SUMMARY
Rust fungi cause devastating diseases on many important food crops, with a damaging stem rust epidemic currently affecting wheat production in Africa and the Middle East. These parasitic fungi propagate exclusively on plants, precluding the use of many biotechnological tools available for other culturable fungi. In particular the lack of a stable transformation system has been an impediment to the genetic manipulation required for molecular analysis of rust pathogenicity. We have developed an Agrobacterium-mediated genetic transformation procedure for the model flax rust fungus Melampsora lini, which infects flax (Linum usitatissimum). Selection of transgenic rust lines is based on silencing of AvrL567, which encodes a rust effector protein that is recognised by the flax L6 immune receptor. The non-transgenic rust line is unable to infect flax plants expressing L6, while silenced transgenic lines are virulent on these plants, providing an effective selection system. This directly confirms that the cloned AvrL567 gene is responsible for flax rust virulence phenotypes, and demonstrates the utility of this system to probe rust gene function.
Keywords: rust, transformation, avirulence, effector, gene silencing, Agrobacterium
INTRODUCTION
Rust fungi (basidiomycetes of the order Uredinales) are a diverse group, with over 7000 species known to cause disease on many different plant host species. They are particularly important pathogens of wheat, with the recent emergence of a new strain of wheat stem rust (Puccinia graminis tritici strain TTKS or Ug99) in East Africa posing a serious threat to global wheat production (Singh et al., 2008). During infection, these pathogens produce specialised infection structures, known as haustoria, which penetrate the host cell wall and are the primary site of nutrient extraction from the host (Voegele and Mendgen, 2003). These structures also deliver effector proteins into the host cell which may facilitate infection and can be recognised by host immune receptors (Dodds et al., 2004, 2006; Catanzariti et al., 2006). Draft genome sequences for two rust species (wheat stem rust and poplar rust, Melampsora larici-populina) have been developed and contain many genes with potential pathogenicity related functions. However, analysis of the functions of these genes in disease requires the development of a reliable stable genetic transformation system to allow manipulation of gene expression in transgenic rust lines.
The interaction between the flax rust fungus, Melampsora lini, and its host plant flax has served as an important model for understanding the plant innate immunity system (Lawrence et al., 2007; Dodds and Thrall, 2009). Flax immune receptors (of the nucleotide binding-leucine rich repeat class) are encoded by highly polymorphic resistance genes, and recognise rust effector proteins that are delivered into flax cells during infection. This triggers a hypersensitive response which involves localised cell death that prevents fungal development (Lawrence et al., 2007). For instance the L6 resistance gene encodes a receptor that recognises members of the AvrL567 rust effector family and triggers host immunity to infection. Transient expression of AvrL567 proteins in L6 plants induces a strong defence response and yeast two-hybrid assays indicate a direct interaction between these proteins (Dodds et al., 2004, 2006). Rust strains that carry an unrecognised variant of AvrL567 can cause disease on host plants containing the L6 resistance gene. However direct evidence that AvrL567 controls the rust avirulence phenotype is not yet available, due to the absence of genetic manipulation techniques for flax rust. We have used the strong resistance response triggered by the AvrL567–L6 interaction as the basis of a selection mechanism to develop a transformation system for flax rust and to confirm the role of AvrL567 in rust avirulence.
Species of fungi that can be cultivated on artificial media are usually amenable to transformation by CaCl2/polyethylene glycol or electroporation-induced DNA uptake into protoplasts or by particle bombardment or Agrobacterium tumefaciens-mediated DNA delivery, with antibiotic resistance or complementation of an auxotrophic mutation usually used to select transgenic cells (Weld et al., 2006). However, obligate biotrophic fungal pathogens, such as rusts, either cannot, or cannot easily, be grown on artificial media, so alternative transformation protocols must be devised. Although transient transformation of rust fungi by particle bombardment has been reported (Webb et al., 2006), stable transformation has remained elusive. We have developed a transformation procedure based on A. tumefaciens delivery of T-DNA into rust hyphae growing in stems of flax plants. The T-DNA contained a hairpin construct designed to silence AvrL567 in the rust and allow subsequent selection of transgenic individuals by inoculation onto flax plants possessing the L6 resistance gene.
RESULTS AND DISCUSSION
The main infection stage of the flax rust life cycle involves dikaryotic (binucleate) urediospores. As flax rust strains heterozygous for avirulence genes can give rise to spontaneous virulent mutants due to mutation or deletion of their single avirulence gene at significant frequencies (Lawrence, 1977), we chose as the T-DNA recipient rust strain CH5–89. This strain is homozygous for a haplotype of the AvrL567 locus with two adjacent genes, AvrL567-A and -B, whose products are both recognised by L6 (Dodds et al., 2004). A binary T-DNA vector (siAvrL567) was designed that contained a silencing construct encoding a 480-bp hairpin RNA that included most of the AvrL567-A coding region, expressed under its own promoter (Figure 1a). An A. tumefaciens strain carrying this binary T-DNA vector was introduced into the stems of Hoshangabad plants (no resistance genes) that had been inoculated 5 days previously with strain CH5–89. Access of the bacteria into the infected stems was achieved by extensively puncturing the stems while immersed in a suspension of the Agrobacterium (Figure S1a). Urediospores were collected from the stems 9 days after Agrobacterium infiltration with two subsequent collections made at 4- or 5-day intervals (Figure S1b). In two separate experiments, a total of 15 pots of rust-infected flax stems were infiltrated with Agrobacterium cultures containing the siAvrL567 construct. Urediospore production from the stems in each pot varied due to differences in initial infection rates and because stem puncture sometimes resulted in the death of the stem or the formation of regions of scar tissue from which no spores were produced. Only spores collected from the six highest yielding pots (three from each experiment) were screened for putative transgenics. Of the three spore collections made from each pot, the first yielded the least amount of spores. Therefore spores from either the second or third collections were used in the screening experiment.
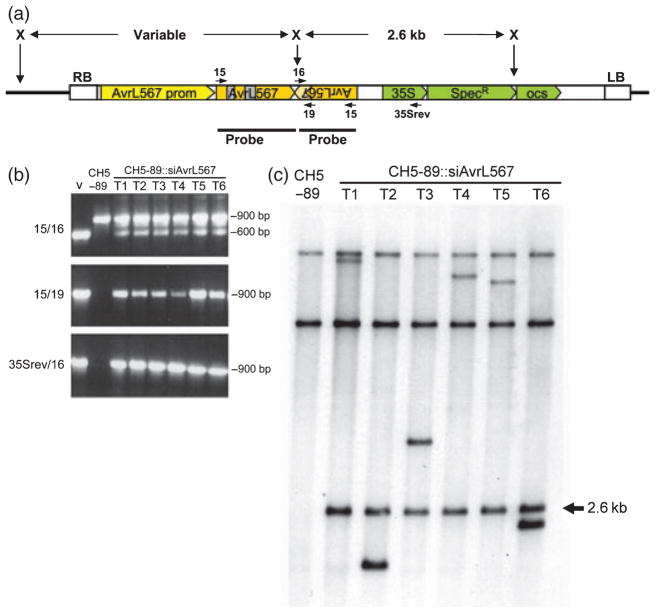
Figure 1. Analysis of transgenic flax rust isolates.
(a) Schematic representation (not to scale) of the siAvrL567 T-DNA construct used to transform flax rust strain CH5–89. The AvrL567 promoter region (yellow) drives expression of the inverted repeat AvrL567-A transcript (orange). Two intron sequences are shown in grey, while a 120-bp non-repeated region is hatched orange. The T-DNA also contains a spectinomycin resistance gene (SpecR) under the control of the 35S promoter and OCS terminator sequences (green). The left (LB) and right (RB) borders of the T-DNA and internal XbaI (X) restriction sites are indicated, as is the location of the AvrL567 probe. The location on the T-DNA of oligonucleotide primers 10-1.15, 10-1.16, 10-1.19 and 35Srev are indicated by small arrows.
(b) Genomic DNA of non-transgenic flax rust strain CH5–89 and six transgenic (T1–T6) isolates, each from a separate pot, was amplified by PCR with primers directed to the siAvrL567 T-DNA construct (v). The primer pair 10–1.15/10–1.16 (15/16) amplifies a 600-bp fragment from the T-DNA and a 900-bp fragment from genomic DNA, while the 10-1.15/10-1.19 (15/19) and 35Srev/10-1.16 (35Srev/16) primer pairs amplify 900-bp fragments from the T-DNA only.
(c) Genomic DNA of non-transgenic flax rust strain CH5–89 and six transgenic (T1–T6) isolates, each from a separate pot, was digested with the restriction enzyme XbaI, separated by agarose gel electrophoresis, transferred to nylon membranes and hybridised with a probe derived from the coding region of AvrL567-A. This probe detects two fragments in CH5–89 derived from the endogenous AvrL567 locus. In the transgenic lines the probe hybridises additionally to a 2.6-kb fragment originating from within the T-DNA and to a single T-DNA–fungal DNA junction fragment of variable size, indicating that each of the transgenic isolates contains a single T-DNA insert.
Urediospores collected from the six high-yielding pots were inoculated separately onto flax plants of the line H3 × Birio, which possesses the L6 resistance gene. Each of these inoculations gave rise to between 18 and 69 virulent pustules (Table 1, Figure S1c) which were considered as putative transgenic lines. Ten pustules originating from the pot 1 collection and two from each of the other five pot collections were multiplied by inoculation onto the susceptible flax line Hoshangabad for two infection cycles. PCR amplification of six isolates, one from each pot and therefore representing independent events, showed that all six harboured the T-DNA construct (Figure 1b). Subsequently, DNA gel blot analysis was used to confirm the presence of an integrated T-DNA. Genomic DNA was digested with XbaI and DNA gel blots were hybridised with an AvrL567-derived probe (Figures 1c and S2a and data not shown). This probe detects two fragments in the untransformed CH5–89 strain, which correspond to the endogenous AvrL567-A and -B genes. All 20 of the virulent isolates contained these two bands, indicating that they still contained undisrupted copies of the endogenous AvrL567-A and -B genes, but they also contained additional fragments corresponding to the introduced T-DNA. These included a single internal T-DNA fragment of 2.6 kb and an external T-DNA-fungal DNA junction fragment whose size varies depending on the distance between the right hand border of the T-DNA and the first XbaI site in the adjacent fungal DNA (Figure 1a). A probe derived from the 35S promoter of the spectinomycin resistance gene cassette hybridised only to the 2.6 kb internal fragment in the transgenic rust strains and did not detect any bands in the untransformed parent line (Figure S2b). Nineteen isolates contained a single junction fragment, indicating the presence of one copy of the T-DNA inserted in the fungal genome, while one isolate contained two copies of the T-DNA (Figure S2, lane pot 1-1). Based on the junction fragment lengths the ten virulent isolates from pot 1 were derived from eight different transgenic events (there were two pairs in which each member of the pair had identical band patterns) while the two isolates tested from each of the other five pots were, in all cases, derived from different transgenic events (Table 1). The isolates with identical fragments are likely to have originated from urediospores that came from the same transgenic sporogenous cell. In the process of urediospore formation, hyphae orientate towards the surface and, after the formation and release of peridial and intercalary cells, produce terminal sporogenous cells which bud off twin cells, one of which develops into a pedicel and the other into an urediospore (Lawrence et al., 2007). As sporogenous cells can bud off a succession of urediospores a transformed sporogenous cell could produce a number of urediospores possessing the same transgenic event. The maintenance of the T-DNA insertions after two asexual generations on a non-selective (no L6 gene) host line indicates stable incorporation of the T-DNA in the original urediospore from which each virulent isolate was derived. The relatively high numbers of putative transformants isolated, and the lack of any apparent untransformed escapes (contaminating spores or spontaneous mutants), suggests that ample transgenic isolates for most investigations could be obtained from transformations performed on ten to fifteen infected stems (two or three pots).
Table 1.
Characterisation of virulent pustules (putative transgenics) recovered from screening flax rust urediospores of strain CH5–89 collected from Agrobacterium-infiltrated flax stems in each of six pots on H3 × Birio (L6) plants
| Spore collection | Number of pustules virulent on H3 × Birio | Number tested by Southern analysis | Number transformed | Number of transgenic events |
|---|---|---|---|---|
| Pot 1 | 23 | 10 | 10 | 8 |
| Pot 2 | 45 | 2 | 2 | 2 |
| Pot 3 | 18 | 2 | 2 | 2 |
| Pot 4 | 48 | 2 | 2 | 2 |
| Pot 5 | 20 | 2 | 2 | 2 |
| Pot 6 | 69 | 2 | 2 | 2 |
Three independent transgenic isolates were inoculated onto a set of 32 flax differential lines containing all known rust resistance genes, to test their virulence phenotypes. In each case, the transgenic lines showed an identical phenotype to the non-transgenic CH5–89, except on lines containing the L5 (Wilden), L6 (Birio) or L7 (Barnes) resistance genes which recognise AvrL567 (Table 2). Whereas the non-transgenic strain is completely avirulent on Wilden and Birio and slightly less than fully virulent on Barnes, the three transgenics were fully virulent on all three of these differential lines. The non-transgenic CH5–89 is not avirulent on Barnes (L7) because CH5–89 contains an inhibitor gene (I) that suppresses the L7–AvrL567 interaction resulting in a slightly less than fully virulent reaction (Lawrence et al., 1981). These results confirm that the transgenic lines are derived from CH5–89, as no other rust strain in our collection displays this pattern of virulence phenotypes. The recovery of transgenic isolates virulent on L5, L6 and L7 suggests that the transgene is triggering an RNAi mechanism leading to a reduction in RNA transcript levels of AvrL567-A and -B. To test whether these genes were indeed silenced, RNA was extracted from Hoshangabad plants infected with non-transgenic CH5–89 and the three transgenic isolates. RNA gel blot analysis detected expression of the rust tubulin gene in all samples, while AvrL567 transcripts were detected in CH5–89, but not in the transgenic lines (Figure 2). The observed high level of silencing in all three transgenic lines probably reflects the strong selection applied by the requirement for growth on plants with the L6 resistance gene – i.e. transgenics with only partial silencing of AvrL567 may not come through the selection screen.
Table 2.
Infection reaction types of 32 flax differential lines to CH5–89 and transgenic rust isolatesa
| Flax lines | R gene | CH5–89 | Rust strains
|
||
|---|---|---|---|---|---|
|
CH5–89::siAvrL567 | |||||
| T1 | T3 | T4 | |||
| Clay | K | + | + | + | + |
| Raja | K1 | +(+/−) | + | + | + |
| Ottawa | L | −− | −− | −− | −− |
| B14 × Burke | L1 | + | + | + | + |
| Stewart | L2 | + | + | + | + |
| P.B.C. | L3 | + | + | + | + |
| Kenya | L4 | − | −(−−) | −(+/−) | −(+/−) |
| Wilden | L5 | −− | + | + | + |
| Birio | L6 | −− | + | + | + |
| Barnes | L7 | +(+/−) | + | + | + |
| B13 × Towner | L8 | + | + | + | + |
| Bison | L9 | + | + | + | + |
| Bolley Golden selection | L10 | + | + | + | + |
| Linore | L11 | + | + | + | + |
| B6 × Kugler C | L12 | −− | −− | −− | −− |
| Lx | Lx | + | + | + | + |
| RL10–1 | RL10 | + | + | + | + |
| Dakota | M | −− | −− | −− | −− |
| Williston Brown | M1 | + | + | + | + |
| Ward | M2 | + | + | + | + |
| Cass | M3 | −− | −− | −− | −− |
| Victory ‘A’ | M4 | −− | −(−−) | −− | − |
| Cortland | M5 | +/− | +/− | +/− | +/− |
| C.I. 2008 | M6 | −− | − | − | − |
| Bombay | N | + | + | + | + |
| Polk | N1 | −− | −− | −− | −− |
| Marshall | N2 | − | −− | −− | −− |
| Koto | P | + | + | + | + |
| Akmolinsk | P1 | −− | −− | −− | −− |
| Abyssinian | P2 | −− | −− | −− | −− |
| Leona | P3 | −− | −− | −− | −− |
| C.I. 1911 | P4 | + | + | + | + |
Reaction types were scored on a four point scale according to Lawrence et al. (1981), ranging from a fully virulent reaction with many large pustules (+) through restricted growth (+/−) and (−) to no growth (−−). Reaction types intermediate between two scores were scored as −(+/−) or +(+/−) etc.
Figure 2. RNA gel blot analysis of transgenic rust lines.
RNA extracted from flax leaves heavily infected with non-transgenic flax rust strain CH5–89 and three transgenic (T1, T3, T4) isolates was separated by gel electrophoresis, transferred to nylon membranes and hybridised with probes derived from AvrL567-A or from the flax rust tubulin gene.
These results confirm that the AvrL567 genes in flax rust are indeed responsible for the conferring the avirulence phenotype on plants containing L5, L6 or L7 resistance genes. Previously this was inferred from the genetic mapping, in-planta expression and yeast two-hybrid protein interaction studies (Dodds et al., 2004, 2006). The AvrL567 protein is presumed to have some effector function in promoting infection in the absence of host resistance genes. However, the AvrL567-silenced transgenic rust lines appear to be just as vigorous with regard to growth rate and urediospore production as the non-transgenic CH5–89 strain when grown on the susceptible flax variety Hoshangabad. Thus, any effector role of AvrL567 does not appear to be essential for flax rust pathogenicity, at least under the environmental conditions employed. This may be due to redundancy in the flax rust effector repertoire, with another protein(s) substituting for the missing AvrL567 function, or may indicate that its function is of only minor consequence for the rust with any subtle effects on urediospore production difficult to quantify. It is also possible that AvrL567 proteins may have an important role in the sexual stage of the rust life cycle.
From the work reported above it is evident that A. tumefaciens can transform cells in flax rust hyphae growing in the stems of flax plants, with the transferred T-DNA being stably integrated into the rust genome and inherited through successive asexual generations. Furthermore, expression of a hairpin RNA triggers a robust gene silencing mechanism that can suppress expression of a rust gene target. These are critical tools for the analysis of rust gene function because they now allow the delivery of novel gene variants, including for instance epitope or fluorescently tagged proteins for localisation or protein interaction analyses. This may require the development of gene expression constructs using homologous promoter and terminator sequences. Robust gene silencing will also allow the targeted ablation of specific gene functions, which would be difficult to achieve by targeted gene knockouts or insertional mutation, as the dikaryotic nature of the rust would require passage through a sexual phase to obtain homozygous strains. Two key factors in this selection system were firstly the use of a rust strain homozygous for the Avr gene silencing target to minimise the isolation of rust pustules that had become virulent due to mutation of the target gene, and therefore reduce the need to screen out these potential contaminants, and secondly the very robust resistance selection afforded by the L6–AvrL567 interaction, which completely prevents any pustule development by the parent rust strain. While the current selection system based on AvrL567 silencing will allow delivery of other genes of interest into flax rust, it does not permit delivery of modified AvrL567 genes. However this could be overcome by developing a selection screen based on the silencing of other cloned avirulence genes in flax rust, such as AvrM, AvrP4 and AvrP123 (Catanzariti et al., 2006), provided that, like AvrL567, they are not essential for rust viability. Our results demonstrate the feasibility of Agrobacterium- mediated rust transformation coupled with selection for a silencing-induced virulence phenotype, which should be adaptable to other rust species including the agronomically important cereal rusts. Many avirulence genes have been genetically defined in wheat stem rust for instance (Zambino et al., 2000; Leonard and Szabo, 2005) and if cloned would serve as gene silencing targets allowing efficient selection for transgenic urediospores by screening on a wheat line carrying the corresponding resistance gene. The demonstration that A. tumefaciens can deliver T-DNA to rust hyphae in infected plants, should also allow the development of alternative selection strategies for rust transformation based on fungicide or herbicide resistance (Anderson and Kolmer, 2005; Feng et al., 2005).
EXPERIMENTAL PROCEDURES
Plant and rust materials
The flax line Birio possesses the L6 resistance gene (Flor, 1956) while the Hoshangabad line contains no known rust resistance genes (Mayo and Shepherd, 1980). The line H3 × Birio, homozygous for L6, was selected amongst the selfed progeny of a second backcross line from the cross [(Birio × Hoshangabad) × Hoshangabad] × Hoshangabad. A set of 32 differential flax lines containing different R genes includes a standard set described by Islam and Mayo (1990) with the addition of two lines containing the recombinant L alleles Lx and RL10 (Luck et al., 2000). Rust strain CH5–89 is an F2 individual from a cross between rust strains C and H (Lawrence et al., 1981), and is homozygous for the AvrL567 haplotype conferring avirulence to L5, L6 and L7 (Dodds et al., 2004). A stock of CH5–89, free of any contaminant spores that might be virulent to L6, was obtained by successively growing the strain on host lines possessing the N, P4, L2 and P resistance genes, while growing no other rust strain. Strain CH5– 89 is the only strain held at this laboratory that is virulent to all four of these resistance genes.
Silencing construct
A silencing construct (siAvrL567) encoding a hairpin RNA was generated in the pTNotTReg T-DNA binary vector (Dodds et al., 2001) (Figure 1a). It consists of a 2.25-kb fragment amplified from flax rust genomic DNA with primers 10–1.28 (GGATGTTCGAGGTAGTGTC) and 10–1.17 (CTTCAATTGTACGGCGAGTC) which contains the AvrL567 promoter (1.5 kbp) and most (750 bp) of the transcribed gene (120 bp at the 3′ end was deleted), including two small introns near its 5′ end. This fragment was fused to an inverted cDNA copy (600 bp) of the same gene amplified with 10–1.15 (AAGCTTGAGAGCTCCGCTC) and 10–1.16 (TAATCCTCGTTGACATCAGTC) primers. Thus the predicted transcript contains a 480-bp inverted repeat separated by a 120-bp linker derived from the 3′ end of the gene. The T-DNA also contained a gene conferring resistance to spectinomycin driven by the 35S promoter.
Transformation protocol
Hoshangabad plants (four or five per pot) were grown in a line in 15 cm pots and kept upright by dowelling and wire supports to ensure straight stems. When approximately 45 cm tall, side stems were removed from each plant and the main stems, stripped of leaves except for the top 5 cm, were inoculated with rust strain CH5– 89 using a small camel’s hair brush dipped in urediospores dispersed on the surface of a few ml of water in a small petri dish. After inoculation plants were sprayed with a fine mist of water and kept at high humidity and below 24°C overnight in large plastic bins before removal to a glasshouse. A. tumefaciens strain GV3101 pMP90 containing the T-DNA silencing construct was grown overnight at 28°C in LB containing 50 μg ml−1 gentamycin and 10 μg ml−1 tetracycline. Acetosyringone (200 μM) was added and cultures grown for a further 5–6 h before harvesting cells by centrifugation (3500 g, Jouan C412 benchtop centrifuge). Agrobacterium cells were resuspended in an equal volume of 5% sucrose containing 200 μM acetosyringone and were introduced into the infected stems 5 days after rust inoculation. To introduce the Agrobacterium into the rust-infected stems, a pot with the infected stems was laid horizontally with one stem lying across the top of a file brush (110 × 50 mm; Figure S1a) immersed in the Agrobacterium suspension in a plastic dish (120 × 80 × 15 mm high). The stem was then punctured by pressing down with both thumbs and rolling the stem across the wire bristles of the brush to puncture all sides of the stem. This procedure was then repeated until the full length of each stem in the pot had been punctured. Plants were kept under high humidity at approximately 23°C for 2 days before removal to a glasshouse. Urediospores were collected from the stems in each pot 9 days after Agrobacterium treatment and stored at 4°C in sealed glass tubes under vacuum. Second and third collections were made at 4- or 5-day intervals.
To screen for transgenic rust spores virulent to L6, H3 × Birio plants were planted in 10 × 10 cm pots (nine plants per pot) and the primary shoot was cut off when a few cm high to promote the side shoot growth to increase leaf area. Spores from one collection (approximately 20–30 mg) were mixed with 1.5 g talc and dusted over 24 pots of H3 × Birio plants (approximately 12 cm tall) and kept at high humidity overnight. Plants were examined for virulent pustules 12–14 days after inoculation. Stems without pustules were removed and pots with pustules separated from each other. Spores collected from single pustules were put through two rounds of increase on Hoshangabad plants to obtain sufficient urediospores for DNA extraction.
DNA extraction, southern analysis, RNA extraction and northern analysis
Rust spores (800–1000 mg) were dispersed over water in two trays (52 × 36 cm) and incubated overnight below 23°C and DNA was extracted from the mat of germinated spores as described (Lawrence et al., 2010). For PCR analysis, genomic DNA was amplified with combinations of primers 10-1.15, 10-1.16, 10-1.19 (CCAGACTACATCAAAATCAAG) and 35Srev (ATTGGCCGTCCGCTCTAC). For gel blot analysis, genomic DNA was digested with restriction enzyme Xba1, separated on 1.0% agarose gels, transferred to Hybond N+ nylon membranes and hybridised with a 32P-dCTP- labelled 10–1.15/16 PCR fragment of AvrL567 as described (Lawrence et al., 2010). RNA was extracted from 1 g of infected flax leaves as described (Jones et al., 1985), separated on 1.5% agarose gels and transferred to Hybond N+ nylon membranes. RNA blots were hybridized with either the 10–1.15/16 AvrL567 fragment or a fragment from the flax rust tubulin gene (Ayliffe et al., 2001) as described (Catanzariti et al., 2006).
Supplementary Material
Figure S1. Rust transformation procedure.
Figure S2. DNA gel blot analysis of transgenic flax rust isolates.
Acknowledgments
Excellent technical assistance was provided by Patricia Moore and Kim Newell. This work was funded by CSIRO and the Australian Grains Research and Development Corporation grant CSP00017.
References
- Anderson JA, Kolmer JA. Rust control in glyphosate tolerant wheat following application of the herbicide glyphosate. Plant Dis. 2005;89:1136–1142. doi: 10.1094/PD-89-1136. [DOI] [PubMed] [Google Scholar]
- Ayliffe MA, Dodds PN, Lawrence GJ. Characterisation of a β-tubulin gene from Melampsora lini and comparison of fungal β-tubulin genes. Mycol Res. 2001;105:818–826. [Google Scholar]
- Catanzariti AM, Dodds PN, Lawrence GJ, Ayliffe MA, Ellis JG. Haustorially expressed secreted proteins from flax rust are highly enriched for avirulence elicitors. Plant Cell. 2006;18:243–256. doi: 10.1105/tpc.105.035980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds P, Thrall P. Recognition events and host–pathogen co-evolution in gene-for-gene resistance to flax rust. Funct Plant Biol. 2009;36:395–408. doi: 10.1071/FP08320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds PN, Lawrence GJ, Ellis JG. Six amino acid changes confined to the leucine-rich repeat beta-strand/beta-turn motif determine the difference between the P and P2 rust resistance specificities in flax. Plant Cell. 2001;13:163–178. doi: 10.1105/tpc.13.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds PN, Lawrence GJ, Catanzariti AM, Ayliffe MA, Ellis JG. The Melampsora lini AvrL567 avirulence genes are expressed in haustoria and their products are recognized inside plant cells. Plant Cell. 2004;16:755–768. doi: 10.1105/tpc.020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds PN, Lawrence GJ, Catanzariti AM, Teh T, Wang CI, Ayliffe MA, Kobe B, Ellis JG. Direct protein interaction underlies gene-for-gene specificity and coevolution of the flax resistance genes and flax rust avirulence genes. Proc Natl Acad Sci USA. 2006;103:8888–8893. doi: 10.1073/pnas.0602577103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng PCC, Baley GJ, Clinton WP, Bunkers GJ, Alibhai MF, Paulitz TC, Kidwell KK. Glyphosate inhibits rust diseases in glyphosate- resistant wheat and soybean. Proc Natl Acad Sci USA. 2005;102:17290–17295. doi: 10.1073/pnas.0508873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor HH. The complementary genic systems in flax and flax rust. Adv Genet. 1956;8:29–54. [Google Scholar]
- Islam MR, Mayo GME. A compendium on host genes in flax conferring resistance to flax rust. Plant Breed. 1990;104:89–100. [Google Scholar]
- Jones JDG, Dunsmuir P, Bedbrook J. High level expression of introduced chimaeric genes in regenerated transformed plants. EMBO J. 1985;4:2411–2418. doi: 10.1002/j.1460-2075.1985.tb03949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence GJ. PhD Thesis. The University of Adelaide; 1977. Genetics of Pathogenicity in Flax Rust. [Google Scholar]
- Lawrence GJ, Mayo GME, Shepherd KW. Interactions between genes controlling pathogenicity in the flax rust fungus. Phytopathology. 1981;71:12–19. [Google Scholar]
- Lawrence GJ, Dodds PN, Ellis JG. Rust of flax and linseed caused by Melampsora lini. Mol Plant Pathol. 2007;8:349–364. doi: 10.1111/j.1364-3703.2007.00405.x. [DOI] [PubMed] [Google Scholar]
- Lawrence GJ, Anderson PA, Dodds PN, Ellis JG. Relationships between rust resistance genes at the M locus in flax. Mol Plant Pathol. 2010 doi: 10.1111/j.1364-3703.2009.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard KL, Szabo LJ. Stem rust of small grains and grasses caused by Puccinia graminis. Mol Plant Pathol. 2005;6:99–111. doi: 10.1111/j.1364-3703.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- Luck JE, Lawrence GJ, Dodds PN, Shepherd KW, Ellis JG. Regions outside of the leucine-rich repeats of flax rust resistance proteins play a role in specificity determination. Plant Cell. 2000;12:1367–1377. doi: 10.1105/tpc.12.8.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo GME, Shepherd KW. Studies of genes controlling specific host–parasite interactions in flax and its rust. I Fine structure analysis of the M group in the host. Heredity. 1980;44:211–227. [Google Scholar]
- Singh RP, Hodson DP, Huerta-Espino J, Jin Y, Njau P, Wanyera R, Herrera-Foessel SA, Ward RW. Will stem rust destroy the world’s wheat crop? Adv Agron. 2008;98:271–309. [Google Scholar]
- Voegele RT, Mendgen K. Rust haustoria: nutrient uptake and beyond. New Phytol. 2003;159:93–100. doi: 10.1046/j.1469-8137.2003.00761.x. [DOI] [PubMed] [Google Scholar]
- Webb CA, Szabo LJ, Bakkeren G, Garry C, Staples RC, Eversmeyer M, Fellers JP. Transient expression and insertional mutagenesis of Puccinia triticina using biolistics. Funct Integr Genomics. 2006;6:250–260. doi: 10.1007/s10142-005-0009-9. [DOI] [PubMed] [Google Scholar]
- Weld RJ, Plummer KM, Carpenter MA, Ridgway HJ. Approaches to functional genomics in filamentous fungi. Cell Res. 2006;16:31–44. doi: 10.1038/sj.cr.7310006. [DOI] [PubMed] [Google Scholar]
- Zambino PJ, Kubelik AR, Szabo LJ. Gene action and linkage of avirulence genes to DNA markers in the rust fungus Puccinia graminis. Phytopathology. 2000;90:819–825. doi: 10.1094/PHYTO.2000.90.8.819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Rust transformation procedure.
Figure S2. DNA gel blot analysis of transgenic flax rust isolates.