Abstract
The development of opioid dependence involves classical neuronal opioid receptor activation and is due in part to engagement of glia causing a proinflammatory response. Such opioid-induced glial activation occurs, at least in part, through a non-classical opioid mechanism involving Toll-like-receptor 4 (TLR4). Among the immune factors released following the opioid-glia-TLR4 interaction, interleukin-1β (IL-1β) plays a prominent role. Previous animal behavioral studies have demonstrated significant heterogeneity of chronic morphine-induced tolerance and dependence between different mouse strains. The aim of this study was to investigate whether the heterogeneity of chronic opioid-induced IL-1β expression contributes to differences in opioid tolerance and withdrawal behaviors. Chronic morphine-induced tolerance and dependence were assessed in 3 inbred wild-type mouse strains (Balb/c, CBA, and C57BL/6) and 2 knockout strains (TLR4 and MyD88). Analysis of brain nuclei (medial prefrontal cortex, cortex, brain stem, hippocampus, and midbrain and diencephalon regions combined) revealed that, of inbred wild-type mice, there are significant main effects of morphine treatment on IL-1β expression in the brain regions analyzed (p < 0.02 for all regions analyzed). A significant increase in hippocampal IL-1β expression was found in C57BL/6 mice after morphine treatment, whilst, a significant decrease was found in the mPFC region of wild-type Balb/c mice. Furthermore, the results of wild-type inbred strains demonstrated that the elevated hippocampal IL-1β expression is associated with withdrawal jumping behavior. Interestingly, knockout of TLR4, but not MyD88 protected against the development of analgesic tolerance. Gene sequence differences of IL-1B and TLR4 genes alone did not explain the heterogeneity of dependence behavior between mouse strains. Together, these data further support the involvement of opioid-induced CNS immune signaling in dependence development. Moreover, this study demonstrated the advantages of utilizing multiple mouse strains and indicates that appropriate choice of mouse strains could enhance future research outcomes.
Keywords: Morphine, glia, interleukin-1β, Toll-like receptor 4, MyD88, tolerance, withdrawal, mice strain differences
1. Introduction
Adding to the well established neuronal opioid actions at classical neuronal opioid receptors, opioid-induced neuroinflammation provides an important complement to current understanding. Opioid-induced glial activation is reported to occur via non-classical opioid mechanisms, engaging the innate immune receptor, Toll-like-receptor 4 (TLR4), expressed on central nervous system (CNS) immune-like cells, glia (Hutchinson et al., 2007; Hutchinson et al., 2009; Watkins et al., 2009). Opioid-induced TLR4 signaling involves the activation of a series of downstream signaling pathways including MyD88-dependent pathways, culminating in a proinflammatory response (Hutchinson et al., 2007; Hutchinson et al., 2009). Among the collection of immune factors that are released following such an opioid-induced TLR4 response, interleukin-1β (IL-1β) plays a prominent role. Chronic morphine dosing increases IL-1β protein expression in the hippocampus, spinal cord and cerebrospinal fluid (Hutchinson et al., 2008; Hutchinson et al., 2009; Johnston et al., 2004). Exogenous administration of a neutral dose of IL-1β abolishes morphine analgesia in mice, and both pharmacological and genetic blockade of IL-1β signaling significantly prolongs and potentiates morphine analgesia (Hutchinson et al., 2008; Shavit et al., 2005). Furthermore, inhibition of opioid-induced glial activation by the glial attenuators minocycline and AV411 inhibits morphine-induced IL-1β expression, potentiates morphine analgesia, suppresses morphine reward, and attenuates withdrawal in rats (Hutchinson et al., 2008b; Hutchinson et al., 2009).
As it is well known, the μ-opioid receptor plays a critical role in the development of opioid dependence. Adding to this, the actions of opioids through glial reactivity are also involved in the development of opioid dependence (Hutchinson et al., 2007; Hutchinson et al., 2008a; Hutchinson et al., 2008b; Hutchinson et al., 2009; Watkins et al., 2007; Watkins et al., 2009) resulting in part from a proinflammatory response. These animal studies are supported by our recent human genetic study that revealed an association between polymorphisms that alter IL-1β expression and risk of opioid dependence (Liu et al., 2009), providing the first human evidence supporting the proinflammatory opioid dependence hypothesis. These non-neuronal glial actions also counter beneficial opioid actions, producing responses such as glial opposition of acute opioid analgesia (Hutchinson et al., 2008) and contributing to the development of analgesic tolerance and opioid-induced atypical pain states such as hyperalgesia and allodynia (Hutchinson et al., 2007; Hutchinson et al., 2008). Interestingly, the glial opposition to the beneficial opioid effects is accompanied by glial-induced increases in both behavioral and neurochemical indices of reward (Bland et al., 2009; Hutchinson et al., 2007; Hutchinson et al., 2008b; Hutchinson et al., 2009; Narita et al., 2006; Narita et al., 2008; Watkins et al., 2005). Critically however, glial reactivity alone will not create a state of dependence, thus engagement of neuronal opioid receptors are obligatory in this phenomena (Narita et al., 2006; Narita et al., 2008).
Previous animal behavioral studies examining neuronal mechanisms of opioid action have demonstrated the heterogeneity of chronic opioid-induced analgesic tolerance and dependence withdrawal severity among different inbred mouse strains, such as greater analgesic response in DBA/2 relative to C57/BL6 mice, and greater withdrawal jumping of C57BL/6 than CBA mice (Belknap et al., 1998; Kest et al., 2002a; Kest et al., 2002b; Liang et al., 2006b; Metten et al., 2009). A factor that has not been examined in these past studies is the differing proinflammatory responses of different mouse strains. Such knowledge is pivotal, as different mouse strains have different regulation of immune functions and potentially different levels of proinflammatory mediators and signaling pathways (Gol’dberg et al., 2005; Masnaya et al., 2002). Furthermore, it is well known that brain regions contribute differently to opioid response and dependence. For example, the motivational aspects of morphine withdrawal is mediated, at least in part, by brain structures such as the nucleus accumbens, nucleus of the amygdala, hippocampus and bed nucleus of the stria terminalis, and the physical withdrawal signs have been linked to stronger activation of some of these brain regions plus involvement of mesencephalic and autonomic brain regions, including the locus coeruleus and central gray (Frenois et al., 2002; Kosten and George, 2002). However, opioid-induced immune response in different brain nuclei in different strains of mice, which have differing propensities to opioid dependence, is yet to be systematically investigated. Finally, the genetic heterogeneity of the opioid-glial-TLR4-IL-1β pathway could also contribute to strain differences in opioid actions; however, there is limited sequencing information available for IL-1B and TLR4 genes in the mouse strains to be used in this study.
Therefore, the present series of experiments aimed to investigate whether the heterogeneity of chronic opioid-induced IL-1β expression contributes to differences in dependence withdrawal behavior. By using multiple inbred wild-type and knockout mouse strains, we have examined the involvement of TLR4 and the MyD88-dependent signaling pathway, and brain region specific IL-1β expression in chronic opioid-induced tolerance and withdrawal behaviors. In addition, in order to reveal any genetic heterogeneity, we also sequenced the IL-1B and TLR4 genes, which encode for the IL-1β and TLR4 proteins, respectively, in all mouse strains studied.
2. Material and methods
2.1 Animals
Male adult mice, weighing between 25–35 g, of the following strains were used in the experiments described below: three inbred wild-type strains, Balb/c, CBA, and C57BL/6, obtained from Laboratory Animal Services of the University of Adelaide (Adelaide, SA, Australia); and two knockout mouse strains from Balb/c background, TLR4 Knockout (TLR4 KO) and MyD88 Knockout (MyD88 KO), kindly sourced from Dr Simon Phipps and Dr Paul Foster from University of Newcastle (Newcastle, NSW, Australia). All mice were housed in groups of 4 – 8 to a cage with the same strain mates, in the Medical School Animal Facility of the University of Adelaide. Mice were allowed free access to food and water in a temperature- controlled environment maintained on a 12:12 h light/dark cycle (lights on at 7:00 h). All testing was performed following an acclimatization period of at least one week after arrival. The experiments reported in these studies were approved by the University of Adelaide Animal Ethics Committee (Ethics approval number M-60-2009).
2.2 Materials
Morphine HCL and naloxone HCL were purchased from GlaxoSmithKline (Melbourne, VIC, Australia) and Sigma-Aldrich (Castle Hill, NSW, Australia), respectively. Lipopolysaccharide (LPS) from Escherichia coli, penicillin-streptomycin solution (10,000 units penicillin, 10 mg streptomycin per ml), and fetal calf serum (FCS) were obtained from Sigma-Aldrich. RPMI 1640 with HEPES modification, L-glutamine medium and Iscove’s medium were purchased from Invitrogen (Mulgrave, VIC, Australia). Optiprep™ was obtained from Axis-Shield (Castle Hill, NSW, Australia).
For in vivo experiments, both morphine HCL and naloxone HCL were dissolved in 0.9% physiological saline (Sigma-Aldrich). Both drugs were administered via the intraperitoneal route in an injection volume of 10 ml/kg (base corrected).
For in vitro experiments, LPS and morphine were firstly dissolved into Milli Q water as stock solutions (LPS 1 mg/ml, morphine 10 mM) and stored at −20 °C until required. A limulus amoebocyte lysate assay (Nachum and Shanbrom, 1981) was performed on the morphine stock prior to experiments to confirm there was no endotoxin contamination.
2.3 In vivo study
2.3.1 Morphine treatment
Chronic morphine dependence was induced by repeated injections for three consecutive days with an escalating dose schedule (Hutchinson et al., 2009). Mice (n = 8 per strain) received morphine twice daily (morning and afternoon) for 2 days (day 1: 7.5 and 15; day 2: 30 and 30 mg/kg). On the testing day (day 3), a final morphine dose (30 mg/kg) was administered. To test for analgesic tolerance, the final dose on day 3 was split into two injections: firstly a 7.5 mg/kg dose followed by the nociceptive test (see below), and then the rest of the dose (22.5 mg/kg). A group of control mice (n = 8 per strain) received an equal number of saline injections over 3 days.
2.3.2 Nociceptive assay
To assess the baseline thermal hypersensitivity and analgesic effects, the hotplate test was employed. In this assay of acute thermal nociception, the mouse was placed on a 50 °C hotplate until jumping, paw shaking, or paw licking behaviors were observed or until the maximum latency time of 60 sec was reached. On day 1 and day 3, baseline sensitivity was measured prior to the morphine injection, and the analgesic effect measured after 20 min of the first morphine dose (7.5 mg/kg). Baseline sensitivity was measured three times, with each determination separated by a minimum of 15 min. The three determinations were later averaged. Analgesia was expressed as a percentage of the maximum possible effect (% MPE) calculated using the following equation:
2.3.3 Naloxone-precipitated withdrawal
A single dose of naloxone (10 mg/kg) was administered to all mice 1 h after the final morphine/saline dose. Immediately after the naloxone injection, animals were placed into individual Plexiglas observation cylinders (25 h × 11 w cm) (Nalgene, Scoresby, VIC, Australia). Withdrawal jumping response symptoms were recorded and the frequency of jumps for each mouse was summed over 30 min. All testing was conducted blind to group assignment.
2.3.4 Brain tissue collection & dissection
Following withdrawal assessment, the mice were sacrificed by overdose with sodium pentobarbital (Abbott Laboratories, North Chicago, IL, USA), and brain tissue collected. To ensure that the IL-1β measured in the brain tissues would not be affected by any potential changes in circulating concentrations of intracellular or extracellular IL-1β post-sacrifice, animals were transcardially perfused with 15 ml of chilled 0.9% saline. Following this, brains were quickly isolated, collected into ice-cold tubes and stored at −70 °C until the time of analysis.
To investigate the possible regions in which morphine could induce glial IL-1β expression changes, gross dissection of the brain was performed to collect the structures of sufficient size to allow analysis. These included the medial prefrontal cortex (mPFC), cortex, brain stem, hippocampus, and midbrain plus diencephalon combined (including midbrain, thalamus and hypothalamus areas).
2.3.5 Sample preparation and IL-1β protein quantification
Procedures for tissue processing were as described previously (Johnston et al., 2004). Briefly, dissected brain tissue was sonicated using a Labsonic 1510 probe sonicator (B. BRAUN, Melsungen, Germany) in ice-cold extraction buffer containing Iscove’s medium with 5% FCS and a cocktail enzyme inhibitor (including: 100 mM amino-n-caproic acid, 10 mM EDTA, 5 mM benzamidine-HCl, and 0.2 mM phenylmethylsulfonyl fluoride) all obtained from Sigma-Aldrich (Castle Hill, NSW, Australia). Sonicated samples were centrifuged, and supernatants were collected and stored at 4 °C until ELISA assays were performed.
IL-1β protein in the sonication supernatant was measured by commercially available mouse-specific ELISA kits (sensitivity: 4 pg/ml, standard curve range: 4 – 2000 pg/ml, OptiEIA ELISA Sets, BD Biosciences, San Diego, CA, USA) in accordance with the manufacturer’s instructions. The ELISA result was corrected for total protein concentration and expressed as pg/mg protein. The total protein was measured by the Bradford assay (sensitivity: 0.1 mg/ml protein, standard curve range: 0.1 – 1.4 mg/ml protein) in accordance with the manufacturer’s instructions (Sigma-Aldrich, Castle Hill, NSW, Australia).
2.4 In vitro study
2.4.1 Leukocyte preparation
A separate group of mice were used to carry out in vitro experiments. Only the wild-type animals Balb/c, CBA, and C57BL/6, (n = 4 per strain) were used in these experiments. Mice were sacrificed by overdose of sodium pentobarbital followed by prompt removal of the spleen. Splenic leukocytes were isolated using Optiprep™ mixer flotation method modified from the manufacturer’s instructions as detailed below. Aseptic techniques were used during the isolation process. The spleen was homogenized using an ice-cold glass homogenizer (Emerald Glass, Adelaide, SA, Australia) and washed with 12 – 15 ml of RPMI 1640. The cells were centrifuged at 4 °C for 5 min at 200 × g, and the supernatant was discarded. The cells were re-suspended in 3.9 ml of RPMI 1640 and thoroughly mixed with 2.1 ml of 66% Optiprep™. One ml of RPMI 1640 was layered on top and samples centrifuged at 1500 × g for 20 min at 4 °C. The leukocytes were harvested from the interface, washed with 12 – 15 ml of RPMI 1640 and centrifuged for 10 min at 400 × g at 4 °C. Supernatant was discarded and the cells were re-suspended in 2 ml of enriched RPMI 1640 (RPMI 1640 supplemented with 10% FCS and 0.8% penicillin-streptomycin solution). The number of viable leukocytes was counted using trypan blue exclusion and a haemocytometer (Crown Scientific, Minto, VIC, Australia).
2.4.2 Cell culture and IL-1β quantification
Seven concentrations of LPS (final concentrations: 100, 10, 1, 0.1, 0.01, 0.001, 0.0001 μg/ml), 7 concentrations of morphine (final concentrations: 100, 10, 1, 0.1, 0.01, 0.001, 0.0001 μM), and unstimulated mitogen negative control (RPMI 1640 only) were separately aliquoted into 96 well plates (Nunc, Noble Park, VIC, Australia). Cells were diluted in enriched RPMI 1640 to 2.5 × 106 cells/ml and 100 μl of this suspension was plated into each well, to a final volume of 200 μl. The plates were incubated at 37 °C, 5% CO2 in a humidified incubator (Thermoline Scientific, NSW, Australia) for 24 h. Supernatants were collected and IL-1β was measured by ELISA as detailed above.
2.5 Gene sequencing
One mouse of each strain was randomly selected for IL-1B and TLR4 gene sequencing. Genomic DNA was isolated from spleen tissue using a QIAamp® DNA Mini kit (Qiagen Pty Ltd, Doncaster, VIC, Australia). The DNA samples were then sent to the Australian Genome Research Facility (AGRF, Brisbane, QLD, Australia) for sequencing using Applied Biosystems 3730×l DNA Analyzer. Each gene with 500 base-pair (bp) flanking sequence either side was sequenced. Reference sequence accessions used for comparisons were NC_000068.6 for IL-1B and NC_000070.5 for TLR4 from the C57BL/6J assembly (sourced from the National Center for Biotechnology Information US: http://www.ncbi.nlm.nih.gov/gene/16176, http://www.ncbi.nlm.nih.gov/gene/21898).
2.6 Statistical analysis
Results for each experiment were expressed as mean ± SEM. One-way ANOVA followed by post-hoc Newman-Keuls testing was used to compare withdrawal jumping frequencies and saline treatment-induced IL-1β expression between strains. Two-way ANOVA was used to examine the effects of strain and drug administration and their interaction, followed by Bonferroni post-hoc analysis where appropriate. To avoid the interference between genetic modification and strain effects, comparisons of each result were carried out firstly between all the inbred wild-type strains (Balb/c, CBA, and C57BL/6) and then between the knockout strains in comparison to their wild-type equivalent Balb/c (TLR4 KO, MyD88 KO, and Balb/c). Association analysis was performed using the Spearman test; p < 0.05 was considered to be statistically significant. For in vitro experiments, potencies (EC50, concentration producing 50% activity) and efficacies (maximal level of activity) were determined by fitting the dose-response curves to a logistic equation (Giraldo et al., 2002).
In addition, statistical mediation analysis was performed to test the effect of morphine-induced brain nuclei IL-1β expression values on withdrawal jumping. Statistical mediation analysis is a powerful method to examine whether the effect of X (independent variable) on Y (dependent variable) may be mediated by a process or mediating variable M. The mediator M is an intervening or process variable. For the current study, this analysis evaluated if morphine treatment (X) affected withdrawal jumping (Y) via changes in brain nuclei IL-1β expression (M). Multiple mediator mediation analysis with 1000 bootstraps was conducted using the SPSS syntax script as previously described (Preacher and Hayes, 2004, 2008).
All analyzes and calculations were conducted with Excel 2007 (Microsoft, Redmond, CA, USA), Prism 5 software (GraphPad, San Diego, CA, USA), and SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA).
3. Results
3.1 In vivo results
3.1.1 Analgesic effect and tolerance
Comparison of the day 1 baseline thermal hypersensitivity between wild-type inbred strains, revealed no statistically significant difference between either strains (p = 0.36) or drug treatment groups (p = 0.07). There was also no significant difference between drug treatment groups when comparing between wild-type Balb/c and two knockout strains (p = 0.27); however, a significant strain effect on baseline thermal hypersensitivity was observed (p = 0.03). At day 3, there was no significant baseline latency change between day 1 and day 3 for both treatment groups of all strains of mice studied (p > 0.19) (Table 1).
Table 1.
No significant baseline nociceptive sensitivity change (ie hyperalgesia) occurred after chronic morphine injection in all strains.
| Strain | Baseline nociceptive sensitivity(s) a | |||
|---|---|---|---|---|
| Day 1 | Day 3 | Change(%) b | p value | |
| Balb/c | 26.3 ± 1.9 | 26.8 ± 2.6 | 1.6 | 0.90 |
| CBA | 27.6 ± 1.3 | 23.3 ± 1.7 | −15.6 | 0.07 |
| C57BL/6 | 22.2 ± 2.7 | 24.5 ± 2.1 | 9.9 | 0.53 |
| TLR4 KO | 30.1 ± 1.8 | 30.0 ± 1.6 | −0.5 | 0.94 |
| MyD88 KO | 31.0 ± 1.8 | 31.8 ± 2.1 | 2.4 | 0.20 |
Mean latency (s) ± SEM to display nociceptive behavior on the 50 °C hotplate.
Average percent reductions on day 3 relative to day 1.
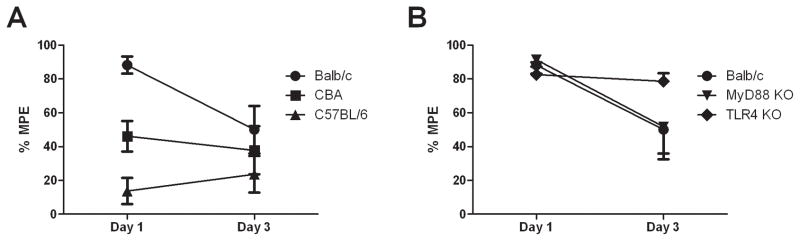
The morphine analgesic effect varied significantly between all strains studied (Figures 1A and B). For acute morphine analgesia (p < 0.0001) (day 1), Balb/c mice experienced the highest level of analgesia (88.2 ± 5.1 % MPE) while C57BL/6 experienced the least (13.7 ± 7.7 % MPE), with CBA in-between (46.2 ± 9.1 % MPE). For the knockout mice, the acute morphine analgesic responses (82.6 ± 7.3 and 91.3 ± 4.1 % MPE for TLR4 KO and MyD88 KO, respectively) were similar to their wild-type equivalents (Balb/c) (p > 0.53) and both experienced near maximal analgesic responses.
Figure 1.
Mouse strains display different morphine analgesic effects. Morphine analgesia (hotplate latency) before (day 1) and after chronic treatment (day 3) on the 50 °C hotplate test. Error bars are SEM with n = 8.
Panel A: Wild-type Balb/c mice displayed significantly greater analgesia (p <0.0001) on day 1 than CBA and C57BL/6 strains. Wild-type Balb/c mice developed significant analgesic tolerance on day 3 (p = 0.02), whilst little/no tolerance developed for CBA and C57BL/6 mice (p > 0.4).
Panel B: TLR4 KO mice were protected against the development of tolerance, whilst MyD88 KO did not protect against tolerance (p = 0.05).
At day 3, significant loss of morphine analgesic response was observed for wild-type Balb/c mice (50.0 ± 14.0 % MPE), with a mean difference (95% confidence interval (CI) of difference) between days 1 and 3 of −38 % [(−75 to −1 %), p = 0.02]; similarly in MyD88 KO mice (52 ± 19 % MPE), with a mean difference (95% CI) of −40 % [(−74 to −5 %), p = 0.05]. No significant loss of morphine analgesia was observed for the remaining strains: C57BL/6 (24 ± 11 % MPE), mean difference (95% CI) of 10 % (−27 to 47 %), p = 0.47; TLR4 KO (79 ± 4.7 % MPE), mean difference (95% CI) of −3.9 % (−41 to 33 %), p = 0.66; and CBA (38 ± 14 % MPE), mean difference (95% CI) of −8.3 % (−45 to 28 %), p = 0.63.
3.1.2 Naloxone-precipitated withdrawal
Figure 2 shows the mean number of jumps over 30 min following naloxone-precipitated withdrawal from chronic morphine treatment and saline-control groups of all five strains studied. There was no significant difference between strains in the saline-control group (p = 0.36), however, significant differences in withdrawal severity from morphine were observed among strains (p < 0.0001). Between mice of inbred wild-type strains, the C57BL/6 mice had the highest jumping scores (98 ± 15), while the CBA mice had the lowest jumping scores (5 ± 2), with Balb/c mice in-between (33 ± 6). There was no significant difference (p = 0.79) between wild-type Balb/c and the two knockout strains (TLR4 KO: 39 ± 8; MyD88 KO: 33 ± 7).
Figure 2.
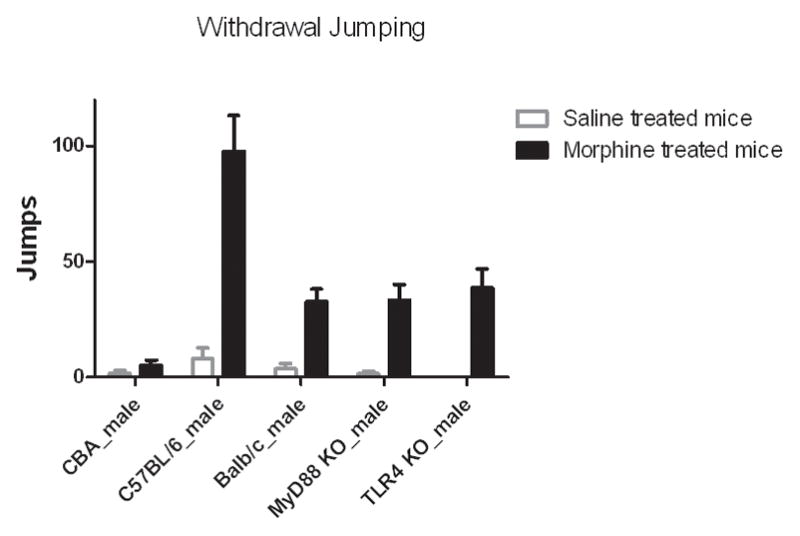
Naloxone-precipitated withdrawal varied substantially between mouse strains. C57BL/6 mice displayed the greatest naloxone-precipitated withdrawal (p < 0.0001). TLR4 KO and MyD88 KO mice experienced similar withdrawal as wild-type Balb/c mice (p = 0.79).
Data represent average jumps in 30 min period with SEM (n = 8).
3.1.3 Chronic morphine-induced IL-1β expression in brain regions
3.1.3.1 Hippocampus
Comparison between mice of inbred wild-type strains
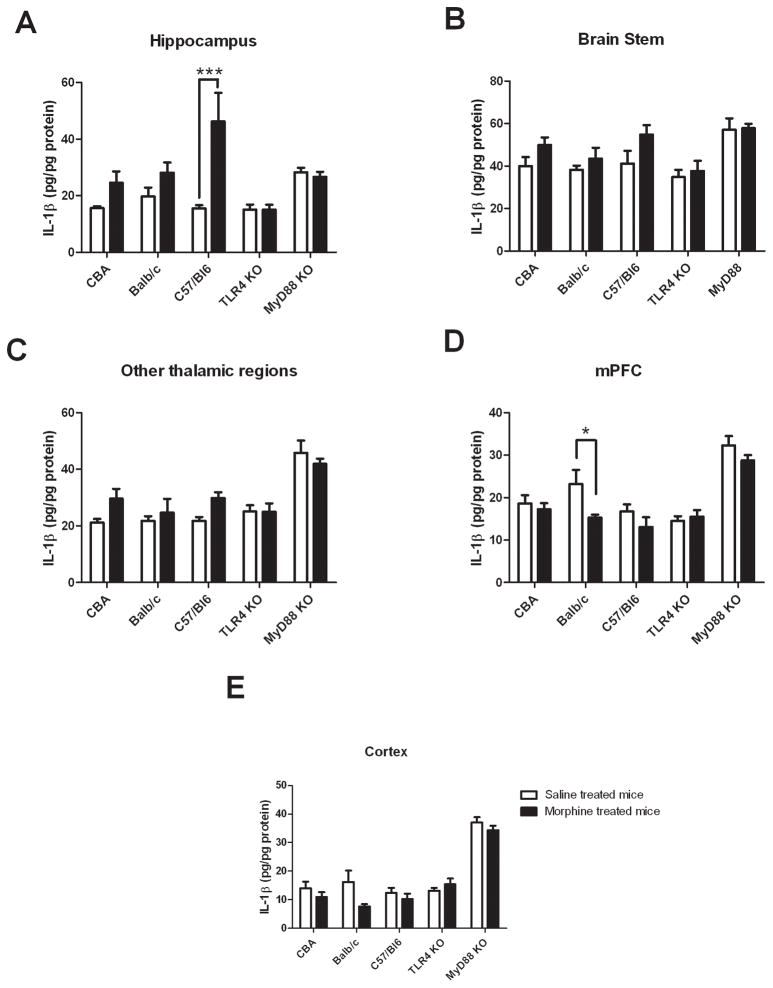
A significant drug effect (F (1, 42) = 16.4, p = 0.0002) on tissue IL-1β expression was found in the inbred wild-type strains (Figure 3A) but no significant strain effect (p = 0.09). A significant interaction between drug administration and strains was found (F (2, 42) = 3.4, p = 0.04). Bonferroni post-hoc tests revealed a significant increase in IL-1β expression values in the chronic morphine treated C57BL/6 mice compared to their saline-control group (p < 0.001), however, there were no significant differences between drug treatment groups for CBA and Balb/c strains (p > 0.2).
Figure 3.
Chronic morphine treatments alter IL-1β expression in different brain regions of mice. Values represent mean ± SEM (n = 8 per group)
Panel A: IL-1β expression in hippocampus significantly increased for wild-type C57BL/6 (p < 0.001) but not for CBA (p > 0.05) and Balb/c (p > 0.05) strains in morphine-treated group, and did not change for TLR4 KO and MyD88 KO strains between treatments (p > 0.05).
Panels B and C: IL-1β expression in brain stem (B) and midbrain plus diencephalon regions (C) - no statistically significant change in IL-1β expression between treatments for all strains (p > 0.05).
Panel D: IL-1β expression in medial prefrontal cortex (mPFC) - significantly decreased for wild-type Balb/c (p < 0.05), but not for CBA (p > 0.05), C57BL/6 (p > 0.05) and MyD88 KO (p > 0.05) mice in morphine treated group and no change for TLR4 KO mice (p > 0.05) between treatments.
Panel E: IL-1β expression in cortex - no statistically significant change of IL-1β expression between treatments for all strains (p > 0.05).
*: p < 0.05, ***: p < 0.001
The rank order of changes of IL-1β expression between the morphine treated and saline-control groups in the wild-type mice was similar to that observed in the withdrawal severity study. To examine the possible relationship between morphine-induced IL-1β expression and dependence severity, a Spearman correlation test was performed between individual mouse’s jumping score and their hippocampal IL-1β expression. A significant positive association was found (rs = 0.44, p = 0.03, Figure 4) with higher hippocampal IL-1β expression associated with greater withdrawal (jumping) score.
Figure 4.
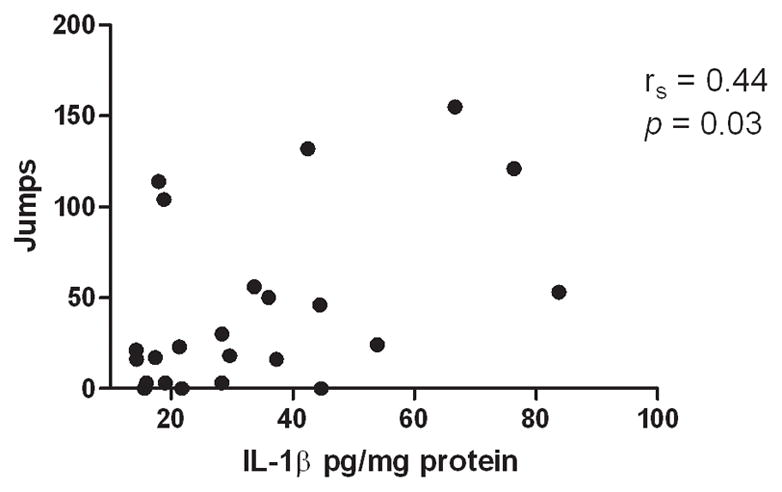
Significant association between naloxone-precipitated withdrawal jumping behavior and hippocampal IL-1β expression (rs= 0.44, p < 0.05). These data suggest higher hippocampal IL-1β expression is associated with worse withdrawal behavior.
Comparison between knockout strains and wild-type Balb/c
A significant strain effect on IL-1β expression (F (2, 42) = 10.5, p = 0.0003) was found when comparing TLR4 KO, MyD88 KO and wild-type Balb/c strains, but no significant drug effect (p = 0.34) or drug-strain interaction (p = 0.16). Bonferroni post-hoc tests revealed no significant difference between treatment groups of TLR4 KO and MyD88 KO strains (p > 0.5). For saline treatment-induced IL-1β expression of saline treated mice, significantly higher expression was observed in MyD88 KO mice compared to Balb/c and TLR4 KO strains (p < 0.05)
3.1.3.2 Brain stem
Comparison between mice of wild-type strains
For the brain stem (Figure 3B), a significant drug effect (F (1, 42) = 7.2, p = 0.01) on tissue IL-1β expression was found in the wild-type strains, but no significant strain effect ( p = 0.28) or drug-strain interaction (p = 0.64). Bonferroni post-hoc tests revealed no significant difference on IL-1β expression between morphine and saline treated mice of each wild-type strain (p > 0.1). There was no association between morphine-induced IL-1β expression and dependence severity (rs = 0.16, p = 0.46, data not shown).
Comparison between knockout strains and wild-type Balb/c
A significant strain effect on IL-1β expression F (2, 42) = 11.0, p = 0.0002) was found when comparing TLR4 KO, MyD88 KO and wild-type Balb/c strains, but no significant drug effect (p = 0.40) or drug-strain interaction (p = 0.88). Bonferroni post-hoc tests revealed no significant difference between treatment groups of TLR4 KO and MyD88 KO strains (p > 0.05). For saline treatment-induced IL-1β expression of saline treated mice, significantly higher expression was found in MyD88 KO mice compared to wild-type Balb/c and TLR4 KO strains (p < 0.05).
3.1.3.2 Midbrain and diencephalon regions
Comparison between mice of wild-type strains
For midbrain and diencephalon regions (Figure 3C), a significant drug effect (F (1, 42) = 8.2, p = 0.007) on tissue IL-1β expression was found in the wild-type strains, but no significant strain effect (p = 0.61) or drug-strain interaction (p = 0.53). Bonferroni post-hoc tests revealed no significant difference on IL-1β expression between morphine and saline treated mice of each wild-type strains (p > 0.1). There was no association between morphine-induced IL-1β expression and dependence severity (rs = 0.07, p = 0.75, data not shown).
Comparison between knockout strains and wild-type Balb/c
A significant strain effect on IL-1β expression (F (2, 42) = 17.7, p < 0.0001) was found when comparing TLR4 KO, MyD88 KO and wild-type Balb/c strains, but no significant drug effect (p = 0.90) or drug-strain interaction (p = 0.66). Bonferroni post-hoc tests revealed no significant difference between treatment groups of TLR4 KO and MyD88 KO strains (p > 0.5). For saline treatment-induced IL-1β expression of saline treated mice, significantly higher expression was observed in MyD88 KO mice compared to wild-type Balb/c and TLR4 KO strains (p < 0.05).
3.1.3.4 Medial prefrontal cortex
Comparison between mice of wild-type strains
For the mPFC region (Figure 3D), a significant drug effect (F (1, 42) = 6.4, p = 0.02) on tissue IL-1β expression was found in the wild-type strain, but no significant strain effect (p = 0.12) or drug-strain interaction (p = 0.28). Bonferroni post-hoc tests revealed a significant decrease of IL-1β expressions in the chronic morphine treated Balb/c mice compared to their saline-control group (p < 0.05), however, there were no significant differences between treatment groups of CBA and C57BL/6 strains (p > 0.5). There was no association between morphine-induced IL-1β expression change and dependence severity (rs = −0.47, p = 0.32, data not shown).
Comparison between knockout strains and wild-type Balb/c
Significant drug effect (F (2, 42) = 4.3, p = 0.05) and strain effect (F (2, 42) = 24.8, p < 0.0001) on IL-1β expression were found when comparing TLR4 KO, MyD88 KO and wild- type Balb/c strains, but no significant drug-strain interaction (p = 0.07). Bonferroni post-hoc tests revealed no significant difference between treatment groups of TLR4 KO and MyD88 KO strains (p > 0.5). For saline treatment-induced IL-1β expression of saline treated mice, significantly higher expression was found in MyD88 KO mice compared to Balb/c and TLR4 KO strains (p < 0.05).
3.1.3.5 Cortex
Comparison between mice of wild-type strains
For the cortex (Figure 3E), a significant drug effect (F (1, 42) = 6.0, p = 0.02) on tissue IL-1β expression was found in the inbred wild-type strains, but no significant strain effect (p = 0.88) or drug-strain interaction (p = 0.32). Bonferroni post-hoc tests revealed no significant difference between morphine and saline treated mice for each inbred wild-type strain (p > 0.05). There was no association between morphine-induced IL-1β expression changes and dependence severity (rs = −0.1, p = 0.38, data not shown).
Comparison between knockout strains and wild-type Balb/c
A significant strain effect on IL-1β expression (F (2, 42) = 47.4, p < 0.0001) was found when comparing TLR4 KO, MyD88 KO and wild-type Balb/c strains, but no significant drug effect (p = 0.14) or drug-strain interaction (p = 0.06). Bonferroni post-hoc tests revealed no significant difference between treatment groups of TLR4 KO and MyD88 KO strains (p > 0.5). For saline treatment-induced IL-1β expression of saline treated mice, significantly higher expression was observed in MyD88 KO mice compared to Balb/c and TLR4 KO strains (p < 0.05).
3.1.4 Statistical mediation analysis results
The analysis demonstrated that the total effect of morphine treatment on withdrawal jumping was statistically significant (coefficient = 31.5, p = 0.003). However, when controlling for the possible effect of the assigned mediator, the direct effect of morphine treatment on withdrawal jumping was no longer statistically significant (coefficient = 19.8, p = 0.09). Upon further examination of the mediator IL-1β expression in each region examined, hippocampal IL-1β expression was responsible for the significant mediation on withdrawal jumping (p = 0.03), and consequently, hippocampal IL-1β expression significantly mediates withdrawal jumping behavior.
3.2 In vitro results
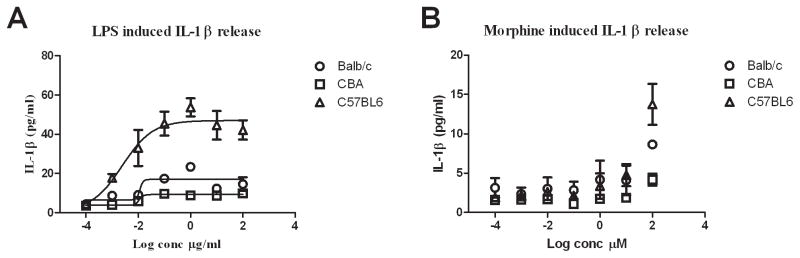
To determine the difference in immune responses among inbred wild-type strains, a wide range of concentrations of LPS (10−4 to 102 μg/ml) and morphine (10−4 to 102 μM) were used to stimulate isolated splenic leukocytes. LPS stimulation yielded clear dose-response curves for all wild-type strains (Figure 5A), with mean EC50 values of IL-1β production of 11.5 ± 0.8 ng/ml for CBA, 13.2 ± 1.8 ng/ml for Balb/c, and 4.8 ± 0.9 ng/ml for C57BL/6. There were statistically significantly different efficacies (Emax) for IL-1β production among the three strains (p < 0.05), where C57BL/6 mice had the highest maximal value (41.6 ± 3.4 pg/ml), followed by Balb/c (16.9 ± 1.4 pg/ml), and CBA had the lowest (9.4 ± 0.6 pg/ml).
Figure 5.
LPS and morphine in vitro stimulated release of IL-1β from splenic leukocytes differed between wild-type strains. Lines represent logistic fitted dose-response curve.
Panel A: LPS stimulation yielded clear dose-response curves with similar EC50 values but different efficacies between strains. C57BL/6 mice displayed the highest maximal response, whilst CBA the least, and Balb/c mice were in-between.
Panel B: Morphine stimulation did not induce IL-1β expression except at 100 μM. C57BL/6 mice displayed the highest response, whilst CBA the least, and Balb/c mice were in-between.
Following morphine exposure (Figure 5B) IL-1β expression was below the lower limit of quantification (< 4 pg/ml) at all concentrations tested except 100 μM, which caused a significant increase in IL-1β expression compared to control for C57BL/6 (13.7 ± 2.6 pg/ml) (p = 0.04) and Balb/c mice (8.6 ± 0.2 pg/ml) (p = 0.05), but no significant difference for CBA mice (4.2 ± 0.8 pg/ml, p = 0.20).
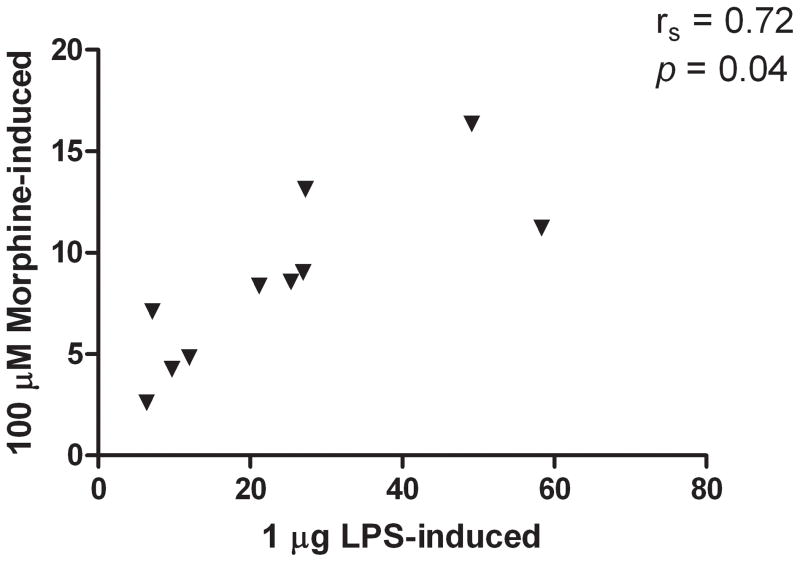
A significant association was found between 1 μg/ml LPS-induced and 100 μM morphine-induced IL-1β expression (rs = 0.72, p = 0.04, Figure 6), suggesting strains with a greater IL-1β expression in response to LPS also had a greater response to morphine stimulation.
Figure 6.
In vitro morphine- (100 μM) and LPS- (1 μg/ml) induced IL-1β expression were significantly associated (rs= 0.72, p=0.04).
3.3 IL-1B and TLR4 gene sequence and SNP analysis
Single nucleotide polymorphism (SNP) analysis results (Table 2, 3) revealed no IL-1B and TLR4 gene sequence differences between the C57BL/6 and CBA wild-type strains. However, differences in 27 SNPs on the IL-1B gene and 9 SNPs on the TLR4 gene were identified between the Balb/c and C57BL/6/CBA wild-type strains. There were no IL-1B gene sequence differences between wild-type Balb/c and its two genetic knockout (TLR4 and MyD88) strains, and no TLR4 gene sequence differences between the wild-type Balb/c and MyD88 KO mouse. However, as expected, gene sequence analysis failed to observe the TLR4 gene in the TLR4 KO strain.
Table 2.
Twenty-seven IL-1B gene SNPs were found between the wild-type Balb/c and C57BL/6/CBA strains. No IL-1B difference between the C57BL/6 and CBA strains.
| No. | SNPs a | CBA | C57BL/6 | Balb/c |
|---|---|---|---|---|
| 1 | 573 | G | G | C |
| 2 | 589 | G | G | C |
| 3 | 599 | A | A | C |
| 4 | 573 | G | G | C |
| 5 | 589 | G | G | C |
| 6 | 599 | A | A | C |
| 7 | 1368 | C | C | T |
| 8 | 1397 | A | A | G |
| 9 | 1474 | A | A | G |
| 10 | 1940 | T | T | C |
| 11 | 2011 | C | C | A |
| 12 | 3397 | T | T | C |
| 13 | 3494 | T | T | A |
| 14 | 3521 | G | G | A |
| 15 | 4185 | A | A | C |
| 16 | 4277 | A | A | G |
| 17 | 4465 | T | T | A |
| 18 | 5701 | A | A | T |
| 19 | 6287 | T | T | C |
| 20 | 6423 | A | A | G |
| 21 | 6579 | C | C | T |
| 22 | 6634 | T | T | G |
| 23 | 6648 | T | T | G |
| 24 | 6651 | G | G | A |
| 25 | 6676 | C | C | T |
| 26 | 6680 | G | G | A |
| 27 | 6681 | G | G | C |
Gene region sequenced included the IL-1B gene, and 500 bp regions up- and down-stream. SNPs positions are relevant to the flanked sequences.
Table 3.
Nine TLR4 gene SNPs were found between the wild-type Balb/c and C57BL/6/CBA strains. No TLR4 difference between the C57BL/6 and CBA strains.
| No. | SNPs a | CBA | C57BL/6 | Balb/c |
|---|---|---|---|---|
| 1 | 12289 | G | G | A |
| 2 | 12422 | G | G | A |
| 3 | 13387 | C | C | T |
| 4 | 13441 | A | A | C |
| 5 | 13819 | C | C | T |
| 6 | 13944 | G | G | A |
| 7 | 14357 | C | C | A |
| 8 | 14441 | A | A | G |
| 9 | 15427 | A | A | G |
Gene region sequenced included the TLR4 gene, and 500 bp regions up- and down-stream.
SNPs positions are relevant to the flanked sequences.
4 Discussion
This is the first study that has investigated chronic morphine-induced proinflammatory cytokine IL-1β expression changes in brain regions of different mouse strains. The data from in vivo experiments demonstrated that there are significant main effects of morphine treatment on IL-1β expression in the brain regions analyzed of inbred wild-type mice. A significant increase in hippocampal IL-1β expression was found in C57BL/6 mice after morphine treatment, whilst, a significant decrease was found in the mPFC region of wild-type Balb/c mice. Consistent with previous behavioral studies (Kest et al., 2002b), significantly different withdrawal severities (withdrawal jumping) were observed between the three inbred wild-type strains; however, there was no significant difference between wild-type Balb/c mice and its two knockout strains. Both association analysis and statistical mediation analysis revealed that the elevation of IL-1β expression in the hippocampus is associated with opioid withdrawal jumping in inbred wild-type mice. Furthermore, comparison of the nociceptive test results between wild-type Balb/c and its two knockout strains demonstrated that, in contrast to MyD88 KO and Balb/c, TLR4 KO mice did not develop analgesic tolerance, suggesting an important role for TLR4 in opioid analgesia following chronic dosing. However, since analgesic tolerance developed in the MyD88 KO, the data suggest the involvement of non-MyD88-dependent TLR4 pathways. In addition, the results from in vitro experiments demonstrated that strains with a greater LPS-induced IL-1β expression (high immune activity) also had a greater response to morphine stimulation. Finally, genetic analysis revealed no IL-1B and TLR4 genetic differences between CBA and C57BL/6 strains, but 27 and 9 SNPs on IL-1B and TLR4 genes, respectively, with wild-type Balb/c strain. Once again, it is critical to emphasize that behaviors reported here are the summation of both classical neuronal opioid receptor actions and these newly identified non-classical proinflammatory signals.
4.1 Morphine analgesic responses
Studies of morphine analgesic tolerance utilizing wild-type inbred strains have shown substantial shifts to the right in the dose-response curve of Balb/c, CBA, and C57BL/6 mice, however, there is no change in analgesic efficacy at lower morphine doses (< 10 mg/kg) for C57BL/6 and CBA mice (Bryant et al., 2006; Kest et al., 2002a; Liang et al., 2006a). In the current study, by using a single morphine challenge dose (7.5 mg/kg), only wild-type Balb/c mice exhibited analgesic tolerance, but not CBA and C57BL/6 mice, which supports previous findings.
When comparing between the wild-type Balb/c and knockout mice, no significant analgesic tolerance was observed for TLR4 KO mice, but, notably, MyD88 KO mice developed a similar degree of tolerance to wild-type Balb/c mice. The former result is consistent with findings from a previous study showing that knockout of TLR4 potentiates neuronal opioid receptor morphine analgesia in mice (Hutchinson et al., 2009). TLR4 has two signaling pathways, MyD88-dependent and -independent pathways (Bell, 2003). Toll/IL-1 receptor-domain-containing adapter-inducing interferon-β (TRIF) and interferon regulatory factor 3 (IRF3) are critical in transducing MyD88-independent pathway signaling. Our results from MyD88 KO mice indicate that MyD88-independent pathways may be involved in tolerance development and as such, TRIF and IRF3 could play a key role; however, further studies are required.
4.2 Naloxone-precipitated withdrawal
Although a different morphine dose regimen was used in the present experiment, similar withdrawal jumping severity rank order was observed between inbred wild-type strains with previous reports (Kest et al., 2002b; Metten et al., 2009).
Naloxone-induced withdrawal jumping in TLR4 KO and MyD88 KO mice has not previously been reported. Based on findings from a previous study that blocking TLR4 receptor by (+)-naloxone attenuated precipitated withdrawal in rats (Hutchinson et al., 2010), we would expect that knockout TLR4 and MyD88 would protect mice from withdrawal. However, this was not the case, as both TLR4 KO and MyD88 KO mice displayed similar degrees of withdrawal behavior as their wild-type equivalent Balb/c mice. This suggests that: firstly, there is no question that the μ-opioid receptor plays a critical role in opioid dependence; secondly, in addition to TLR4, other neuroimmune pathways are likely to contribute to opioid withdrawal. One of the possible candidates is the TLR2 pathway, as (+)-naloxone may block both TLR4 and TLR2 receptors, and recent evidence suggests that morphine can also mediate neuronal cell function through TLR2 (Li et al., 2010). Therefore, further study is required to examine the role of other pathways, such as TLR2, and opioid-TLR signaling interactions in opioid-induced withdrawal.
4.3 Chronic morphine-induced brain IL-1β expression of wild-type strains
The involvement of CNS glia and proinflammatory cytokines in opioid response (Hutchinson et al., 2008; Hutchinson et al., 2010; Johnston et al., 2004; Raghavendra et al., 2002; Shavit et al., 2005; Song and Zhao, 2001) is now well established and complements and extends the classical neuronal opioid receptor actions of opioids. Here we provide further evidence of opioid-induced proinflammatory response in the CNS by demonstrating that chronic morphine significantly affects IL-1β expression in brain regions in multiple strains of mice.
4.3.1 IL-1β expression in hippocampus
A significant main effect of morphine treatment on IL-1β expression was found for the hippocampus. This is consistent with previous observations in rats that IL-1β levels are altered in the hippocampus after chronic morphine (Hutchinson et al., 2009). IL-1β levels of morphine treated mice were significantly increased for C57BL/6 mice only. The reason why IL-1β expression was not significantly altered in Balb/c and CBA mice could be due to the lower immune response in these strains compared to C57BL/6 mice, as in vitro experiments have shown that immune cells from Balb/c and CBA mice have a lower response to morphine stimuli compared to cells from C57BL6 mice.
4.3.2 IL-1β expression in other brain regions
Although significant main effects of morphine treatment on IL-1β expression were found in other brain regions, the difference between saline and morphine treatment groups did not reach statistical significance when comparing within the same strain. Interestingly, a decrease in IL-1β expression was observed in the mPFC of wild-type Balb/c mice after chronic opioid treatment, which supports a previous study in rats that demonstrated reduced glial activation in the mPFC after chronic morphine treatment (Hutchinson et al., 2009). There is currently no rational explanation for these observations, and further studies are required to examine the functional consequences.
4.4 Chronic morphine-induced brain IL-1β expression of TLR4 KO and MyD88 KO mice
No significant IL-1β expression changes were observed in any of the regions examined in TLR4 KO mice, which suggests that without TLR4, morphine could not induce the IL-1β signal and confirms the role of TLR4 in opioid-glia activation pathway. In contrast, for MyD88 KO mice, there was also no IL-1β level change in morphine treated animals. However, significantly higher IL-1β expression than wild-type Balb/c mice within saline treatment group was observed. This may be caused by a disrupted TLR4/IL-1R pathway and lack of negative feedback; however, further investigations are required.
4.5 Relationship between brain IL-1β and opioid withdrawal
Both statistical mediation analysis and correlation analysis between individual IL-1β expression and morphine withdrawal jumping frequency revealed that hippocampal IL-1β expression is associated with withdrawal severity. Inbred wild-type mice with higher morphine-induced hippocampal IL-1β expression had more severe withdrawal jumping behavior. This finding suggests a role of IL-1β in opioid dependence. However, surprisingly, TLR4 KO or MyD88 KO mice experienced a similar degree of precipitated withdrawal to that observed with wild-type Balb/c mice. In addition, there was no significant IL-1β expression change between different treatment groups, indicating that TLR4-MyD88-IL-1β signaling pathway alone is not obligatory for withdrawal jumping behavior, emphasizing the critical role neuronal opioid receptors have in this response. Interestingly, although significantly higher IL-1β expression was found for MyD88 KO mice than wild-type Balb/c mice within the saline treatment group, no hyperactive behavior was observed for those mice. This can be explained by the critical role of MyD88 in the IL-1 signaling pathway (Weber et al., 2010). It has been shown that mice lacking MyD88 show severe defects in IL-1 signaling (Adachi et al., 1998). Therefore, the signaling efficiency of IL-1β in MyD88 KO mice is far lower, to completely absent, than that in wild-type Balb/c mice. In addition, this also indicates that IL-1β signaling alone, in the absence of opioid receptor activation, is not sufficient to induce withdrawal behaviors, since naloxone administration did not induce withdrawal jumping in the saline-treated MyD88 KO mice where IL-1β levels are already elevated. It is acknowledged that the development of opioid dependence involves complicated neuronal opioid receptor dependent and neuroimmune signaling pathways and their interactions. These data from the knockout mice suggest that even without the TLR4-MyD88-IL-1β signaling pathway, other factors such as TLR2, the MyD88-independent pathway, and other proinflammatory cytokines and chemokines alone or in combination may function to compensate to induce withdrawal behavior.
4.6 Relationship between in vivo and in vitro morphine/LPS induced IL-1β expression of different mice strains
The in vivo morphine-induced hippocampal IL-1β expression and LPS/morphine-induced in vitro immune response differences between the inbred wild-type strains confirm previous findings that immune cells from mice of different strains show significant differences in response (Gol’dberg et al., 2005; Masnaya et al., 2002). The rank order of in vitro stimuli (LPS Emax and morphine maximum) on IL-1β response was in accord with the rank order of in vivo chronic morphine-induced hippocampal IL-1β expression among three wild-type strains. This suggests an association between peripheral and CNS proinflammatory response, and that peripheral immune activity may reflect brain immune activity, at least following a TLR4 trigger. One point that we did not address in this study is the cellular origin of the brain IL-1β expression. We hypothesize that the major source of IL-1β expression is from activated glial cells, and that morphine-induced central neuroinflammation has a similar mechanism to that in the peripheral immune system. However, further investigations are required to identify specific cellular sources of inflammatory mediators and the possible similarities and differences in the signaling pathway activation between CNS and peripheral immunocompetent cells.
4.7 IL-1B/TLR4 genetic differences between strains
Differences in 27 SNPs on the IL-1B gene and 9 SNPs on the TLR4 gene were found between wild-type Balb/c and CBA/C57BL/6 strains. Currently, there is no functional information available for these SNPs. However, these SNPs did not appear to significantly influence opioid-induced IL-1β expression, since behavior and IL-1β expression values for wild-type Balb/c mice were between those of the other two wild-type strains. Consequently, further examination of the IL-1B or TLR4 sequence/structure to determine the functional consequences of these SNPs is warranted.
4.8 Utilization of multiple mouse strains
The utilization of multiple strains of mice strengthens the conclusions drawn from this study. As shown, different mouse strains had different proinflammatory responses to morphine treatment; therefore, the choice of specific strains could be a useful tool for future research. Specifically, for investigation of neuronal pathways, selection of CBA mice could minimize immune effects; while, for investigation of immune function-related mechanisms, the C57BL/6 strain would be appropriate. Furthermore, such an approach could be used as a graded inflammation model for future studies.
4.9 Limitations of current study
A limitation of the present study is that only withdrawal jumping behavior was quantitatively recorded in the experiment. During the withdrawal period, it was also observed qualitatively that TLR4 KO and MyD88 KO opioid-dependent mice appeared more alert and displayed less other withdrawal signs (such as wet dog shaking, diarrhea) than their wild-type counterparts. This suggests that differences in opioid-induced neuroinflammation may affect other withdrawal behaviors not classically recorded in mouse withdrawal studies. However, this study was not designed to record other behaviors initially, therefore, further investigation will be required. Furthermore, IL-1β expression changes in the spinal cord were not analyzed in this study. Since expression of IL-1β in spinal cord is involved in tolerance development (Shavit et al., 2005), future examination of spinal tissue IL-1β expression is necessary and may reveal whether IL-1β expression differences between strains contribute to different magnitudes of tolerance. In addition, an important question that has not been addressed in this study is, what is the role of the opioid receptor in the regulation of brain IL-1β expression. As opioid receptors are a key site of opioid action in the CNS, and previous studies have shown that β-opioid receptor facilitate proinflammatory response (Rock et al., 2006; Wetzel et al., 2000), a future study using opioid receptor KO mice is required to address this issue. Finally, only a single dose of naloxone was used to induce withdrawal signs. To more comprehensively evaluate the withdrawal behaviors, testing of various doses of naloxone may be necessary in future studies (Bohn et al., 2000).
5. Conclusion
This is the first study to demonstrate that chronic morphine alters brain proinflammatory cytokine IL-1β production in mice and that the pattern of change is brain region-specific. Comparison between multiple strains of mice showed that the elevated hippocampal IL-1β expression is associated with, but is not obligatory for withdrawal jumping behavior. All these data support the involvement of opioid-induced CNS immune signaling in dependence development. Moreover, this study demonstrated the advantage of utilization of multiple mouse strains and suggests that appropriate choice of mouse strains could boost future research outcomes.
Research Highlight.
Chronic morphine-induced hippocampal IL-1β expression is associated with withdrawal jumping, and knockout of TLR4 protects against the development of analgesic tolerance.
Acknowledgments
This work was supported by University of Adelaide Postgraduate Scholarship (L.L.), University of Adelaide Medical Endowment Funds FTT Fricker Research Fellowship (J.K.C), National Health and Medical Research Council CJ Martin Fellowship (ID 465423; M.R.H.), University of Adelaide Faculty of Health Science Project grant, NIH Grants DA015642, DA017670, DA024044, DE017782, and DE017782 and NHMRC Grant. Thanks to Dr Simon Phipps and Dr Paul Foster, University of Newcastle, Newcastle, Australia for providing the TLR4 KO and MyD88 KO mice. Thanks to the Akira Research group, Osaka University, Osaka, Japan for permission to use the knockout mice.
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Riggan J, Cross S, Young ER, Gallaher EJ, Crabbe JC. Genetic determinants of morphine activity and thermal responses in 15 inbred mouse strains. Pharmacol Biochem Behav. 1998;59:353–360. doi: 10.1016/s0091-3057(97)00421-8. [DOI] [PubMed] [Google Scholar]
- Bell E. TLR signalling. Nat Rev Immunol. 2003;3:692–692. [Google Scholar]
- Bland ST, Hutchinson MR, Maier SF, Watkins LR, Johnson KW. The glial activation inhibitor AV411 reduces morphine-induced nucleus accumbens dopamine release. Brain Behav Immun. 2009;23:492–497. doi: 10.1016/j.bbi.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- Bryant CD, Roberts KW, Byun JS, Fanselow MS, Evans CJ. Morphine analgesic tolerance in 129P3/J and 129S6/SvEv mice. Pharmacol Biochem Behav. 2006;85:769–779. doi: 10.1016/j.pbb.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo J, Vivas NM, Vila E, Badia A. Assessing the (a)symmetry of concentration-effect curves: empirical versus mechanistic models. Pharmacol Ther. 2002;95:21–45. doi: 10.1016/s0163-7258(02)00223-1. [DOI] [PubMed] [Google Scholar]
- Gol’dberg E, Masnaya N, Churin A. Immunity parameters in mice of different strains. Bull Exp Biol Med. 2005;140:219–221. doi: 10.1007/s10517-005-0450-8. [DOI] [PubMed] [Google Scholar]
- Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. Scientific World Journal. 2007;7:98–111. doi: 10.1100/tsw.2007.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Coats BD, Lewis SS, Zhang Y, Sprunger DB, Rezvani N, Baker EM, Jekich BM, Wieseler JL, Somogyi AA, Martin D, Poole S, Judd CM, Maier SF, Watkins LR. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav Immun. 2008a;22:1178–1189. doi: 10.1016/j.bbi.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Northcutt AL, Chao LW, Kearney JJ, Zhang Y, Berkelhammer DL, Loram LC, Rozeske RR, Bland ST, Maier SF, Gleeson TT, Watkins LR. Minocycline suppresses morphine-induced respiratory depression, suppresses morphine-induced reward, and enhances systemic morphine-induced analgesia. Brain Behav Immun. 2008b;22:1248–1256. doi: 10.1016/j.bbi.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Lewis SS, Coats BD, Skyba DA, Crysdale NY, Berkelhammer DL, Brzeski A, Northcutt A, Vietz CM, Judd CM, Maier SF, Watkins LR, Johnson KW. Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast) Brain Behav Immun. 2009;23:240–250. doi: 10.1016/j.bbi.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler J, Hughes TS, Landgraf KE, Chan S, Fong S, Phipps S, Falke JJ, Leinwand LA, Maier SF, Yin H, Rice KC, Watkins LR. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010;24:83–95. doi: 10.1016/j.bbi.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, Langer S, Martin D, Green P, Fleshner M, Leinwand L, Maier SF, Watkins LR. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci. 2004;24:7353–7365. doi: 10.1523/JNEUROSCI.1850-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kest B, Hopkins E, Palmese CA, Adler M, Mogil JS. Genetic variation in morphine analgesic tolerance: a survey of 11 inbred mouse strains. Pharmacol Biochem Behav. 2002a;73:821–828. doi: 10.1016/s0091-3057(02)00908-5. [DOI] [PubMed] [Google Scholar]
- Kest B, Palmese CA, Hopkins E, Adler M, Juni A, Mogil JS. Naloxone-precipitated withdrawal jumping in 11 inbred mouse strains: evidence for common genetic mechanisms in acute and chronic morphine physical dependence. Neuroscience. 2002b;115:463–469. doi: 10.1016/s0306-4522(02)00458-x. [DOI] [PubMed] [Google Scholar]
- Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13–20. doi: 10.1151/spp021113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li H, Zhang Y, Sun X, Hanley GA, LeSage G, Zhang Y, Sun S, Peng Y, Yin D. Toll-like receptor 2 is required for opioids-induced neuronal apoptosis. Biochem Biophys Res Commun. 2010;391:426–430. doi: 10.1016/j.bbrc.2009.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang DY, Guo T, Liao G, Kingery WS, Peltz G, Clark JD. Chronic pain and genetic background interact and influence opioid analgesia, tolerance, and physical dependence. Pain. 2006a;121:232–240. doi: 10.1016/j.pain.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Liang DY, Liao G, Wang J, Usuka J, Guo Y, Peltz G, Clark JD. A genetic analysis of opioid-induced hyperalgesia in mice. Anesthesiology. 2006b;104:1054–1062. doi: 10.1097/00000542-200605000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Hutchinson MR, White JM, Somogyi AA, Coller JK. Association of IL-1B genetic polymorphisms with an increased risk of opioid and alcohol dependence. Pharmacogenet Genomics. 2009;19:869–876. doi: 10.1097/FPC.0b013e328331e68f. [DOI] [PubMed] [Google Scholar]
- Masnaya NV, Churin AA, Borsuk OS, Sherstoboev EY. Immune reactions in different mouse strains. Bull Exp Biol Med. 2002;134:376–378. doi: 10.1023/a:1021916500286. [DOI] [PubMed] [Google Scholar]
- Metten P, Crabbe JC, Belknap JK. Genetic correlates of morphine withdrawal in 14 inbred mouse strains. Drug Alcohol Depend. 2009;99:123–131. doi: 10.1016/j.drugalcdep.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachum R, Shanbrom E. Rapid detection of Gram-negative bacteriuria by Limulus amoebocyte lysate assay. J Clin Microbiol. 1981;13:158–162. doi: 10.1128/jcm.13.1.158-162.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Miyatake M, Shibasaki M, Shindo K, Nakamura A, Kuzumaki N, Nagumo Y, Suzuki T. Direct evidence of astrocytic modulation in the development of rewarding effects induced by drugs of abuse. Neuropsychopharmacology. 2006;31:2476–2488. doi: 10.1038/sj.npp.1301007. [DOI] [PubMed] [Google Scholar]
- Narita M, Suzuki M, Kuzumaki N, Miyatake M, Suzuki T. Implication of activated astrocytes in the development of drug dependence. Ann N Y Acad Sci. 2008;1141:96–104. doi: 10.1196/annals.1441.032. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Rutkowski MD, DeLeo JA. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J Neurosci. 2002;22:9980–9989. doi: 10.1523/JNEUROSCI.22-22-09980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavit Y, Wolf G, Goshen I, Livshits D, Yirmiya R. Interleukin-1 antagonizes morphine analgesia and underlies morphine tolerance. Pain. 2005;115:50–59. doi: 10.1016/j.pain.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Song P, Zhao ZQ. The involvement of glial cells in the development of morphine tolerance. Neurosci Res. 2001;39:281–286. doi: 10.1016/s0168-0102(00)00226-1. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Johnston IN, Maier SF. Glia: novel counter-regulators of opioid analgesia. Trends Neurosci. 2005;28:661–669. doi: 10.1016/j.tins.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Glia as the “bad guys”: implications for improving clinical pain control and the clinical utility of opioids. Brain Behav Immun. 2007;21:131–146. doi: 10.1016/j.bbi.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Rice KC, Maier SF. The “toll” of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol Sci. 2009;30:581–591. doi: 10.1016/j.tips.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A, Wasiliew P, Kracht M. Interleukin-1 (IL-1) pathway. Sci Signal. 2010;3:cm1. doi: 10.1126/scisignal.3105cm1. [DOI] [PubMed] [Google Scholar]