II-B phosphorylation: A novel signaling pathway regulating filament assembly. Mol. Biol. Cell. 2006;17:2869–2881. doi: 10.1091/mbc.E05-11-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagotto F, Gumbiner BM. Cell contact-dependent signaling. Dev. Biol. 1996;180:445–454. doi: 10.1006/dbio.1996.0318. [DOI] [PubMed] [Google Scholar]
- Farooqui R, Fenteany G. Multiple rows of cells behind an epithelial wound edge extend cryptic lamellipodia to collectively drive cell-sheet movement. J. Cell. Sci. 2005;118:51–63. doi: 10.1242/jcs.01577. [DOI] [PubMed] [Google Scholar]
- Feng Q, Albeck JG, Cerione RA, Yang W. Regulation of the Cool/Pix Proteins. Key binding partners of the Cdc42/Rac targets the p21-activated kinases. J. Biol. Chem. 2002;277:5644–5650. doi: 10.1074/jbc.M107704200. [DOI] [PubMed] [Google Scholar]
- Feng Q, Baird D, Cerione RA. Novel regulatory mechanisms for the Dbl family guanine nucleotide exchange factor Cool-2/alpha-Pix. EMBO J. 2004;23:3492–3504. doi: 10.1038/sj.emboj.7600331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q, Baird D, Peng X, Wang J, Ly T, Guan JL, Cerione RA. Cool-1 functions as an essential regulatory node for EGF receptor- and Src-mediated cell growth. Nat. Cell Biol. 2006;8:945–956. doi: 10.1038/ncb1453. [DOI] [PubMed] [Google Scholar]
- Fenteany G, Janmey PA, Stossel TP. Signaling pathways and cell mechanics involved in wound closure by epithelial cell sheets. Curr. Biol. 2000;10:831–838. doi: 10.1016/s0960-9822(00)00579-0. [DOI] [PubMed] [Google Scholar]
- Fincham VJ, James M, Frame MC, Winder SJ. Active ERK/MAP kinase is targeted to newly forming cell-matrix adhesions by integrin engagement and v-Src. EMBO J. 2000;19:2911–2923. doi: 10.1093/emboj/19.12.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank SR, Adelstein MR, Hansen SH. GIT2 represses Crk- and Rac1-regulated cell spreading and Cdc42-mediated focal adhesion turnover. EMBO J. 2006;25:1848–1859. doi: 10.1038/sj.emboj.7601092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost JA, Khokhlatchev A, Stippec S, White MA, Cobb MH. Differential effects of PAK1-activating mutations reveal activity-dependent and -independent effects on cytoskeletal regulation. J. Biol. Chem. 1998;273:28191–28198. doi: 10.1074/jbc.273.43.28191. [DOI] [PubMed] [Google Scholar]
- Fukata M, Nakagawa M, Kaibuchi K. Roles of Rho-family GTPases in cell polarisation and directional migration. Curr. Opin. Cell Biol. 2003;15:590–597. doi: 10.1016/s0955-0674(03)00097-8. [DOI] [PubMed] [Google Scholar]
- Furmaniak-Kazmierczak E, Crawley SW, Carter RL, Maurice DH, Cote GP. Formation of extracellular matrix-digesting invadopodia by primary aortic smooth muscle cells. Circ. Res. 2007;100:1328–1336. doi: 10.1161/CIRCRESAHA.106.147744. [DOI] [PubMed] [Google Scholar]
- Galisteo M, Chernoff J, Su Y, Skolnik E, Schlessinger J. The adaptor protein Nck links receptor tyrosine kinases with the serine-threonine kinase Pak1. J. Biol. Chem. 1996;271:20997–21000. doi: 10.1074/jbc.271.35.20997. [DOI] [PubMed] [Google Scholar]
- Grunert S, Jechlinger M, Beug H. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat. Rev. Mol. Cell Biol. 2003;4:657–665. doi: 10.1038/nrm1175. [DOI] [PubMed] [Google Scholar]
- Gunning PW, Schevzov G, Kee AJ, Hardeman EC. Tropomyosin isoforms: Divining rods for actin cytoskeleton function. Trends Cell Biol. 2005;15:333–341. doi: 10.1016/j.tcb.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Hai CM, Gu Z. Caldesmon phosphorylation in actin cytoskeletal remodeling. Eur J. Cell Biol. 2006;85:30530–30539. doi: 10.1016/j.ejcb.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Hakeda-Suzuki S, Ng J, Tzu J, Dietzl G, Sun Y, Harms M, Nardine T, Luo L, Dickson BJ. Rac function and regulation during Drosophila development. Nature. 2002;416:438–442. doi: 10.1038/416438a. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Harden N. Signaling pathways directing the movement and fusion of epithelial sheets: lessons from dorsal closure in Drosophila. Differentiation. 2002;70:181–203. doi: 10.1046/j.1432-0436.2002.700408.x. [DOI] [PubMed] [Google Scholar]
- Harden N, Lee J, Loh H, Ong Y, Tan I, Leung T, Manser E, Lim L. A Drosophila homolog of the Rac- and Cdc42-activated serine/threonine kinase Pak is a potential focal adhesion and focal complex protein that colocalizes with dynamic actin structures. Mol. Cell Biol. 1996;16:1896–1908. doi: 10.1128/mcb.16.5.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden N, Loh HY, Chia W, Lim L. A dominant inhibitory version of the small GTP-binding protein Rac disrupts cytoskeletal structures and inhibits developmental cell shape changes in Drosophila. Development. 1995;121:903–914. doi: 10.1242/dev.121.3.903. [DOI] [PubMed] [Google Scholar]
- Harden N, Ricos M, Ong YM, Chia W, Lim L. Participation of small GTPases in dorsal closure of the Drosophila embryo: Distinct roles for Rho subfamily proteins in epithelial morphogenesis. J. Cell. Sci. 1999;112(Pt 3):273–284. doi: 10.1242/jcs.112.3.273. [DOI] [PubMed] [Google Scholar]
- Harris MJ, McLeod MJ. Eyelid growth and fusion in fetal mice. A scanning electron microscope study. Anat. Embryol. (Berl.) 1982;164:207–220. doi: 10.1007/BF00318505. [DOI] [PubMed] [Google Scholar]
- Harrison-Balestra C, Eaglstein WH, Falabela AF, Kirsner RS. Recombinant human platelet-derived growth factor for refractory nondiabetic ulcers: A - retrospective series. Dermatol. Surg. 2002;28:755–759. doi: 10.1046/j.1524-4725.2002.02004.x. discussion 759–760. [DOI] [PubMed] [Google Scholar]
- Hay ED, Zuk A. Transformations between epithelium and mesenchyme: normal, pathological, and experimentally induced. Am. J. Kidney Dis. 1995;26:678–690. doi: 10.1016/0272-6386(95)90610-x. [DOI] [PubMed] [Google Scholar]
- He H, Levitzki A, Zhu HJ, Walker F, Burgess A, Maruta H. Platelet-derived growth factor requires epidermal growth factor receptor to activate p21-activated kinase family kinases. J. Biol. Chem. 2001;276:26741–26744. doi: 10.1074/jbc.C100229200. [DOI] [PubMed] [Google Scholar]
- Hing H, Xiao J, Harden N, Lim L, Zipursky SL. Pak functions downstream of Dock to regulate photoreceptor axon guidance in Drosophila. Cell. 1999;97:853–863. doi: 10.1016/s0092-8674(00)80798-9. [DOI] [PubMed] [Google Scholar]
- Hirsch DS, Shen Y, Wu WJ. Growth and motility inhibition of breast cancer cells by epidermal growth factor receptor degradation is correlated with inactivation of Cdc42. Cancer Res. 2006;66:3523–3530. doi: 10.1158/0008-5472.CAN-05-1547. [DOI] [PubMed] [Google Scholar]
- Hoffman GR, Cerione RA. Flipping the switch: The structural basis for signaling through the CRIB motif. Cell. 2000;102:403–406. doi: 10.1016/s0092-8674(00)00045-3. [DOI] [PubMed] [Google Scholar]
- Hofmann C, Shepelev M, Chernoff J. The genetics of Pak. J. Cell. Sci. 2004;117:4343–4354. doi: 10.1242/jcs.01392. [DOI] [PubMed] [Google Scholar]
- Holly SP, Blumer KJ. PAK-family kinases regulate cell and actin polarization throughout the cell cycle of Saccharomyces cerevisiae. J. Cell Biol. 1999;147:845–856. doi: 10.1083/jcb.147.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe AK. Cell adhesion regulates the interaction between Nck and p21-activated kinase. J. Biol. Chem. 2001;276:14541–14544. doi: 10.1074/jbc.C000797200. [DOI] [PubMed] [Google Scholar]
- Huang R, Lian JP, Robinson D, Badwey JA. Neutrophils stimulated with a variety of chemoattractants exhibit rapid activation of p21-activated kinases (Paks): Separate signals are required for activation and inactivation of paks. Mol. Cell Biol. 1998;18:7130–7138. doi: 10.1128/mcb.18.12.7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hullinger TG, Panek RL, Xu X, Karathanasis SK. p21-activated kinase-1 (PAK1) inhibition of the human scavenger receptor class B, type I promoter in macrophages is independent of PAK1 kinase activity, but requires the GTPase-binding domain. J. Biol. Chem. 2001;276:46807–46814. doi: 10.1074/jbc.M103176200. [DOI] [PubMed] [Google Scholar]
- Humbert PO, Dow LE, Russell SM. The Scribble and Par complexes in polarity and migration: Friends or foes? Trends Cell Biol. 2006;16:622–630. doi: 10.1016/j.tcb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Iyer VR, Eisen MB, Ross DT, Schuler G, Moore T, Lee JC, Trent JM, Staudt LM, Hudson J, Jr, Boguski MS, Lashkari D, Shalon D, et al. The transcriptional program in the response of human fibroblasts to serum. Science. 1999;283:83–87. doi: 10.1126/science.283.5398.83. [DOI] [PubMed] [Google Scholar]
- Jacinto A, Martinez-Arias A, Martin P. Mechanisms of epithelial fusion and repair. Nat. Cell Biol. 2001;3:E117–E123. doi: 10.1038/35074643. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Rho GTPases: Biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Jaffer ZM, Chernoff J. p21-activated kinases: Three more join the Pak. Int. J. Biochem. Cell Biol. 2002;34:713–717. doi: 10.1016/s1357-2725(01)00158-3. [DOI] [PubMed] [Google Scholar]
- Jaffer ZM, Chernoff J. The cross-Rho’ds of cell-cell adhesion. J. Biol. Chem. 2004;279:35123–35126. doi: 10.1074/jbc.R400010200. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K, Kuroda S, Fukata M, Nakagawa M. Regulation of cadherin-mediated cell-cell adhesion by the Rho family GTPases. Curr. Opin. Cell Biol. 1999;11:591–596. doi: 10.1016/s0955-0674(99)00014-9. [DOI] [PubMed] [Google Scholar]
- Kang MJ, Seo JS, Park WY. Caveolin-1 inhibits neurite growth by blocking Rac1/Cdc42 and p21-activated kinase 1 interactions. Neuroreport. 2006;17:823–827. doi: 10.1097/01.wnr.0000220139.83671.60. [DOI] [PubMed] [Google Scholar]
- Keely P, Parise L, Juliano R. Integrins and GTPases in tumour cell growth, motility and invasion. Trends Cell Biol. 1998;8:101–106. doi: 10.1016/s0962-8924(97)01219-1. [DOI] [PubMed] [Google Scholar]
- King AJ, Sun H, Diaz B, Barnard D, Miao W, Bagrodia S, Marshall MS. The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature. 1998;396:180–183. doi: 10.1038/24184. [DOI] [PubMed] [Google Scholar]
- King CC, Gardiner EM, Zenke FT, Bohl BP, Newton AC, Hemmings BA, Bokoch GM. p21-activated kinase (PAK1) is phosphorylated and activated by 3-phosphoinositide-dependent kinase-1 (PDK1) J. Biol. Chem. 2000a;275:41201–41209. doi: 10.1074/jbc.M006553200. [DOI] [PubMed] [Google Scholar]
- King CC, Zenke FT, Dawson PE, Dutil EM, Newton AC, Hemmings BA, Bokoch GM. Sphingosine is a novel activator of 3-phosphoinositide-dependent kinase 1. J. Biol. Chem. 2000b;275:18108–18113. doi: 10.1074/jbc.M909663199. [DOI] [PubMed] [Google Scholar]
- Kiosses WB, Daniels RH, Otey C, Bokoch GM, Schwartz MA. A role for p21-activated kinase in endothelial cell migration. J. Cell Biol. 1999;147:831–844. doi: 10.1083/jcb.147.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiosses WB, Hood J, Yang S, Gerritsen ME, Cheresh DA, Alderson N, Schwartz MA. A dominant-negative p65 Pak peptide inhibits angiogenesis. Circ. Res. 2002;90:697–702. doi: 10.1161/01.res.0000014227.76102.5d. [DOI] [PubMed] [Google Scholar]
- Kissil JL, Johnson KC, Eckman MS, Jacks T. Merlin phosphorylation by p21-activated kinase 2 and effects of phosphorylation on merlin localization. J. Biol. Chem. 2002;277:10394–10399. doi: 10.1074/jbc.M200083200. [DOI] [PubMed] [Google Scholar]
- Kissil JL, Wilker EW, Johnson KC, Eckman MS, Yaffe MB, Jacks T. Merlin, the product of the Nf2 tumor suppressor gene, is an inhibitor of the p21-activated kinase, Pak1. Mol. Cell. 2003;12:841–849. doi: 10.1016/s1097-2765(03)00382-4. [DOI] [PubMed] [Google Scholar]
- Knaus U, Morris S, Dong H, Chernoff J, Bokoch G. Regulation of human leukocyte p21-activated kinases through G protein-coupled receptors. Science. 1995;269:221–223. doi: 10.1126/science.7618083. [DOI] [PubMed] [Google Scholar]
- Knaus UG, Wang Y, Reilly AM, Warnock D, Jackson JH. Structural requirements for Pak activation by Rac GTPases. J. Biol. Chem. 1998;273:21512–21518. doi: 10.1074/jbc.273.34.21512. [DOI] [PubMed] [Google Scholar]
- Koh CG, Tan EJ, Manser E, Lim L. The p21-activated kinase Pak is negatively regulated by POPX1 and POPX2, a pair of serine/threonine phosphatases of the PP2C family. Curr. Biol. 2002;12:317–321. doi: 10.1016/s0960-9822(02)00652-8. [DOI] [PubMed] [Google Scholar]
- Krendel M, Zenke FT, Bokoch GM. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat. Cell. Biol. 2002;4:294–301. doi: 10.1038/ncb773. [DOI] [PubMed] [Google Scholar]
- Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat. Rev. Cancer. 2006;6:459–471. doi: 10.1038/nrc1892. [DOI] [PubMed] [Google Scholar]
- Lee SH, Eom M, Lee SJ, Kim S, Park HJ, Park D. BetaPix-enhanced p38 activation by Cdc42/Rac/Pak/MKK3/6-mediated pathway. Implication in the regulation of membrane ruffling. J. Biol. Chem. 2001;276:25066–25072. doi: 10.1074/jbc.M010892200. [DOI] [PubMed] [Google Scholar]
- Lei M, Lu W, Meng W, Parrini MC, Eck MJ, Mayer BJ, Harrison SC. Structure of PAK1 in an autoinhibited conformation reveals a multistage activation switch. Cell. 2000;102:387–397. doi: 10.1016/s0092-8674(00)00043-x. [DOI] [PubMed] [Google Scholar]
- Lei M, Robinson MA, Harrison SC. The active conformation of the PAK1 kinase domain. Structure. 2005;13:769–778. doi: 10.1016/j.str.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Li F, Adam L, Vadlamudi RK, Zhou H, Sen S, Chernoff J, Mandal M, Kumar R. p21-activated kinase 1 interacts with and phosphorylates histone H3 in breast cancer cells. EMBO Rep. 2002;3:767–773. doi: 10.1093/embo-reports/kvf157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Chong H, Guan KL. Function of the Rho family GTPases in Ras-stimulated Raf activation. J. Biol. Chem. 2001;276:34728–34737. doi: 10.1074/jbc.M103496200. [DOI] [PubMed] [Google Scholar]
- Loo TH, Ng YW, Lim L, Manser E. GIT1 activates p21-activated kinase through a mechanism independent of p21 binding. Mol. Cell. Biol. 2004;24:3849–3859. doi: 10.1128/MCB.24.9.3849-3859.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Katz S, Gupta R, Mayer B. Activation of Pak by membrane localization mediated by an SH3 domain from the adaptor protein Nck. Curr. Biol. 1997;7:85–94. doi: 10.1016/s0960-9822(06)00052-2. [DOI] [PubMed] [Google Scholar]
- Lu W, Mayer BJ. Mechanism of activation of Pak1 kinase by membrane localization. Oncogene. 1999;18:797–806. doi: 10.1038/sj.onc.1202361. [DOI] [PubMed] [Google Scholar]
- Luders J, Stearns T. Microtubule-organizing centres: a re-evaluation. Nat. Rev. Mol. Cell. Biol. 2007;8:161–167. doi: 10.1038/nrm2100. [DOI] [PubMed] [Google Scholar]
- Mak GZ, Kavanaugh GM, Buschmann MM, Stickley SM, Koch M, Goss KH, Waechter H, Zuk A, Matlin KS. Regulated synthesis and functions of laminin 5 in polarized madin-darby canine kidney epithelial cells. Mol. Biol. Cell. 2006;17:3664–3677. doi: 10.1091/mbc.E05-11-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammen JM, Matthews JB. Mucosal repair in the gastrointestinal tract. Crit Care Med. 2003;31:S532–S537. doi: 10.1097/01.CCM.0000081429.89277.AF. [DOI] [PubMed] [Google Scholar]
- Manabe Ri R, Kovalenko M, Webb DJ, Horwitz AR. GIT1 functions in a motile, multi-molecular signaling complex that regulates protrusive activity and cell migration. J. Cell. Sci. 2002;115:1497–1510. doi: 10.1242/jcs.115.7.1497. [DOI] [PubMed] [Google Scholar]
- Manser E, Huang H, Loo T, Chen X, Dong J, Leung T, Lim L. Expression of constitutively active alpha-Pak reveals effects of the kinase on actin and focal complexes. Mol. Cell Biol. 1997;17:1129–1143. doi: 10.1128/mcb.17.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, Tan I, Leung T, Lim L. Pak kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol. Cell. 1998;1:183–192. doi: 10.1016/s1097-2765(00)80019-2. [DOI] [PubMed] [Google Scholar]
- Marshall C. How do small GTPase signal transduction pathways regulate cell cycle entry? Curr. Opin. Cell Biol. 1999;11:732–736. doi: 10.1016/s0955-0674(99)00044-7. [see comments]. [DOI] [PubMed] [Google Scholar]
- Martin GA, Bollag G, McCormick F, Abo A. A novel serine kinase activated by rac1/CDC42Hs-dependent autophosphorylation is related to PAK65 and STE20. EMBO J. 1995;14:4385. doi: 10.1002/j.1460-2075.1995.tb00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. Wound healing–aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Martin P, Parkhurst SM. Parallels between tissue repair and embryo morphogenesis. Development. 2004;131:3021–3034. doi: 10.1242/dev.01253. [DOI] [PubMed] [Google Scholar]
- Masszi A, Fan L, Rosivall L, McCulloch CA, Rotstein OD, Mucsi I, Kapus A. Integrity of cell-cell contacts is a critical regulator of TGF-beta 1-induced epithelial-to-myofibroblast transition: role for beta-catenin. Am. J. Pathol. 2004;165:1955–1967. doi: 10.1016/s0002-9440(10)63247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi Y, Ebisuya M, Honjoh S, Nishida E. ERK activation propagates in epithelial cell sheets and regulates their migration during wound healing. Curr. Biol. 2004;14:731–735. doi: 10.1016/j.cub.2004.03.060. [DOI] [PubMed] [Google Scholar]
- Menard RE, Mattingly RR. Gbetagamma subunits stimulate p21-activated kinase 1 (PAK1) through activation of PI3-kinase and Akt but act independently of Rac1/Cdc42. FEBS Lett. 2004;556:187–192. doi: 10.1016/s0014-5793(03)01406-6. [DOI] [PubMed] [Google Scholar]
- Middleton CA. Contact inhibition of locomotion in cultures of pigmented retina epithelium. Exp. Cell Res. 1972;70:91–96. doi: 10.1016/0014-4827(72)90185-1. [DOI] [PubMed] [Google Scholar]
- Mira JP, Benard V, Groffen J, Sanders LC, Knaus UG. Endogenous, hyperactive Rac3 controls proliferation of breast cancer cells by a p21-activated kinase-dependent pathway. Proc. Natl. Acad. Sci. USA. 2000;97:185–189. doi: 10.1073/pnas.97.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T, Mayanagi T, Yoshio T, Sobue K. Changes in the balance between caldesmon regulated by p21-activated kinases and the Arp2/3 complex govern podosome formation. J. Biol. Chem. 2007;282:8454–8463. doi: 10.1074/jbc.M609983200. [DOI] [PubMed] [Google Scholar]
- Murdoch JN, Henderson DJ, Doudney K, Gaston-Massuet C, Phillips HM, Paternotte C, Arkell R, Stanier P, Copp AJ. Disruption of scribble (Scrb1) causes severe neural tube defects in the circletail mouse. Hum. Mol. Genet. 2003;12:87–98. doi: 10.1093/hmg/ddg014. [DOI] [PubMed] [Google Scholar]
- Nayal A, Webb DJ, Brown CM, Schaefer EM, Vicente-Manzanares M, Horwitz AR. Paxillin phosphorylation at Ser273 localizes a GIT1-PIX-Pak complex and regulates adhesion and protrusion dynamics. J. Cell Biol. 2006;173:587–589. doi: 10.1083/jcb.200509075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ. Adaptation of core mechanisms to generate cell polarity. Nature. 2003;422:766–774. doi: 10.1038/nature01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neudauer CL, Joberty G, Tatsis N, Macara IG. Distinct cellular effects and interactions of the Rho-family GTPase TC10. Curr. Biol. 1998;8:1151–1160. doi: 10.1016/s0960-9822(07)00486-1. [DOI] [PubMed] [Google Scholar]
- Nheu T, He H, Hirokawa Y, Walker F, Wood J, Maruta H. Pak is essential for RAS-induced upregulation of cyclin D1 during the G1 to S transition. Cell Cycle. 2004;3:71–74. [PubMed] [Google Scholar]
- Nikolic M, Chou MM, Lu W, Mayer BJ, Tsai LH. The p35/Cdk5 kinase is a neuron-specific Rac effector that inhibits Pak1 activity. Nature. 1998;395:194–198. doi: 10.1038/26034. [DOI] [PubMed] [Google Scholar]
- Nishimura I, Yang Y, Lu B. PAR-1 kinase plays an initiator role in a temporally ordered phosphorylation process that confers tau toxicity in Drosophila. Cell. 2004;116:671–682. doi: 10.1016/s0092-8674(04)00170-9. [DOI] [PubMed] [Google Scholar]
- Nishiya N, Kiosses WB, Han J, Ginsberg MH. An alpha4 integrin-paxillin-Arf-GAP complex restricts Rac activation to the leading edge of migrating cells. Nat. Cell Biol. 2005;7:343–352. doi: 10.1038/ncb1234. [DOI] [PubMed] [Google Scholar]
- O’Brien LE, Zegers MM, Mostov KE. Opinion: Building epithelial architecture: insights from three-dimensional culture models. Nat. Rev. Mol. Cell Biol. 2002;3:531–537. doi: 10.1038/nrm859. [DOI] [PubMed] [Google Scholar]
- Obermeier A, Ahmed S, Manser E, Yen SC, Hall C, Lim L. Pak promotes morphological changes by acting upstream of Rac. EMBO J. 1998;17:4328–4339. doi: 10.1093/emboj/17.15.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Lopez-Lago M, Giancotti FG. Merlin/NF-2 mediates contact inhibition of growth by suppressing recruitment of Rac to the plasma membrane. J. Cell Biol. 2005;171:361–371. doi: 10.1083/jcb.200503165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, You L, Giancotti FG. Shedding light on Merlin’s wizardry. Trends Cell Biol. 2007;17:222–229. doi: 10.1016/j.tcb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Olson MF, Ashworth A, Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science. 1995;269:1270–1272. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- Orr AW, Stockton R, Simmers MB, Sanders JM, Sarembock IJ, Blackman BR, Schwartz MA. Matrix-specific p21-activated kinase activation regulates vascular permeability in atherogenesis. J. Cell Biol. 2007;176:719–727. doi: 10.1083/jcb.200609008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmani N, Vitale N, Borg JP, Etienne-Manneville S. Scrib controls Cdc42 localization and activity to promote cell polarization during astrocyte migration. Curr. Biol. 2006;16:2395–2405. doi: 10.1016/j.cub.2006.10.026. [DOI] [PubMed] [Google Scholar]
- Palazzo AF, Gundersen GG. Microtubule-actin cross-talk at focal adhesions. Sci. STKE. 2002;2002:PE31. doi: 10.1126/stke.2002.139.pe31. [DOI] [PubMed] [Google Scholar]
- Papanas N, Maltezos E. Growth factors in the treatment of diabetic foot ulcers: new technologies, any promises? Int. J. Low Extrem Wounds. 2007;6:37–53. doi: 10.1177/1534734606298416. [DOI] [PubMed] [Google Scholar]
- Paris S, Longhi R, Santambrogio P, de Curtis I. Leucine-zipper-mediated homo- and hetero-dimerization of GIT family p95-ARF GTPase-activating protein, PIX-, paxillin-interacting proteins 1 and 2. Biochem. J. 2003;372:391–398. doi: 10.1042/BJ20030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrini MC, Lei M, Harrison SC, Mayer BJ. Pak1 Kinase Homodimers Are Autoinhibited in trans and Dissociated upon Activation by Cdc42 and Rac1. Mol. Cell. 2002;9:73–83. doi: 10.1016/s1097-2765(01)00428-2. [DOI] [PubMed] [Google Scholar]
- Pedersen TX, Leethanakul C, Patel V, Mitola D, Lund LR, Dano K, Johnsen M, Gutkind JS, Bugge TH. Laser capture microdissection-based in vivo genomic profiling of wound keratinocytes identifies similarities and differences to squamous cell carcinoma. Oncogene. 2003;22:3964–3976. doi: 10.1038/sj.onc.1206614. [DOI] [PubMed] [Google Scholar]
- Peterson JR, Chernoff J. Src transforms in a Cool way. Nat. Cell Biol. 2006;8:905–907. doi: 10.1038/ncb0906-905. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Premont RT, Claing A, Vitale N, Freeman JL, Pitcher JA, Patton WA, Moss J, Vaughan M, Lefkowitz RJ. beta2-Adrenergic receptor regulation by GIT1, a G protein-coupled receptor kinase-associated ADP ribosylation factor GTPase-activating protein. Proc. Natl. Acad. Sci. USA. 1998;95:14082–14087. doi: 10.1073/pnas.95.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premont RT, Claing A, Vitale N, Perry SJ, Lefkowitz RJ. The GIT family of ADP-ribosylation factor GTPase-activating proteins. Functional diversity of GIT2 through alternative splicing. J. Biol. Chem. 2000;275:22373–22380. doi: 10.1074/jbc.275.29.22373. [DOI] [PubMed] [Google Scholar]
- Price L, Leng J, Schwartz M, Bokoch G. Activation of rac and cdc42 by integrins mediates cell spreading. Mol. Biol. Cell. 1998;9:1863–1871. doi: 10.1091/mbc.9.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullikuth A, McKinnon E, Schaeffer HJ, Catling AD. The MEK1 scaffolding protein MP1 regulates cell spreading by integrating PAK1 and Rho signals. Mol. Cell Biol. 2005;25:5119–5133. doi: 10.1128/MCB.25.12.5119-5133.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puto LA, Pestonjamasp K, King CC, Bokoch GM. p21-activated kinase 1 (PAK1) interacts with the Grb2 adapter protein to couple to growth factor signaling. J. Biol. Chem. 2003;278:9388–9393. doi: 10.1074/jbc.M208414200. [DOI] [PubMed] [Google Scholar]
- Qin Y, Capaldo C, Gumbiner BM, Macara IG. The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J. Cell Biol. 2005;171:1061–1071. doi: 10.1083/jcb.200506094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev. Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- Rhee S, Grinnell F. P21-activated kinase 1: Convergence point in PDGF- and LPA-stimulated collagen matrix contraction by human fibroblasts. J. Cell Biol. 2006;172:423–432. doi: 10.1083/jcb.200505175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: Integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Roig J, Traugh JA. Cytostatic p21 G protein-activated protein kinase gamma-Pak. Vitam. Horm. 2001;62:167–198. doi: 10.1016/s0083-6729(01)62004-1. [DOI] [PubMed] [Google Scholar]
- Rosenberger G, Gal A, Kutsche K. AlphaPIX associates with calpain 4, the small subunit of calpain, and has a dual role in integrin-mediated cell spreading. J. Biol. Chem. 2005;280:6879–6889. doi: 10.1074/jbc.M412119200. [DOI] [PubMed] [Google Scholar]
- Royal I, Lamarche-Vane N, Lamorte L, Kaibuchi K, Park M. Activation of cdc42, rac, Pak, and rho-kinase in response to hepatocyte growth factor differentially regulates epithelial cell colony spreading and dissociation. Mol. Biol. Cell. 2000;11:1709–1725. doi: 10.1091/mbc.11.5.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel T, Bokoch GM. Membrane and morphological changes in apoptotic cells regulated by caspase-mediated activation of PAK2. Science. 1997;276:1571–1574. doi: 10.1126/science.276.5318.1571. [DOI] [PubMed] [Google Scholar]
- Salaycik KJ, Fagerstrom CJ, Murthy K, Tulu US, Wadsworth P. Quantification of microtubule nucleation, growth and dynamics in wound-edge cells. J. Cell. Sci. 2005;118:4113–4122. doi: 10.1242/jcs.02531. [DOI] [PubMed] [Google Scholar]
- Sanders LC, Matsumura F, Bokoch GM, de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase. Science. 1999;283:2083–2085. doi: 10.1126/science.283.5410.2083. [see comments]. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes. Dev. 2002;16:1587–1609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- Schmidt MH, Husnjak K, Szymkiewicz I, Haglund K, Dikic I. Cbl escapes Cdc42-mediated inhibition by downregulation of the adaptor molecule betaPix. Oncogene. 2006;25:3071–3078. doi: 10.1038/sj.onc.1209329. [DOI] [PubMed] [Google Scholar]
- Schmitz AA, Govek EE, Bottner B, Van Aelst L. Rho GTPases: Signaling, migration, and invasion. Exp. Cell Res. 2000;261:1–12. doi: 10.1006/excr.2000.5049. [DOI] [PubMed] [Google Scholar]
- Schneider H, Muhle C, Pacho F. Biological function of laminin-5 and pathogenic impact of its deficiency. Eur. J. Cell Biol. 2007;86:701–717. doi: 10.1016/j.ejcb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Sells M, Knaus U, Bagrodia S, Ambrose D, Bokoch G, Chernoff J. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr. Biol. 1997;7:202–210. doi: 10.1016/s0960-9822(97)70091-5. [DOI] [PubMed] [Google Scholar]
- Sells MA, Boyd JT, Chernoff J. p21-activated kinase 1 (Pak1) regulates cell motility in mammalian fibroblasts. J. Cell Biol. 1999;145:837–849. doi: 10.1083/jcb.145.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sells MA, Pfaff A, Chernoff J. Temporal and spatial distribution of activated Pak1 in fibroblasts. J. Cell Biol. 2000;151:1449–1458. doi: 10.1083/jcb.151.7.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settleman J. Getting in shape with Rho. Nat. Cell Biol. 2000;2:E7–E9. doi: 10.1038/71390. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Paez JG, Curto M, Yaktine A, Pruitt WM, Saotome I, O’Bryan JP, Gupta V, Ratner N, Der CJ, Jacks T, McClatchey AI. The Nf2 tumor suppressor, merlin, functions in Rac-dependent signaling. Dev. Cell. 2001;1:63–72. doi: 10.1016/s1534-5807(01)00009-0. [DOI] [PubMed] [Google Scholar]
- Shin EY, Shin KS, Lee CS, Woo KN, Quan SH, Soung NK, Kim YG, Cha CI, Kim SR, Park D, Bokoch GM, Kim EG. Phosphorylation of p85 beta PIX, a Rac/Cdc42-specific guanine nucleotide exchange factor, via the Ras/ERK/PAK2 pathway is required for basic fibroblast growth factor-induced neurite outgrowth. J. Biol. Chem. 2002;277:44417–44430. doi: 10.1074/jbc.M203754200. [DOI] [PubMed] [Google Scholar]
- Shin EY, Woo KN, Lee CS, Koo SH, Kim YG, Kim WJ, Bae CD, Chang SI, Kim EG. Basic fibroblast growth factor stimulates activation of Rac1 through a p85 betaPIX phosphorylation-dependent pathway. J. Biol. Chem. 2004;279:1994–2004. doi: 10.1074/jbc.M307330200. [DOI] [PubMed] [Google Scholar]
- Siegrist SE, Doe CQ. Microtubule-induced cortical cell polarity. Genes. Dev. 2007;21:483–496. doi: 10.1101/gad.1511207. [DOI] [PubMed] [Google Scholar]
- Singer AJ, Clark RA. Cutaneous wound healing. N. Engl. J. Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Slack-Davis JK, Eblen ST, Zecevic M, Boerner SA, Tarcsafalvi A, Diaz HB, Marshall MS, Weber MJ, Parsons JT, Catling AD. PAK1 phosphorylation of MEK1 regulates fibronectin-stimulated MAPK activation. J. Cell Biol. 2003;162:281–291. doi: 10.1083/jcb.200212141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small JV, Geiger B, Kaverina I, Bershadsky A. How do microtubules guide migrating cells? Nat. Rev. Mol. Cell Biol. 2002;3:957–964. doi: 10.1038/nrm971. [DOI] [PubMed] [Google Scholar]
- Small JV, Kaverina I. Microtubules meet substrate adhesions to arrange cell polarity. Curr. Opin. Cell Biol. 2003;15:40–47. doi: 10.1016/s0955-0674(02)00008-x. [DOI] [PubMed] [Google Scholar]
- Stockton R, Reutershan J, Scott D, Sanders J, Ley K, Schwartz MA. Induction of vascular permeability: betaPIX and GIT1 scaffold the activation of extracellular signal-regulated kinase by Pak. Mol. Biol. Cell. 2007;18:2346–2355. doi: 10.1091/mbc.E06-07-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stofega MR, Sanders LC, Gardiner EM, Bokoch GM. Constitutive p21-activated Kinase (Pak) Activation in Breast Cancer Cells as a Result of Mislocalization of Pak to Focal Adhesions. Mol. Biol. Cell. 2004;15:2965–2977. doi: 10.1091/mbc.E03-08-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker MG, Rubin H. Density dependent inhibition of cell growth in culture. Nature. 1967;215:171–172. doi: 10.1038/215171a0. [DOI] [PubMed] [Google Scholar]
- Stoletov KV, Ratcliffe KE, Spring SC, Terman BI. NCK and Pak participate in the signaling pathway by which vascular endothelial growth factor stimulates the assembly of focal adhesions. J. Biol. Chem. 2001;276:22748–22755. doi: 10.1074/jbc.M009720200. [DOI] [PubMed] [Google Scholar]
- Sun H, King AJ, Diaz HB, Marshall MS. Regulation of the protein kinase Raf-1 by oncogenic Ras through phosphatidylinositol 3-kinase, Cdc42/Rac and Pak. Curr. Biol. 2000;10:281–284. doi: 10.1016/s0960-9822(00)00359-6. [DOI] [PubMed] [Google Scholar]
- Sundberg-Smith LJ, Doherty JT, Mack CP, Taylor JM. Adhesion stimulates direct PAK1/ERK2 association and leads to ERK-dependent PAK1 Thr212 phosphorylation. J. Biol. Chem. 2005;280:2055–2064. doi: 10.1074/jbc.M406013200. [DOI] [PubMed] [Google Scholar]
- Talukder AH, Meng Q, Kumar R. CRIPak, a novel endogenous Pak1 inhibitor. Oncogene. 2006;25:1311–1319. doi: 10.1038/sj.onc.1209172. [DOI] [PubMed] [Google Scholar]
- Tang Y, Chen Z, Ambrose D, Liu J, Gibbs J, Chernoff J, Field J. Kinase-deficient Pak1 mutants inhibit Ras transformation of Rat-1 fibroblasts. Mol. Cell Biol. 1997;17:4454–4464. doi: 10.1128/mcb.17.8.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Zhou H, Chen A, Pittman RN, Field J. The Akt proto-oncogene links Ras to Pak and cell survival signals. J. Biol. Chem. 2000;275:9106–9109. doi: 10.1074/jbc.275.13.9106. [DOI] [PubMed] [Google Scholar]
- Tao W, Pennica D, Xu L, Kalejta RF, Levine AJ. Wrch-1, a novel member of the Rho gene family that is regulated by Wnt-1. Genes. Dev. 2001;15:1796–1807. doi: 10.1101/gad.894301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel DA, Reeder MK, Pfaff A, Coleman TR, Sells MA, Chernoff J. Cell cycle-regulated phosphorylation of p21-activated kinase 1. Curr. Biol. 2002;12:1227–1232. doi: 10.1016/s0960-9822(02)00931-4. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- Thullberg M, Gad A, Beeser A, Chernoff J, Stromblad S. The kinase-inhibitory domain of p21-activated kinase 1 (PAK1) inhibits cell cycle progression independent of PAK1 kinase activity. Oncogene. 2007;26:1820–1828. doi: 10.1038/sj.onc.1209983. [DOI] [PubMed] [Google Scholar]
- Tscharntke M, Pofahl R, Chrostek-Grashoff A, Smyth N, Niessen C, Niemann C, Hartwig B, Herzog V, Klein HW, Krieg T, Brakebusch C, Haase I. Impaired epidermal wound healing in vivo upon inhibition or deletion of Rac1. J. Cell. Sci. 2007;120:1480–1490. doi: 10.1242/jcs.03426. [DOI] [PubMed] [Google Scholar]
- Tu H, Wigler M. Genetic evidence for Pak1 autoinhibition and its release by Cdc42. Mol. Cell Biol. 1999;19:602–611. doi: 10.1128/mcb.19.1.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CE, Brown MC, Perrotta JA, Riedy MC, Nikolopoulos SN, McDonald AR, Bagrodia S, Thomas S, Leventhal PS. Paxillin LD4 motif binds Pak and PIX through a novel 95-kDa ankyrin repeat, ARF-GAP protein: A role in cytoskeletal remodeling. J. Cell Biol. 1999;145:851–863. doi: 10.1083/jcb.145.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadlamudi RK, Adam L, Wang RA, Mandal M, Nguyen D, Sahin A, Chernoff J, Hung MC, Kumar R. Regulatable expression of p21-activated kinase-1 promotes anchorage-independent growth and abnormal organization of mitotic spindles in human epithelial breast cancer cells. J. Biol. Chem. 2000;275:36238–36244. doi: 10.1074/jbc.M002138200. [DOI] [PubMed] [Google Scholar]
- Vadlamudi RK, Barnes CJ, Rayala S, Li F, Balasenthil S, Marcus S, Goodson HV, Sahin AA, Kumar R. p21-activated kinase 1 regulates microtubule dynamics by phosphorylating tubulin cofactor B. Mol. Cell Biol. 2005;25:3726–3736. doi: 10.1128/MCB.25.9.3726-3736.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadlamudi RK, Li F, Barnes CJ, Bagheri-Yarmand R, Kumar R. p41-Arc subunit of human Arp2/3 complex is a p21-activated kinase-1-interacting substrate. EMBO Rep. 2004;5:154–160. doi: 10.1038/sj.embor.7400079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aelst L, Symons M. Role of Rho family GTPases in epithelial morphogenesis. Genes. Dev. 2002;16:1032–1054. doi: 10.1101/gad.978802. [DOI] [PubMed] [Google Scholar]
- van Leeuwen FN, van Delft S, Kain HE, van der Kammen RA, Collard JG. Rac regulates phosphorylation of the myosin-II heavy chain, actinomyosin disassembly and cell spreading. Nat. Cell Biol. 1999;1:242–248. doi: 10.1038/12068. [DOI] [PubMed] [Google Scholar]
- Walter BN, Huang Z, Jakobi R, Tuazon PT, Alnemri ES, Litwack G, Traugh JA. Cleavage and activation of p21-activated protein kinase gamma-Pak by CPP32 (caspase 3). Effects of autophosphorylation on activity. J. Biol. Chem. 1998;273:28733–28739. doi: 10.1074/jbc.273.44.28733. [DOI] [PubMed] [Google Scholar]
- Wang J, Frost JA, Cobb MH, Ross EM. Reciprocal signaling between heterotrimeric G proteins and the p21-stimulated protein kinase. J. Biol. Chem. 1999;274:31641–31647. doi: 10.1074/jbc.274.44.31641. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Noritake J, Kaibuchi K. Regulation of microtubules in cell migration. Trends Cell Biol. 2005;15:76–83. doi: 10.1016/j.tcb.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Webb BA, Eves R, Crawley SW, Zhou S, Cote GP, Mak AS. PAK1 induces podosome formation in A7r5 vascular smooth muscle cells in a Pak-interacting exchange factor-dependent manner. Am. J. Physiol. Cell Physiol. 2005;289:C898–C907. doi: 10.1152/ajpcell.00095.2005. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Kovalenko M, Whitmore L, Horwitz AF. Phosphorylation of serine 709 in GIT1 regulates protrusive activity in cells. Biochem. Biophys. Res. Commun. 2006;346:1284–1288. doi: 10.1016/j.bbrc.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Weisz Hubsman M, Volinsky N, Manser E, Yablonski D, Aronheim A. Autophosphorylation-dependent degradation of Pak1, triggered by the Rho-family GTPase. Chp. Biochem. J. 2007;404:487–497. doi: 10.1042/BJ20061696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann T, Bokoch GM, Waterman-Storer CM. Regulation of leading edge microtubule and actin dynamics downstream of Rac1. J. Cell Biol. 2003;161:845–851. doi: 10.1083/jcb.200303082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann T, Bokoch GM, Waterman-Storer CM. Regulation of microtubule destabilizing activity of Op18/stathmin downstream of Rac1. J. Biol. Chem. 2004;279:6196–6203. doi: 10.1074/jbc.M307261200. [DOI] [PubMed] [Google Scholar]
- Woolner S, Jacinto A, Martin P. The small GTPase Rac plays multiple roles in epithelial sheet fusion–dynamic studies of Drosophila dorsal closure. Dev. Biol. 2005;282:163–173. doi: 10.1016/j.ydbio.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochim. Biophys. Acta. 2004;1692:103–119. doi: 10.1016/j.bbamcr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Wu WJ, Tu S, Cerione RA. Activated Cdc42 sequesters c-Cbl and prevents EGF receptor degradation. Cell. 2003;114:715–725. doi: 10.1016/s0092-8674(03)00688-3. [DOI] [PubMed] [Google Scholar]
- Xia C, Ma W, Stafford LJ, Marcus S, Xiong WC, Liu M. Regulation of the p21-activated kinase (Pak) by a human Gbeta-like WD-repeat protein, hPIP1. Proc Natl. Acad. Sci. USA. 2001;98:6174–6179. doi: 10.1073/pnas.101137298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao GH, Beeser A, Chernoff J, Testa JR. p21-activated kinase links Rac/Cdc42 signaling to merlin. J. Biol. Chem. 2002;277:883–886. doi: 10.1074/jbc.C100553200. [DOI] [PubMed] [Google Scholar]
- Xiao GH, Gallagher R, Shetler J, Skele K, Altomare DA, Pestell RG, Jhanwar S, Testa JR. The NF2 tumor suppressor gene product, merlin, inhibits cell proliferation and cell cycle progression by repressing cyclin D1 expression. Mol. Cell Biol. 2005;25:2384–2394. doi: 10.1128/MCB.25.6.2384-2394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Yin G, Haendeler J, Yan C, Berk BC. GIT1 functions as a scaffold for MEK1-extracellular signal-regulated kinase 1 and 2 activation by angiotensin II and epidermal growth factor. Mol. Cell Biol. 2004;24:875–885. doi: 10.1128/MCB.24.2.875-885.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin G, Zheng Q, Yan C, Berk BC. GIT1 is a scaffold for ERK1/2 activation in focal adhesions. J. Biol. Chem. 2005;280:27705–27712. doi: 10.1074/jbc.M502271200. [DOI] [PubMed] [Google Scholar]
- Yoshii S, Tanaka M, Otsuki Y, Wang DY, Guo RJ, Zhu Y, Takeda R, Hanai H, Kaneko E, Sugimura H. alphaPIX nucleotide exchange factor is activated by interaction with phosphatidylinositol 3-kinase. Oncogene. 1999;18:5680–5690. doi: 10.1038/sj.onc.1202936. [DOI] [PubMed] [Google Scholar]
- Yu W, Datta A, Leroy P, O’Brien LE, Mak G, Jou TS, Matlin KS, Mostov KE, Zegers MM. β1-Integrin orients epithelial polarity via rac1 and laminin. Mol. Biol. Cell. 2005;16:433–445. doi: 10.1091/mbc.E04-05-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel-Bar R, Ballestrem C, Kam Z, Geiger B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J. Cell. Sci. 2003;116:4605–4613. doi: 10.1242/jcs.00792. [DOI] [PubMed] [Google Scholar]
- Zarbalis K, May SR, Shen Y, Ekker M, Rubenstein JL, Peterson AS. A focused and efficient genetic screening strategy in the mouse: identification of mutations that disrupt cortical development. PLoS Biol. 2004;2:E219. doi: 10.1371/journal.pbio.0020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegers MM, Forget MA, Chernoff J, Mostov KE, ter Beest MB, Hansen SH. Pak1 and PIX regulate contact inhibition during epithelial wound healing. EMBO J. 2003a;22:4155–4165. doi: 10.1093/emboj/cdg398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegers MM, O’Brien LE, Yu W, Datta A, Mostov KE. Epithelial polarity and tubulogenesis in vitro. Trends Cell Biol. 2003b;13:169–176. doi: 10.1016/s0962-8924(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat. Med. 2003;9:964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- Zenke FT, King CC, Bohl BP, Bokoch GM. Identification of a central phosphorylation site in p21-activated kinase regulating autoinhibition and kinase activity. J. Biol. Chem. 1999;274:32565–32573. doi: 10.1074/jbc.274.46.32565. [DOI] [PubMed] [Google Scholar]
- Zenke FT, Krendel M, DerMardirossian C, King CC, Bohl BP, Bokoch GM. p21-activated kinase 1 phosphorylates and regulates 14-3-3 binding to GEF-H1, a microtubule-localized Rho exchange factor. J. Biol. Chem. 2004;279:18392–183400. doi: 10.1074/jbc.M400084200. [DOI] [PubMed] [Google Scholar]
- Zhao ZS, Lim JP, Ng YW, Lim L, Manser E. The GIT-associated kinase Pak targets to the centrosome and regulates Aurora-A. Mol. Cell. 2005;20:237–249. doi: 10.1016/j.molcel.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Zhao ZS, Manser E. Pak and other Rho-associated kinases—Effectors with surprisingly diverse mechanisms of regulation. Biochem. J. 2005;386:201–214. doi: 10.1042/BJ20041638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZS, Manser E, Chen XQ, Chong C, Leung T, Lim L. A conserved negative regulatory region in alphaPAK: inhibition of Pak kinases reveals their morphological roles downstream of Cdc42 and Rac1. Mol. Cell Biol. 1998;18:2153–2163. doi: 10.1128/mcb.18.4.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZS, Manser E, Lim L. Interaction between Pak and nck: A template for Nck targets and role of Pak autophosphorylation. Mol. Cell Biol. 2000a;20:3906–3917. doi: 10.1128/mcb.20.11.3906-3917.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZS, Manser E, Loo TH, Lim L. Coupling of Pak-interacting exchange factor PIX to GIT1 promotes focal complex disassembly. Mol. Cell Biol. 2000b;20:6354–6363. doi: 10.1128/mcb.20.17.6354-6363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong JL, Banerjee MD, Nikolic M. Pak1 and its T212 phosphorylated form accumulate in neurones and epithelial cells of the developing rodent. Dev. Dyn. 2003;228:121–127. doi: 10.1002/dvdy.10351. [DOI] [PubMed] [Google Scholar]
- Zhou GL, Zhuo Y, King CC, Fryer BH, Bokoch GM, Field J. Akt phosphorylation of serine 21 on Pak1 modulates Nck binding and cell migration. Mol. Cell Biol. 2003;23:8058–8069. doi: 10.1128/MCB.23.22.8058-8069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Kramer RH. Integrin engagement differentially modulates epithelial cell motility by RhoA/ROCK and PAK1. J. Biol. Chem. 2005;280:10624–10635. doi: 10.1074/jbc.M411900200. [DOI] [PubMed] [Google Scholar]