Abstract
Background and objective
Endothelial progenitor cells (EPCs) contribute to postnatal neovascularization, thus promoting wide interest in their therapeutic potential in vascular injury and prevention of their dysfunction in cardiovascular diseases. Cleaved high molecular weight kininogen (HKa), an activation product of the plasma kallikrein-kinin system (KKS), inhibits the functions of differentiated endothelial cells including in vitro and in vivo angiogenesis. In this study, our results provided the first evidence that HKa is able to target EPCs and inhibits their tube forming capacity.
Methods and results
We determined the effect of HKa on EPCs using a three-dimensional vasculogenesis assay. Upon stimulation with vascular endothelial growth factor (VEGF) alone, EPCs formed vacuoles and tubes, and differentiated into capillary-like networks. As detected by gelatinolytic activity assay, VEGF stimulated secretion and activation of matrix metallopeptidase 2 (MMP-2), but not MMP-9, in the conditioned medium of 3D culture of EPCs. Specific inhibition or gene ablation of MMP-2, but not MMP-9, blocked the vacuole and tube formation by EPCs. Thus, MMP-2 is selectively required for EPC vasculogenesis. In a concentration-dependent manner, HKa significantly inhibited tube formation by EPCs and the conversion of pro-MMP-2 to MMP-2. Moreover, HKa completely blocked the association between pro-MMP- 2 and αvβ3 integrin, and its inhibition of MMP-2 activation was dependent on the presence of αvβ3 integrin. In a purified system, HKa did not directly inhibit MMP-2 activity.
Conclusions
HKa inhibits tube forming capacity of EPCs by suppression of MMP-2 activation, which may constitute a novel link between activation of the KKS and EPC dysfunction.
Keywords: endothelial progenitor cells, kininogen, matrix metalloproteinase, vasculogenesis
Introduction
Circulating endothelial progenitor cells (EPCs) are a hierarchy of pluripotent cells in peripheral blood capable of differentiating into mature endothelial cells destined for blood vessel formation(1). They are a major determinant of a postnatal mechanism for neovascularization and vascular remodeling(2). In patients with atherosclerosis and cardiovascular disease, EPCs are reduced in number and impaired in function(3). Although EPCs successfully restore endothelial function and enhance angiogenesis after tissue ischemia in animal models(4), the clinical administration of EPCs to patients has had limited efficacy(3). Likely, EPCs are targets of endogenous angiogenic inhibitors elaborated in the setting of atherosclerosis. Therefore, understanding the mechanisms, that regulate EPC function, may not only provide new insights into the pathogenesis of vasculogenesis, but also promote development of specific therapies to ultimately correct EPC dysfunction and prevent progression of atherosclerosis.
The plasma kallikrein–kinin system (KKS) consists of the proteins factor XII, prekallikrein, and high molecular weight kininogen (HK) (5). This system may widely participate in maintenance of the cardiovascular phenotype, and displays multiple physiologic activities such as blood pressure adjustment, modulation of thrombosis, regulation of endothelial cell proliferation and angiogenesis. Activation of the KKS is triggered in vivo by tissue destruction or by thrombus development (5) (6), and results in cleavage of HK by kallikrein and generates two-chain HK (HKa). Plasma HK, which is synthesized and released from liver, is a major component of the KKS and is responsible for the association of the KKS with cell surface(5). The plasma membrane of endothelial cells is an important site for the assembly and activation of the KKS. HKa inhibits endothelial cell function and exhibits potent antiangiogenic activity(7). The inhibitory effect of HKa may result from its inhibition of αvβ3 integrin function (8), and induction of apoptosis via its interaction with uPAR. Although other membrane molecules, such as cytokeratin-1 and gC1qR, also bind to HKa, their role in mediating HKa’s effect remains to be elucidated. Because EPCs express high levels of uPAR(9), which is a HKa receptor(10), we tested whether HKa also exerts inhibitory effect on EPCs. The results provide initial understanding of the contribution of the KKS cascade to EPC dysfunction.
Methods
Antibodies and Reagents
HKa was purchased from Enzyme Research Laboratories (South Bend, Indiana). Human VEGF was from R&D Systems (Minneapolis, MN). Anti-αvβ3 integrin (LM609) and anti-MMP- 2 monoclonal antibodies were from Chemicon (Temecula, CA). Rabbit polyclonal antibody against integrin β3 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). MMP inhibitors were purchased from Calbiochem (San Diego, CA). The peptide c(RGDfK), cyclo(Arg-Gly-Asp-D-Phe-Lys), was purchased from Peptides International (Louisville, KY). The plasmid expressing human MMP-2 and recombinant human full length proMMP-2 protein were generated as previously described (11). All other reagents were obtained from Sigma (St Louis, MO) unless otherwise specified.
Cell preparation
In this study EPCs refer to endothelial colony-forming cells (ECFCs) and their progenitor cell capacities were characterized as previously described (1, 12). ECFCs with robust proliferative potential, colony-forming and vessel-forming activity in vitro are defined EPCs and used in the experiments of this study. Under protocols approved by the IRB at Indiana University and Temple University and after informed consent was obtained in accordance with the Declaration of Helsinki, human blood was collected from healthy volunteer donors. After dilution with HBSS (1:1), blood was overlaid onto Ficoll-Paque and centrifuged at 740g for 30 minutes. Buffy coat mononuclear cells (MNCs) were collected and resuspended in complete endothelial growth culture medium -2 (EGM-2, Cambrex, Wakersville) with additives and 10% fetal bovine serum (FBS). Cells were cultured in tissue culture plate precoated with type I collagen (BD Bioscience, San Diego) at 37°C. Colonies appeared between 5 and 22 days of culture were identified as well-circumscribed monolayer of cobblestone-appearing cells. The phenotypic and functional analysis of EPCs at early passages (passage 2 to 4 ) was performed as previously described(1, 12).
Human umbilical vein endothelial cells (HUVECs) and human aortic endothelial cells (HAECs) were purchased from Cambrex and Invitrogen, respectively. The hamster CS-1 melanoma cell line expressing αvβ3 heterodimer was generated and characterized previously(13).
Three-dimensional (3D) vasculogenesis assay
EPCs were resuspended in endothelial cell basal medium (EBM-2, 2 ×106 cells/mL) and mixed with neutralized type-I collagen (4 mg/mL) at a volume ratio of 1:1. One hundred microliters of mixture was transferred to one well of the 96-well culture plate and incubated at 37°C for 20 minutes. The gel matrices were then overlaid with EBM-2 containing growth factor and Serum Replacement 1 and cultured at 37°C. After fixation with 2% paraformaldehyde (PFA), the gels were stained with 0.1% toluidine blue and visualized with a microscope. The tube length was quantified as recently described(14).
Confocal laser-scanning microscopy
EPCs embedded in collagen gels were permeabilized with 0.2% Triton X-100, and stained with rhodamine phalloidin. The SlowFade® Antifade (Invitrogen) was used to protect against fading. The gels were placed on a slide and imaged with a Leica SP1 Scanning Confocal Microscope. Optical sections were obtained and the 3D structure was reconstructed using NIH Image-J digital photo editing software (Image>Stacks>Z-Project>Max Intensity).
Cell Proliferation, viability and adhesion assay
Cells were seeded in EGM-2 at 600 cells/cm2 in separate culture plates coated with collagen and cultured at 37°C with 5% CO2. The medium was replaced every 24 hours. Cell growth was determined by cell counts at 24 hours intervals. For cell viability and adhesion assay in 96-well plates, the numbers of cells were determined by CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega)(Madison, WI)(13).
Gene silencing of matrix metalloproteinase-2 (MMP-2) and MMP-9
The sequence of human MMP-2 5′-CCAGATGTGGCCAACTACAACTTCT-3′ or human MMP-9 5′-AAGATGTTCACGTTGCAGGCATCGT-3′ was the targeting region for siRNA, respectively. A non-silencing sequence 5′-AACCUGCGGGAAGAAGUGG was used as control siRNA. SiRNA was introduced into EPCs by Lipofectamine 2000 (Invitrogen)(13).
Immunoprecipitation and Western blotting
The cells were solubilized using extraction buffer as described previously (8). The lysates were centrifuged at 15,000g for 10 minutes. For immunoprecipitation, supernatants with equal amount of protein were incubated with antibody at 4°C for 2 hours, followed by incubation with protein G Sepharose (Santa Cruz Biotechnology) at 4°C for 1 hour. For Western blotting, the samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDSPAGE), and transferred onto PVDF membranes (Millipore, Billerica, CA). The membranes were blocked with 5% milk in Tris-buffered saline (50 mmol/L Tris-HCl pH 7.4, 150 mmol/L NaCl), before incubation with primary antibodies. Antibody binding was detected using HRPconjugated secondary antibodies, and visualized using an enhanced chemiluminescent substrate (Super Signal West Pico Luminal reagents, Pierce).
Gelatin zymography assay
The conditioned culture medium was collected and resolved in nonreducing Laemmli-buffer. The gelatinolytic activity was detected by zymography using 8% SDS-PAGE, containing 1.0 mg/mL gelatin.
Gelatinase activity assay
MMP-2 catalytic activity was measured using a thiopeptide as a chromogenic substrate in a Colorimetric QuantiZyme™ MMP-2 Assay System (Biomol, Plymouth Meeting, PA). Absorbance values were measured at A412nm in a microplate reader (Molecular Devices Thermo Max). For MMP activation assay using 2-aminophenylmercuric acetate (APMA), 50 ng pro-MMP-2 was incubated with 1mM APMA in a reaction buffer (50mM Tris/HCl pH 7.5, 1mM CaCl2, 0.05% Triton X-100).
Measurement of mRNA expression by reverse transcription - polymerase chain reaction (RT-PCR)
Total RNA was isolated from EPCs using Trizol® reagent (Invitrogen). For RT-PCR, primer sequences were as follows: for human MMP-9: forward primer 5′-CCTGCCAGTTTCCATTCATC-3′ and reverse primer 5′-GCCATTCACGTCGTCCTTAT-3′; for human MMP-2: forward primer 5′-ACCTGGATGCCGTCGTGGAC-3′ and reverse primer 5′-TGTGGCAGCACCAGGGCAGC-3′. RNA was used as template in a SuperScript One-Step RT-PCR reaction (Invitrogen), performed according to the manufacturer’s instructions.
Data analysis
The data were calculated as average ± SEM from experiments done at least 3 times, and statistically analyzed by Students’ t test (two groups only) or One Way Analysis of variance (ANOVA) and Student-Newman-Keuls test (multiple groups). Difference with probability values below 0.05 were considered significant.
Results
EPCs exhibit strong capacity to form vacuoles and tubes in the presence of VEGF
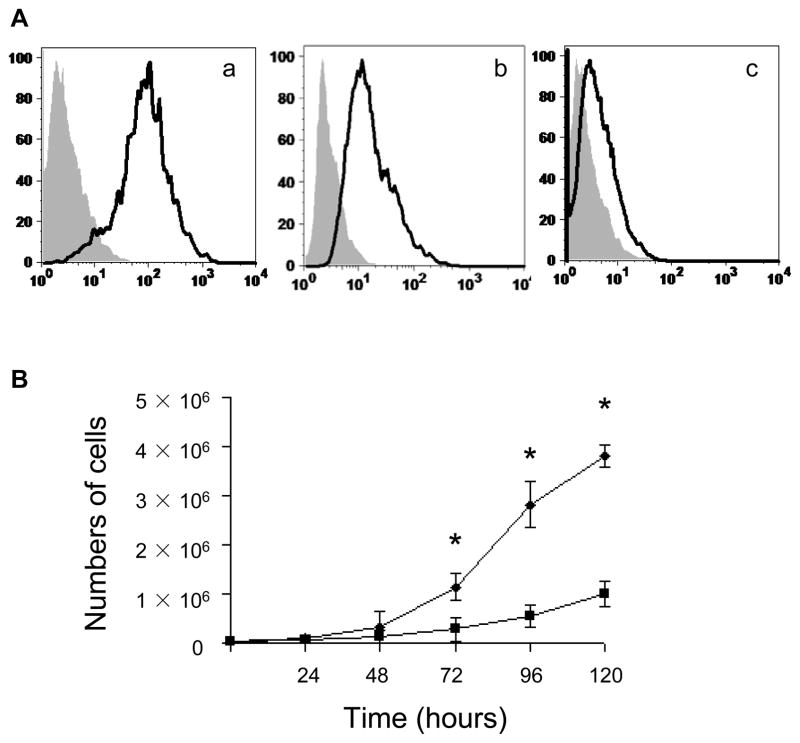
The current study was aimed at determining whether HKa inhibits EPC function. In order to determine if activation of the KKS serves as an endogenous factor contributing to EPC dysfunction, we thus studied EPCs isolated from adult circulation, instead of cord blood cells. EPCs exhibited a homogenous population and uniformly expressed pan-endothelial cell-surface antigens, CD31 (>90% positive) and VE-cadherin (>65% positive), whereas only a small percentage of the cells expressed AC133 (Figure 1A). They grew significantly faster than differentiated HAECs when cultured for 72 to 120 hours (Figure 1B).
Figure 1. Immunophenotyping and high proliferation capacity of EPCs.
(A) Immunophenotyping of cell-surface by flow cytometry (a, CD-31; b, VE-cadherin; c, AC133). Shown are representative data from 4 independent experiments. Isotype controls are indicated in gray shadow. (B) Growth of EPCs (diamonds) and HAECs (squares) in EGM-2 was evaluated in equivalently seeded in vitro. *p<0.01.
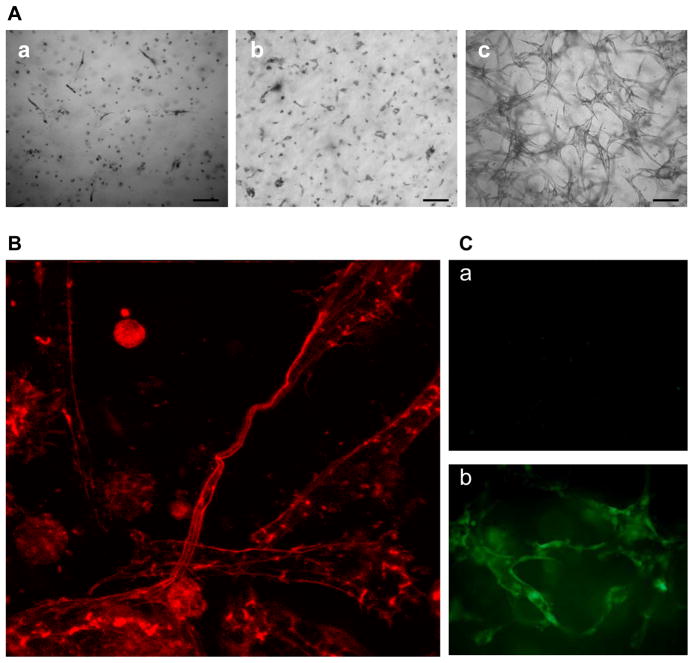
If EPCs are to be defined as true progenitor cells with postnatal vasculogenic potential, the evidence should be presented that EPCs give rise to differentiated progeny with the capacity of vessel formation(1). In a 3D collage gel, EPCs, but not HAECs and HUVECs, displayed a hierarchy of tube formation potentials in response to VEGF (Figure 2A), suggesting that EPCs are unique in their stronger potential for tubular morphogenesis and are more sensitive to physiological growth factor stimulation, compared with differentiated endothelial cells. The latter such as HUVECs require phorbol myristate acetate (PMA) to form tubes(14). The confocal image further indicates that EPCs differentiated into 3D capillary-like network (Figure 2B), with endothelial origins as evident by anti-vWF staining (Figure 2C).
Figure 2. EPCs exhibit potent capacity to form tubular structures.
(A) HAECs (a), HUVECs (b) and EPCs (c) were cultured in the presence of VEGF (25 ng/mL) in 3D collagen gel for 48 hours. Bar = 100 μm. The images are representative of three independent experiments. (B) Formation of capillary-like networks by EPCs. After culture for 48 hours, EPCs in collagen gel were stained with rhodamine-phalloidin and visualized by confocal microscope (400×). The image represents a maximum projection of the stacked optical sections. (C) Tube structure of EPCs in 3D was visualized by immunostaining with anti-vWF (b) or control IgG (a) plus Alexa Fluor@ 488-GαM antibody.
MMP-2 is selectively required for tube formation of EPCs
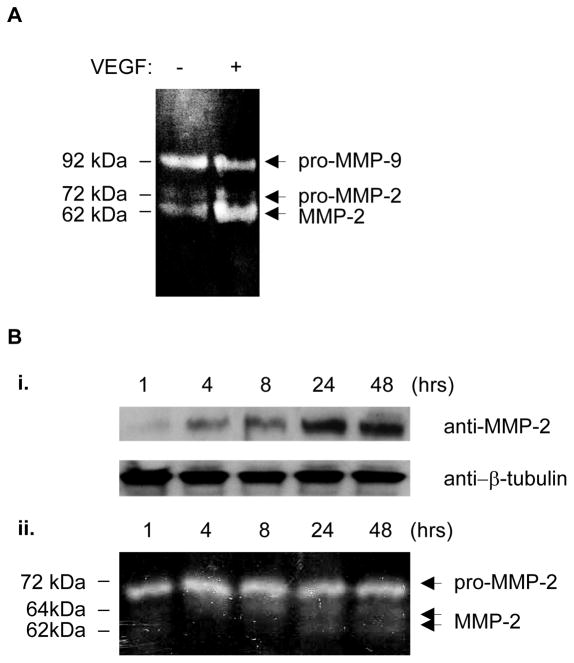
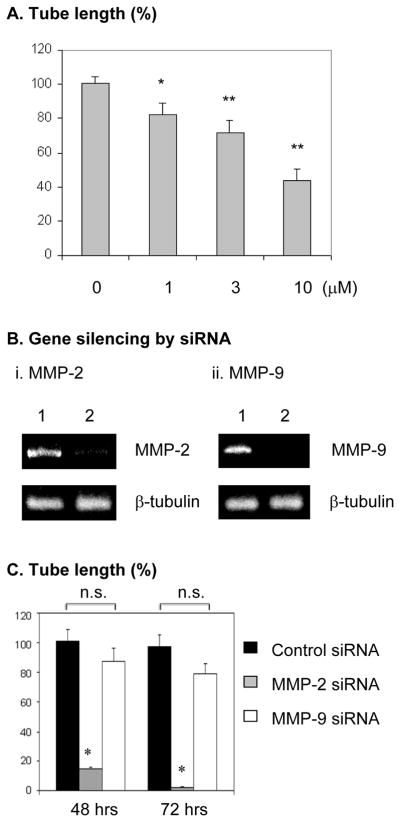
MMP-2 and MMP-9 participate in the mobilization of EPCs (13, 15). However, their contribution to VEGF-stimulated vasculogenic differentiation of EPCs remains unknown. In the conditioned culture media of EPCs embedded in 3D collagen gel, VEGF selectively stimulated the secretion and activation of MMP-2, but not MMP-9 (Figure 3A). Figure 3B further indicates that VEGF-stimulated proMMP-2 expression (i) and secretion (ii) was time-dependent, the secreted proMMP-2 was meanwhile converted into active form. We further determined the role of MMP-2 and MMP-9 activities in tube formation by EPCs. The specific MMP-2 inhibitor 444244 concentration-dependently attenuated VEGF-stimulated tube formation by EPCs (Figure 4A), whereas the specific MMP-9 inhibitor (444278) did not have inhibitory effect (data not shown). Moreover, we determined the requirement of MMP-2 and MMP-9 expression for the tube formation by EPCs. Specific siRNA oligonucleotides markedly downregulated MMP-2 and MMP-9 expression (Figure 4B). VEGF-stimulated tube formation by EPCs was almost completely suppressed by gene silencing of MMP-2, but not of MMP-9, at 48 hours and 72 hours, respectively, whereas the tube formation by EPCs became saturated at 48 hours (Figure 4C). Thus, MMP-2 is selectively required for EPC formation of tubular structures. The inhibitory effect of the MMP-2 gene silencing (Figure 4C) was more potent than that of the enzymatic inhibitor (Figure 4A), suggesting that both of the catalytic activity and expression of MMP-2 are necessary for the tubular morphogenesis of EPCs. Indeed, pro-MMP-2 possesses enzymatic activity-independent functions, its interaction with cell-surface proteins may mediate intracellular activation signals(16).
Figure 3. MMP-2, but not MMP-9, is activated during VEGF-stimulated EPC differentiation.
(A) Zymography assay. After EPCs embedded in collagen gel were cultured with or without 25 ng/mL of VEGF for 24 hours, the gelatinolytic activity in the conditioned culture media was analyzed by zymography. (B) VEGF (25 ng/ml)-stimulated expression and secretion of MMP-2 is time-dependent. The expression of cytosolic MMP-2 in EPCs as determined by Western-blot with β-tubulin serving as a loading control (i); Zymography of the conditioned culture media (ii). The results are representative of three independent experiments.
Figure 4. MMP-2 activity and expression is selectively required for EPC differentiation in response to VEGF stimulation.
(A) EPCs were cultured in 3D collagen gel for 48 hours in the presence o f 25 ng/mL VEGF plus specific MMP-2 inhibitor (444244) at the indicated concentrations. As described in the Methods, the sum of the tube length in six digital images per well was indicated as micrometer per square millimeter. Results are given as percentage of tube length/area compared with the control (0 μM) (which was set as 100%). Experiments were done in triplicates. * p< 0.05; ** p<0.01. (B) Gene silencing of MMP-2 (i) and MMP-9 (ii). The knockdown efficiency by siRNA was evaluated by RT-PCR. Lane 1, control non-silencing siRNA, lane 2, MMP-2 or MMP-9 siRNA. (C) After transfection with siRNA, EPCs in 3D collagen gel were cultured with VEGF for 48 and 72 hours, respectively. Experiments at each time point were done in triplicates. Quantitation of tube length was done as described in A. Results are given as percentage of tube length/area compared with the control group at 48 hours (which was set as 100%). *p<0.0001; n.s: not significant, compared with control.
HKa inhibits EPC tube formation and MMP-2 activation
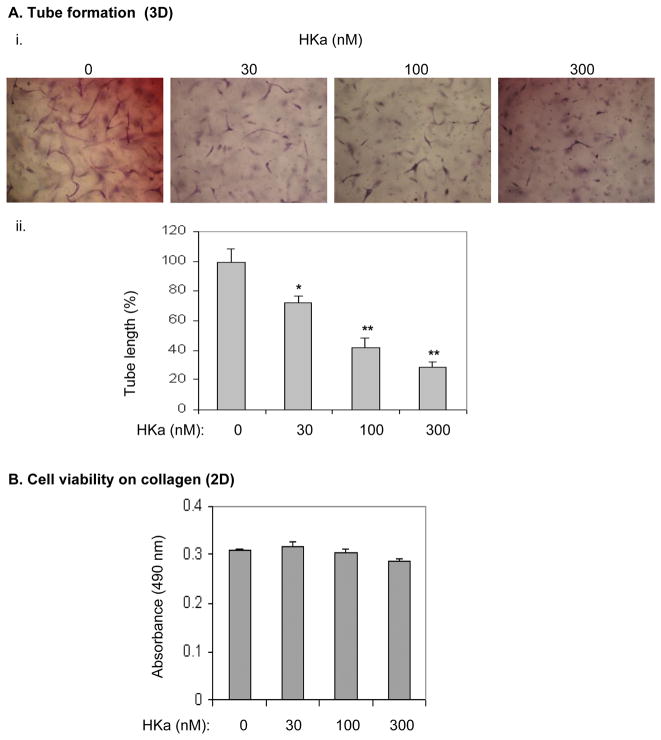
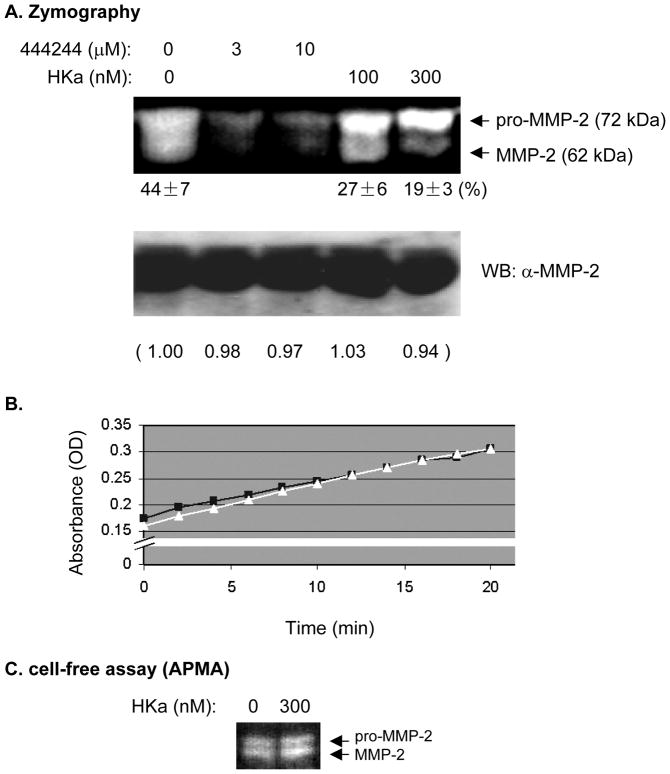
EPCs are able to differentiate into vacuoles and tubes upon stimulation with VEGF alone in the 3D system (Figure 1A). By using this system we found that HKa significantly decreased VEGF-stimulated tube formation by EPCs in a concentration-dependent fashion (Figure 5A, i and ii). These concentrations of HKa, 30 nM, 100 nM, and 300 nM, represent cleavage of HK of 4.5%, 15% and 45% respectively, which occur in experimental inflammatory bowel disease and arthritis(17). Because MMP-2 is necessary for tubular morphogenesis of EPCs (Figure 4), we tested whether HKa inhibits their tube formation by suppression of MMP-2 activation. VEGFstimulated MMP-2 secretion and activation was not detected in the medium of EPCs cultured on collagen-coated surfaces (data not shown), suggesting that MMP-2 is not involved in EPC activation in a 2D system. HKa did not inhibit endothelial cell function on collagen surfaces (Figure 5B and ref.(18)). Thus, HKa inhibition of tube formation by EPCs in collagen gel reveals a novel mechanism for HKa antiangiogenic activities. We postulated that MMP-2 activation in a 3D system is very likely a target for HKa. As shown in Figure 6B, the MMP-2 Inhibitor I (444244) markedly abolished the gelatinolytic activity of both proform and active form of MMP- 2. In contrast, HKa treatment only reduced the ratio of active MMP-2 to total MMP-2 (Figure 6A, 44 ± 7% at 0 nM versus 27 ± 6% and 19 ± 3 % at 100 nM and 300 nM, respectively). Neither the MMP-2 Inhibitor nor HKa reduced MMP-2 secretion, as detected by Western-blotting using anti-MMP-2 mAb which recognizes both MMP-2 and pro-MMP-2 (Figure 6A, lower panel: the numbers under the gel indicate the relative band density). Thus, HKa is able to inhibit the conversion of pro-MMP-2 to active MMP-2. In purified systems, HKa at 300 nM did not have apparent inhibition of catalytic activity of MMP-2 (Figure 6B) and the conversion of pro-MMP-2 to MMP-2 (Figure 6C). Collectively, HKa inhibits the conversion of pro-MMP-2 to MMP-2 in EPCs without directly affecting MMP-2 secretion and activity.
Figure 5. HKa inhibits EPC differentiation without affecting cell viability.
(A) i. Morphogenesis of EPCs in collagen gel. EPCs were suspended in EBM-2 containing 25 μM ZnCl2 and incubated with or without HKa on ice for 20 minutes. After mixture with the collagen gel solution, EPCs were cultured in the presence of VEGF (25 ng/mL) for 48 hours. ii. Tube length was measured and analyzed as described in the Figure 4A legend. Experiments were done in triplicate. * p<0.01; **p<0.005. (B) EPCs were cultured on collagen-coated plate in the presence of VEGF (25 ng/ml) plus HKa at the indicated concentrations for 48 hours (n=4). The cell number was determined as described in the Methods.
Figure 6. HKa inhibits the conversion of proMMP-2 to MMP-2.
(A) EPCs were cultured in 3D collagen matrices in the presence of VEGF (25 ng/mL) plus MMP-2 Inhibitor I (444244) or HKa as indicated. The conditioned medium was collected for zymography assay (upper panel) and anti-MMP-2 Western-blot (lower panel). Intensity of pro-MMP-2 and MMP-2 bands in zymography gel was quantified using software Quantity One, version 4.1.1 (Bio-Rad) after background subtraction. The number under the zymography gel indicates the band intensity ratio of MMP-2/(MMP-2 + pro-MMP-2) (%), average ± SEM, n=3. (B) Gelatinase activity assay. Recombinant human MMP-2 catalytic domain was incubated with a thiopeptide as a chromogenic substrate in the presence of 0.1% BSA or 300 nM HKa. Absorbance values were read as described in the Methods. The results are representative of three independent experiments. (■, BSA; △, HKa). (C) Recombinant pro-MMP-2 was incubated with APMA with 0 nM or 300 nM HKa at 37°C for 60 min. Native and processed MMP-2 were detected by zymography.
HKa inhibition of MMP-2 activation is dependent on αvβ3 integrin
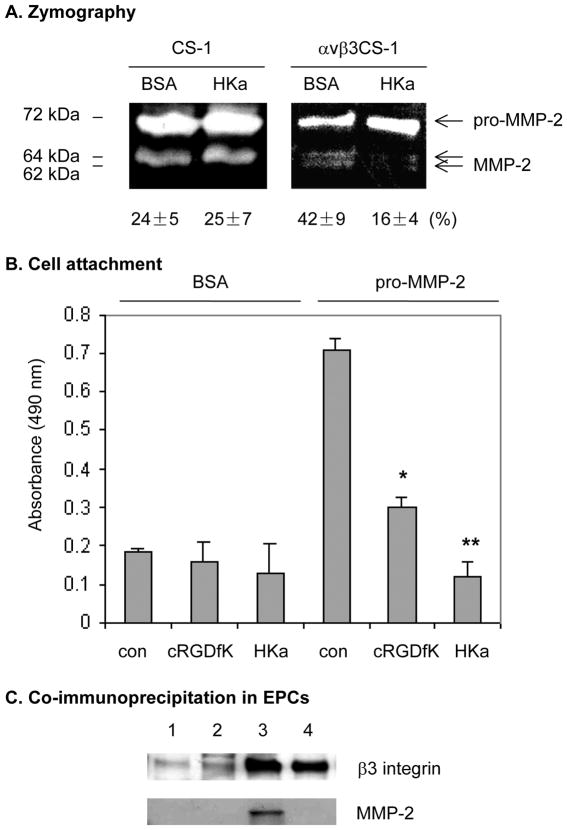
EPCs expressed high level of αvβ3 integrin (data not shown and ref.(19)) and HKa downregulates αvβ3 integrin ligand binding affinity in endothelial cells(13), we were motivated to test whether HKa inhibition of MMP-2 activation is dependent on αvβ3 integrin. Because EPCs express several integrins other than αvβ3, such as αvβ5, α5β1, α2β1, which may also play a role in MMP-2 activation, we tested whether HKa inhibits MMP-2 activation through αvβ3 integrin by using an αvβ3CS-1 cell line. This cell line expresses the heterodimer of αvβ3 but not αvβ5 and have been characterized in our previous studies (13) (20). Using the CS-1 cells, we have demonstrated that HKa specifically downregulates the ligand binding affinity of αvβ3 integrin, which supports that HKa inhibits this integrin function in endothelial cells. In the conditioned medium of wild-type CS-1 cells which do not express αvβ3 integrin, HKa did not block MMP-2 activation (Figure 7A, 24 ± 5 % versus 25 ± 7 %). As shown in the Figure 7A, the expression of αvβ3 integrin on cell surface not only increased MMP-2 activation (24 ± 5 % verse 42 ± 9 %), but also stimulated autoactivation of MMP-2 (conversion of 64-kDa to 62-kDa form). However, in the presence of HKa, the conversion of proMMP-2 to 64- and 62-kDa forms was inhibited (Figure 7A, 42 ± 9 % versus 16 ± 4 %). Since direct binding of proMMP-2 to αvβ3 was not detected using a coimmuoprecipitation method (21), HKa inhibition of MMP-2 activation seems to be mediated by αvβ3 integrin, but only indirectly. Because tumor cells (CS- 1) are resistant to apoptosis, HKa inhibition of MMP-2 activation in EPCs and CS-1 cells is not subject to the induction of apoptosis, whereas depletion of αvβ3 integrin by gene silencing induced EPC apoptosis (data not shown). In an adhesion assay, we found that pro-MMP-2 mediates attachment of αvβ3CS-1 cells (Figure 7B), but not that of wild-type CS-1 cells (data not shown). Pretreatment of αvβ3CS-1 cells with cRGDfK, a specific inhibitory peptide against αvβ3, significantly suppressed attachment of αvβ3CS-1 cells, but not wild-type CS-1 cells, to pro-MMP-2 surfaces (Figure 7B and data not shown). Thus, αvβ3CS-1 cell attachment to pro- MMP-2 is mainly mediated by αvβ3 integrin. We further found that HKa at 300 nM completely prevented αvβ3CS-1 cell attachment to proMMP-2 surfaces to a greater extent than cRGDfK (Figure 7B). Correspondingly, HKa blocked the formation of avβ3 integrin-MMP2 complex in EPCs cultured in a 3D gel (Figure 7C). HKa disrupts the association between αvβ3 integrin and proMMP-2, which may account for its inhibition of MMP-2 activation. Taken together with other observations that MMP-2-αvβ3 integrin interaction is critical for angiogenesis (30)(31), HKa inhibition of MMP-2 activation and tube formation by EPCs is, at least in part, mediated through αvβ3 integrin. The residual activation of MMP-2 by HKa treatment in both EPCs (Figure 6A) and αvβ3CS-1 cells (Figure 7B) supports the existence of alternative mechanisms of MMP-2 activation (22).
Figure 7. HKa inhibition of MMP-2 activation is dependent on αvβ3 integrin.
(A) CS-1 and αvβ3CS-1 cells were transfected with full length human MMP-2 cDNA for 48 hours. After incubation with 0.1% BSA or 300 nM HKa in serum-free medium overnight, the conditioned medium was collected for zymography assay. The band intensity was calculated as described in the Figure 4B legend and the number shown under the gel represents average ± SEM, n=3. (B) Cell attachment. The 96-well cell culture plates were coated with 0.3% BSA and 10 μg/mL recombinant proMMP-2 protein. The αvβ3CS-1 cells (5×104 cells/well) were cultured at 37°C in the presence of 0.1% BSA, 10 μM cRGDfK, or 300 nM HKa for 2 hours. After unattached cells were removed, the attached cell number was determined as described in the Methods. Experiments were done in triplicate. *p<0.05; **p<0.01. (C) Effect of HKa on αvβ3 integrin- MMP-2 complex in EPCs. As described in the legend to Figure 5A, EPCs were incubated in a 3- D gel with (lane 2 and lane 4) or without (lane 1 and lane 3) 300 nM HKa for 48 hours. Cells were harvested by the addition of extraction buffer. Cell lysates were subjected to immunoprecipitation with mouse IgG (lane 1 and lane 2) or LM609 (lane 3 and 4). The immunoprecipitates were probed by antibodies against β3 integrin and MMP-2, respectively. Data are representative of 3 independent experiments.
Discussion
The previous studies from our laboratory and others have indicated that HKa inhibits the function of differentiated endothelial cells such as HUVECs as well as in vivo angiogenesis (7, 23). This study further indicates that HKa inhibits tube forming capacity of EPCs. We have recently shown that HKa inhibits endothelial cell functions by suppressing αvβ3 integrin activation(13), the current study further demonstrated the novel activities of HKa in the downstream activation events of αvβ3 integrin, indicating that HKa targets activation of MMP-2 for tube formation.
To evaluate the tubular differentiation, we used a 3D collagen culture system (24) and found that VEGF alone was sufficient to induce differentiation of EPCs into tubes and lumens (Figure 1A), demonstrating that EPCs exhibit greater tube forming capacity than differentiated endothelial cells. This system without addition of PMA thus allowed us to determine the effects of HKa on EPCs in a more physiologically relevant system. In this modified system, HKa is able to inhibit VEGF-stimulated EPC formation of tubular structures (Figure 5A), whereas HKa inhibition of differentiated endothelial cell function is compromised on 2D collagen surfaces (Figure 5B) (18, 25). Thus, HKa inhibition of tube formation by EPCs in a 3D system reveals its additional activities. Since the KKS activation may occur during inflammation and thrombosis, HKa as an activation product of this system may induce EPC dysfunction in the setting of pathological conditions. During the process of EPCs forming vacuoles and vessels in a 3D system, but not in a 2D condition such as matrix surfaces, MMP-2 activation is specifically involved; HKa inhibits EPC function through blocking MMP-2 activation.
In the process of neovascularization, invasive endothelial cells secrete MMPs to remodel the extracellular matrix (ECM) and the basal lamina - an important physical barrier between the endothelial and connective tissue (26). Two members of the MMP family, MMP-2 and MMP-9, display the highest enzymatic activities against the ECM components important in angiogenesis. In the 3D system, VEGF stimulates secretion and activation of MMP-2, but not MMP-9 (Figure 3), suggesting that MMP-2 is selectively required for EPC differentiation. By using pharmaceutical inhibitors and gene ablation, we provide evidence that both the enzymatic activity and expression of MMP-2 are required for EPC differentiation (Figure 4). MMP-9 is, however, dispensable in this process. This observation is consistent with a recent study that MMP-2 deficiency reduces the functional activities of EPCs, leading to impaired vasculogenesis (13). Interestingly, HKa markedly inhibits conversion of pro-MMP-2 to active MMP-2 in EPCs without affecting MMP-2 secretion (Figure 6). This activity of HKa may, at least in part, account for its inhibition of tubular morphogenesis of EPCs.
We further found that the inhibitory effect of HKa on MMP-2 is dependent on the presence of αvβ3 integrin (Fig. 7A) and mediated by the dissociation of proMMP-2 from αvβ3 integrin (Fig. 7B). The αvβ3 integrin is necessary for vascular cell survival, proliferation, and invasion during angiogenesis(27). MMP-2 activation has been suggested to be downstream of αvβ3 integrin activation, and MMP2- αvβ3 binding is required for proper MMP2 function. This is consistent with MMP-2 and αvβ3 integrin being colocalized in a particular membrane fraction caveolae(28), which enhances cellular protrusive activity (29). The disruption of MMP-2 binding to αvβ3 integrin blocks angiogenesis in vitro and in vivo(30)(31), αvβ3 integrin may serve as an MMP-2 receptor in the process of angiogenesis. However, the specificity of the MMP2-αvβ3 integrin binding has been questioned(21), and other molecules may also be involved in cell surface localization of MMP-2(22, 32). Thus, the mechanisms for activation of MMP-2 are complex. In endothelial cells, the primary target for HKa is uPAR and HKa occupancy of uPAR disrupts uPAR-αvβ3 integrin complex formation(8). HKa seems to indirectly modulate αvβ3 integrin function. In platelets, αIIbβ3 integrin also interacts with C-terminal hemopexin-like domain of MMP-2(33), suggesting that MMP-2 is likely associated with β3 subunit. In a purified system, we could not observeαvβ3 integrin forming a complex with pro-MMP2 or active MMP- 2 (data not shown), supporting that MMP-2, which does not have a RGD sequence, indirectly interacts with αvβ3 integrin via a mediatory RGD-bearing molecule (21). Alternatively, MMP-2 may bind to αvβ3 integrin in a RGD-independent manner, as cRGDfK failed to completely prevent the association between proMMP-2 and αvβ3 integrin (Fig. 7B). Strikingly, HKa possesses stronger inhibition of both pathways. Taken together, HKa, by interfering with αvβ3 integrin function, decreases the ligation of proMMP2 or its associated molecule to αvβ3 integrin, thereby suppressing MMP-2 activation. The inhibitory effect of HKa on MMP-2, at least in part, accounts for its inhibition of EPC vasculogenic differentiation.
The finding in this study that HKa inhibits tube forming capacity of EPCs reveals a novel activity of the components of the KKS in vascular dysfunction and expands our understanding of the pathophysiological activities of this system. Local EPCs recruited after early arterial injury incorporate into foci of postnatal vasculogenesis and exhibit strong potential for vascular repair. A study using the kininogen-deficient mice indicates that the KKS activation, in the process of vascular injury, induces arterial thrombosis(34). Thus, developing thrombus in atherosclerotic lesions may form a surface that promotes the assembly of the components of the KKS, leading to a secondary activation of this system and further production of HKa(35). Our current observations that HKa inhibits EPC tube forming capacity propose a novel concept that activation of the KKS may contribute to EPC dysfunction. Although it remains to be determined whether and how HKa inhibition of EPCs is involved in the disordered vascular remodeling in vivo, recognition of components of plasma KKS modulation of EPC function allows for the beginning of understanding its pathophysiologic activities in atherosclerosis.
Acknowledgments
This work was supported by the following grants: R01CA083121, R01AR051713 and T32HL007777 from NIH (to R.W.C.), and R03AR057542 from NIH (to Y.W.).
References
- 1.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–9. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prater DN, Case J, Ingram DA, Yoder MC. Working hypothesis to redefine endothelial progenitor cells. Leukemia. 2007;21:1141–9. doi: 10.1038/sj.leu.2404676. [DOI] [PubMed] [Google Scholar]
- 3.Werner N, Nickenig G. Endothelial progenitor cells in health and atherosclerotic disease. Annals of Medicine. 2007;39:82–90. doi: 10.1080/07853890601073429. [DOI] [PubMed] [Google Scholar]
- 4.Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761–8. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- 5.Colman RW, Schmaier AH. Contact System: A Vascular Biology Modulator With Anticoagulant, Profibrinolytic, Antiadhesive, and Proinflammatory Attributes. Blood. 1997;90:3819–43. [PubMed] [Google Scholar]
- 6.Schmaier AH, McCrae KR. The plasma kallikrein-kinin system: its evolution from contact activation. J Thromb Haemost. 2007;5:2323–9. doi: 10.1111/j.1538-7836.2007.02770.x. [DOI] [PubMed] [Google Scholar]
- 7.Colman RW, Jameson BA, Lin Y, Johnson D, Mousa SA. Domain 5 of high molecular weight kininogen (kininostatin) down-regulates endothelial cell proliferation and migration and inhibits angiogenesis. Blood. 2000;95:543–50. [PubMed] [Google Scholar]
- 8.Wu Y, Rizzo V, Liu Y, Sainz IM, Schmuckler NG, Colman RW. Kininostatin Associates With Membrane Rafts and Inhibits {alpha}v{beta}3 Integrin Activation in Human Umbilical Vein Endothelial Cells. Arterioscler Thromb Vasc Biol. 2007;27:1968–75. doi: 10.1161/ATVBAHA.107.148759. [DOI] [PubMed] [Google Scholar]
- 9.Basire A, Sabatier F, Ravet S, Lamy E, Mialhe A, Zabouo G, Paul P, Gurewich V, Sampol J, Dignat-George F. High urokinase expression contributes to the angiogenic properties of endothelial cells derived from circulating progenitors. Thromb Haemost. 2006;95:678–88. [PubMed] [Google Scholar]
- 10.Colman RW, Pixley RA, Najamunnisa S, Yan W, Wang J, Mazar A, McCrae KR. Binding of high molecular weight kininogen to human endothelial cells is mediated via a site within domains 2 and 3 of the urokinase receptor. J Clin Invest. 1997;100:1481–7. doi: 10.1172/JCI119669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallon UM, Overall CM. The Hemopexin-like Domain (C Domain) of Human Gelatinase A (Matrix Metalloproteinase-2) Requires Ca2+ for Fibronectin and Heparin Binding. J Biol Chem. 1997;272:7473–81. doi: 10.1074/jbc.272.11.7473. [DOI] [PubMed] [Google Scholar]
- 12.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–60. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 13.Cheng XW, Kuzuya M, Nakamura K, Maeda K, Tsuzuki M, Kim W, Sasaki T, Liu Z, Inoue N, Kondo T, Jin H, Numaguchi Y, Okumura K, Yokota M, Iguchi A, Murohara T. Mechanisms underlying the impairment of ischemia-induced neovascularization in matrix metalloproteinase 2-deficient mice. Circ Res. 2007;100:904–13. doi: 10.1161/01.RES.0000260801.12916.b5. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Sainz IM, Wu Y, Pixley R, Espinola RG, Hassan S, Khan MM, Colman RW. The inhibition of tube formation in a collagen-fibrinogen, three-dimensional gel by cleaved kininogen (HKa) and HK domain 5 (D5) is dependent on Src family kinases. Experimental Cell Research. 2008;314:774–88. doi: 10.1016/j.yexcr.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, Werb Z, Rafii S. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–37. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stefanidakis M, Koivunen E. Cell-surface association between matrix metalloproteinases and integrins: role of the complexes in leukocyte migration and cancer progression. Blood. 2006;108:1441–50. doi: 10.1182/blood-2006-02-005363. [DOI] [PubMed] [Google Scholar]
- 17.Isordia-Salas I, Pixley RA, Li F, Sainz I, Sartor RB, Adam A, Colman RW. Kininogen deficiency modulates chronic intestinal inflammation in genetically susceptible rats. Am J Physiol Gastrointest Liver Physiol. 2002;283:G180–6. doi: 10.1152/ajpgi.00514.2001. [DOI] [PubMed] [Google Scholar]
- 18.Sun D, McCrae KR. Endothelial-cell apoptosis induced by cleaved high-molecular-weight kininogen (HKa) is matrix dependent and requires the generation of reactive oxygen species. Blood. 2006;107:4714–20. doi: 10.1182/blood-2005-09-3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deb A, Skelding KA, Wang S, Reeder M, Simper D, Caplice NM. Integrin profile and in vivo homing of human smooth muscle progenitor cells. Circulation. 2004;110:2673–7. doi: 10.1161/01.CIR.0000139842.15651.B2. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y, Singh S, Georgescu MM, Birge RB. A role for Mer tyrosine kinase in alphavbeta5 integrin-mediated phagocytosis of apoptotic cells. J Cell Sci. 2005;118:539–53. doi: 10.1242/jcs.01632. [DOI] [PubMed] [Google Scholar]
- 21.Nisato RE, Hosseini G, Sirrenberg C, Butler GS, Crabbe T, Docherty AJ, Wiesner M, Murphy G, Overall CM, Goodman SL, Pepper MS. Dissecting the role of matrix metalloproteinases (MMP) and integrin alpha(v)beta3 in angiogenesis in vitro: absence of hemopexin C domain bioactivity, but membrane-Type 1-MMP and alpha(v)beta3 are critical. Cancer Res. 2005;65:9377–87. doi: 10.1158/0008-5472.CAN-05-1512. [DOI] [PubMed] [Google Scholar]
- 22.Steffensen B, Bigg HF, Overall CM. The involvement of the fibronectin type II-like modules of human gelatinase A in cell surface localization and activation. J Biol Chem. 1998;273:20622–8. doi: 10.1074/jbc.273.32.20622. [DOI] [PubMed] [Google Scholar]
- 23.Zhang JC, Claffey K, Sakthivel R, Darzynkiewicz Z, Shaw DE, Leal J, Wang YC, Lu FM, McCrae KR. Two-chain high molecular weight kininogen induces endothelial cell apoptosis and inhibits angiogenesis: partial activity within domain 5. Faseb J. 2000;14:2589–600. doi: 10.1096/fj.99-1025com. [DOI] [PubMed] [Google Scholar]
- 24.Davis GE, Camarillo CW. An [alpha]2[beta]1 Integrin-Dependent Pinocytic Mechanism Involving Intracellular Vacuole Formation and Coalescence Regulates Capillary Lumen and Tube Formation in Three-Dimensional Collagen Matrix. Experimental Cell Research. 1996;224:39–51. doi: 10.1006/excr.1996.0109. [DOI] [PubMed] [Google Scholar]
- 25.Guo YL, Wang S, Cao DJ, Colman RW. Apoptotic effect of cleaved high molecular weight kininogen is regulated by extracellular matrix proteins. J Cell Biochem. 2003;89:622–32. doi: 10.1002/jcb.10536. [DOI] [PubMed] [Google Scholar]
- 26.Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002;2:657–72. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- 27.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–71. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 28.Puyraimond A, Fridman R, Lemesle M, Arbeille B, Menashi S. MMP-2 colocalizes with caveolae on the surface of endothelial cells. Exp Cell Res. 2001;262:28–36. doi: 10.1006/excr.2000.5069. [DOI] [PubMed] [Google Scholar]
- 29.Rupp PA, Visconti RP, Czirok A, Cheresh DA, Little CD. Matrix metalloproteinase 2- integrin alpha(v)beta3 binding is required for mesenchymal cell invasive activity but not epithelial locomotion: a computational time-lapse study. Mol Biol Cell. 2008;19:5529–40. doi: 10.1091/mbc.E07-05-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silletti S, Kessler T, Goldberg J, Boger DL, Cheresh DA. Disruption of matrix metalloproteinase 2 binding to integrin alpha vbeta 3 by an organic molecule inhibits angiogenesis and tumor growth in vivo. Proc Natl Acad Sci U S A. 2001;98:119–24. doi: 10.1073/pnas.011343298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brooks PC, Silletti S, von Schalscha TL, Friedlander M, Cheresh DA. Disruption of angiogenesis by PEX, a noncatalytic metalloproteinase fragment with integrin binding activity. Cell. 1998;92:391–400. doi: 10.1016/s0092-8674(00)80931-9. [DOI] [PubMed] [Google Scholar]
- 32.Morrison CJ, Overall CM. TIMP independence of matrix metalloproteinase (MMP)-2 activation by membrane type 2 (MT2)-MMP is determined by contributions of both the MT2- MMP catalytic and hemopexin C domains. J Biol Chem. 2006;281:26528–39. doi: 10.1074/jbc.M603331200. [DOI] [PubMed] [Google Scholar]
- 33.Choi WS, Jeon OH, Kim HH, Kim DS. MMP-2 regulates human platelet activation by interacting with integrin alphaIIbbeta3. J Thromb Haemost. 2008;6:517–23. doi: 10.1111/j.1538-7836.2007.02871.x. [DOI] [PubMed] [Google Scholar]
- 34.Merkulov S, Zhang W-M, Komar AA, Schmaier AH, Barnes E, Zhou Y, Lu X, Iwaki T, Castellino FJ, Luo G, McCrae KR. Deletion of murine kininogen gene 1 (mKng1) causes loss of plasma kininogen and delays thrombosis. Blood. 2008;111:1274–81. doi: 10.1182/blood-2007-06-092338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmaier AH. Assembly, activation, and physiologic influence of the plasma kallikrein/kinin system. International Immunopharmacology. 2008;8:161–5. doi: 10.1016/j.intimp.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]