Abstract
A prevalent T helper type 1 (Th1) subset of lymphocytes has been described in Hashimoto's thyroiditis (HT), but whether a similar polarization may characterize HT when associated with non-endocrine autoimmune disorders (NEAD) is not known. The aim of the present study was to analyse the intracellular Th1 and Th2 distinctive cytokines in patients with isolated HT or associated with non-endocrine autoimmune disorders. Intracellular cytokine expression was assessed in peripheral blood lymphocytes (PBL) of 68 out-patients (females = 55; males = 13; median age = 36 years) with HT : 33 had isolated HT and 35 had a concurrent NEAD. The percentage of interferon (IFN)-γ and interleukin (IL)-2 Th1- and IL-4 Th2-positive cells was measured by flow cytometric analysis. We found an increased percentage of IL-2-positive cells in all patients, without differences between patients with isolated HT or associated with NEAD. IFN-γ+ cells were also increased in both groups, but the median percentage of those with isolated HT was lower than in patients with HT+NEAD (19·0 versus 29·9%; P = 0·0082). An increased number of IL-4-positive cells was observed in three of 33 (9·1%) patients with isolated HT and in 25 of 35 patients with NEAD [71%; P < 0·0001; relative risk (RR) = 3·18]. The median values of IL-4+ cells (HT = 5·0% versus HT + NEAD = 16·8%) confirmed this large difference (P < 0·0001). A clear-cut increase of IL-4+ lymphocytes characterizes patients with autoimmune thyroiditis who have associated non-endocrine autoimmune disorders. These findings may represent an initial tool to detect patients with autoimmune thyroiditis in which additional non-endocrine autoimmune disorders may be awaited.
Keywords: autoimmune thyroiditis, cytokines, interleukin-4, PAS III, Th1 lymphocytes, Th2 lymphocytes
Introduction
Chronic autoimmune thyroiditis may occur as a single disease or associated with further endocrine autoimmune diseases [1–3]. These polyglandular autoimmune syndromes (PAS) are classified as juvenile form (PAS I) or adult form (PAS II) [1,2]. The association of autoimmune thyroiditis with non-endocrine autoimmune disorders has also been recognized [4] (identified throughout as NEAD), sometimes included in PA III syndrome [5]. The association of NEAD with autoimmune thyroiditis includes atrophic gastritis/pernicious anaemia [6,7], coeliac disease (CD) [8], vitiligo [9], anti-phospholipid syndrome [10] and many other autoimmune diseases (see [5] for a review). Such an association may reflect common genetic [11] and environmental factors [12], but shared immunological features also seem to be involved [13]. The immunological characterization of these associations was often based on the presence of co-existing organ-specific autoantibodies in serum [4], but their pathogenesis is, as yet, incompletely understood. In recent years, the role of cellular immune responses has been characterized in some of these diseases when in isolated form [13–16]. Multi-parameter flow cytometry permits simultaneous detection of two or more cytokines, allowing direct T helper type 1 (Th1) versus Th2 determination, and has emerged as the premier technique for studying cytokine production at the single-cell level [17,18]. By using this technique, a prevalent Th1-driven autoimmune response has been clearly recognized in Hashimoto's thyroiditis (HT) [19] and this assumption has been validated in studies where the Th1-distinctive cytokines [interferon (IFN)-γ, interleukin (IL)-2] have been measured in serum [20] and in intrathyroidal lymphocytes [21]. Recently, a mild increase in the synthesis of Th-17 cytokines in patients with HT has also been reported [22]. A Th1 lymphocyte polarization even characterizes some related autoimmune disorders (CD, atrophic gastritis, type 1 diabetes) when occurring in isolated form [14–16]. Whether a similar lymphocytes polarization characterizes autoimmune thyroiditis when associated with other non-endocrine autoimmune disorders is not known. Our study is aimed at analysing and comparing distinctive intracellular cytokines in patients with autoimmune thyroiditis associated or not with selected non-endocrine autoimmune diseases.
Materials and methods
Patients
A total of 78 Caucasian patients agreed to participate in this study. The inclusion criteria were a definite diagnosis of HT associated or not with the most representative non-endocrine autoimmune diseases (chronic atrophic gastritis, CD, generalized vitiligo and Sjögren's syndrome). Exclusion criteria were: (a) the presence of anti-thyrotrophin (TSH)-receptor antibodies or ultrasonographic evidence of thyroid atrophy; (b) clinical history of hyperthyroidism; (c) evidence of infectious diseases in the last 3 months; (d) treatment with drugs known to interfere with the immune system, namely cytokines, interferon, corticosteroids, non-steroidal anti-inflammatory drugs (NSAIDs), amiodarone, lithium; (e) pregnancy and lactation over the previous 6 months; and (f) presence of acute or chronic systemic diseases other than those included above. Ten patients were subsequently excluded because they took drugs for concomitant diseases, became pregnant or because they had simultaneous infectious diseases. Of the remaining 68 (55 female, 13 male; mean age = 40 ± 16 years), 33 met the criteria for isolated chronic lymphocytic thyroiditis (28 females, five males; mean age = 34 ± 13 years). The remaining 35 patients (27 females, eight males; mean age = 47 ± 16 years), besides chronic lymphocytic thyroiditis, also had chronic atrophic gastritis (n = 18; seven patients also with pernicious anaemia), CD (n = 7), generalized vitiligo (n = 6) and Sjögren's syndrome (n = 4). The study was conducted with written informed consent and as part of the diagnostic work-up of the patients involved, according to the local ethical rules and the guidelines in the Declaration of Helsinki.
Materials
RPMI-1640 supplemented with 25 mm Hepes buffer, 2 mm glutamine, 100 U/ml penicillin, heat-inactivated fetal calf serum (FCS) and phosphate-buffered saline (PBS) Dulbecco's medium without calcium and magnesium and sodium bicarbonate were purchased from Gibco (Grand Island, NY, USA). Fycoll Hypaque (Lymphoprep) density 1·077 ± 0·001 g/ml, osmolality 280 ± 15 mOsm, was from Axis-Shield (Oslo, Norway). Phorbol-12-myristate-13-acetate (PMA), ionomycin, monensin and digitonin were purchased from Sigma (St Louis, MO, USA). Paraformaldehyde (PFA) was from Merck (Darmstadt, Germany). Monoclonal antibodies (anti-CD4, anti-CD8, anti-CD2, anti-IL-2, anti-IL-4, anti-IFN-γ fluorescein isothiocyanate (FITC)-conjugate and anti-CD8 phycoerythrin (PE)-conjugate) and isotype-matched antibodies were purchased from IL-Coulter (Hialeah, FL, USA).
Methods
Cell preparation
Blood samples were sampled from all patients at the same time of day and processed immediately. Peripheral blood lymphocytes (PBL) were separated from 10 ml heparinized venous blood and then mixed with 20 ml RPMI-1640, using Ficoll Hypaque density gradient centrifugation. PBL were washed twice and resuspended in RPMI-1640 supplemented with 10% FCS. The cell suspension was adjusted to a concentration of 1 × 106/ml and cultured in 24-well plates at 37°C and 5% CO2 for 24 h.
Intracellular cytokine study
PBL were stimulated with PMA (16 nm), ionomycin (1 µm) and monensin (20 µm) during the last 18 h of incubation and were then collected, washed in PBS and then fixed with paraformaldehyde 2% for 20 min at room temperature. The cellular suspension was washed with cold PBS and permeabilized with digitonin (60 µm) in the presence of specific monoclonal antibodies FITC-conjugate (anti-IL-2, anti-IL-4, anti-IFN-γ) and isotype-matched antibody [17]. After staining, all samples were washed with cold PBS and resuspended in PBS for flow cytometric analysis.
Flow cytometric analysis
Fluorescence of each sample was analysed on an EPICS XL Flow cytometer (Coulter), equipped with an argon laser at 488 nm. PBL were gated on the basis of forward-angle light-scatter (FS) and 90° light-scatter parameters (SS) and the percentage of purity was analysed using monoclonal antibody (mAb) anti-CD2. For every histogram, 10 000 events were counted in PBL gate CD2-positive. Samples were also examined using a Zeiss laser scanner microscope to localize the intracellular distribution of cytokines.
Surface staining
Surface staining of PBL was performed, before PMA and ionomycin stimulation, with mAb (anti-CD4, anti-CD8) FITC-conjugated. Alternatively, because the down-regulation of surface phenotype markers is particularly severe with CD4 in PMA and ionomycin-stimulated human T cells [23], PBL were fixed, permeabilized and stained with FITC-conjugated anti-IL in the presence of digitonin. After washing they were stained with anti-CD8 PE-conjugated and finally washed for flow cytometric analysis.
Diagnostic procedures
Diagnostic procedures for thyroid disease
Procedures to diagnose thyroid disease included routine clinical examinations, serum iodothyronines and TSH measurements, anti-thyroperoxidase antibodies (anti-TPOAb) and anti-thyroglobulin (anti-TgAb) detection and ultrasonography. Diagnosis of autoimmune thyroiditis was based on the particular ultrasonographic pattern [24], the presence of anti-TPOAb and, when present, of mild or overt hypothyroidism. FT4 levels were assayed by commercial radioimmunoassay (normal range = 10–23 pmol/l). TSH levels were assayed by immunoradiometric assay (normal range = 0·2–4 mU/l). The anti-TPO autoantibodies (negative: < 30 UI/ml) were measured by a immunoradiometric assay (intra-assay variation 7·2–13%; interassay variation 8·3–16·4%; Radim, Pomezia, Italy). The anti-Tg autoantibodies (negative: < 30 UI/ml) were measured by immunoradiometric assay (intra-assay variation 5·7–8·3%; interassay variation 9·3–12·8%; Radim).
Diagnosis of chronic atrophic gastritis (CAG)
The diagnosis of CAG was suspected on the basis of unexplained anaemia and the presence of anti-parietal cell antibodies and hypergastrinaemia and confirmed by histological examination of multiple specimens obtained at gastroscopy, as described previously [6]. Atrophy of the gastric mucosa was defined as focal or complete loss of glands and/or replacement by metaplastic, pyloric or intestinal glands. The degree of gastritis was assessed according to the updated Sydney System and its relative score [25]. Fasting plasma gastrin levels were evaluated by a specific radioimmunoassay using antibody 4562 (courtesy of Professor J. F. Rehfeld), as described [6].
Diagnosis of CD
The diagnosis of CD was suspected on clinical grounds (abdominal discomfort, unexplained iron-deficiency anaemia, low weight) and on positive serological screening tests, such as the measurement of serum anti-transglutaminase (tTgAb) and anti-endomysium antibodies (EMAb). CD was confirmed by histological examination of duodenal specimens obtained by upper intestinal endoscopy. The Marsh classification has been adopted to describe the degree of the abnormalities in the intestinal mucosa [26]. Half of the patients with CD had histological damage classified as Marsh type II and the remaining as Marsh type IIIa lesions.
Diagnosis of vitiligo
Only generalized vitiligo was considered, and the diagnosis was made on clinical grounds [27].
Diagnosis of Sjögren's syndrome
Diagnosis of primary Sjögren' syndrome was based on the presence of any four of six criteria according to American–European Consensus [28].
Statistics
Data are expressed as median value (interquartile range, IQ). Data were analysed by non-parametric Mann–Whitney U-test and/or correlated by Spearman's correlation test. Subgroup percentages were compared using Fisher's exact test. Analysis of variance (anova) was used to compare three or more variables. instat Graphpad™ version 3·06 (Graphpad Inc., San Diego, CA, USA) statistical software for Windows was used.
Results
Cytofluorometric analysis was performed on all patients to characterize surface lymphocytic antigens. No differences in the clusters of differentiation were recorded between patients with isolated HT and those with NEAD (data not shown). IFN-γ, but not IL-2 and/or IL-4, has been shown to correlate with surface lymphocytic antigens (Table 1). In particular, IFN-γ correlated fairly with CD8+ T lymphocytes (r = 0·37; P = 0·0039) and well with total natural killer (NK) (r = 0·56; P < 0·0001). The analysis of cytokines in peripheral blood lymphocytes showed a significantly increased percentage of IL-2+ cells (Th1) subset in all patients studied. The median results were similar in patients with isolated lymphocytic thyroiditis (34·4%) and in those with an associated autoimmune disease [36·3%; P = not significant (n.s.)] (Fig. 1a). Th1 polarization was confirmed by the increased IFN-γ-positive PBL in almost all patients from both groups. Normal to borderline percentages of IFN-γ+ cells were found in only five of 33 patients with isolated lymphocytic thyroiditis and in one of 35 patients with NEAD. However, the median percentage of IFN-γ-positive PBL was significantly lower in those with isolated lymphocytic thyroiditis (19·0%) than in patients with NEAD (29·9%; P = 0·0082) (Fig. 1b). With regard to the Th2 subset, most patients with isolated lymphocytic thyroiditis, as expected, had a normal percentage of IL-4+ cells and only three of 33 patients showed increased IL-4+ PBL. Interestingly, two of these three patients were relatives of patients with HT+NEAD. In contrast, most of the patients with NEAD (71%) had a significantly increased percentage of IL-4+ cells [Fisher's exact test: P < 0·0001; relative risk (RR) = 3·182]. The median values (16·8% versus 5·0%; P < 0·0001) were also significantly different (Fig. 2). These differences were independent from autoimmune disease associated with HT because, with one exception, the percentage of positive cells for each cytokine was not dissimilar in all subgroups (Table 2). Overall, the Th1/Th2 ratio was 3·8 in patients with isolated HT and decreased to 1·78 in those with NEAD.
Table 1.
Mathematical correlation† between selected cytokines and surface lymphocytic antigens
| CD3 | CD4 | CD8 | CD56 NK | CD16 56 | CD19 | CD4/CD8 | |
|---|---|---|---|---|---|---|---|
| IL-2 | 0·13 | 0·36 | −0·35 | −0·4 | −0·16 | 0·31 | 0·35 |
| IL-4 | −0·11 | −0·13 | 0·11 | 0·11 | −0·08 | −0·16 | −0·21 |
| IFN-γ | −0·23 | −0·4 | 0·37‡ | 0·56§ | 0·22 | −0·27 | −0·41 |
Spearman's correlation test has been used as statistical analysis; r values shown in bold type are statistically significant.
P = 0·0039.
P < 0·0001. IL, interleukin; IFN, interferon; NK, natural killer.
Fig. 1.
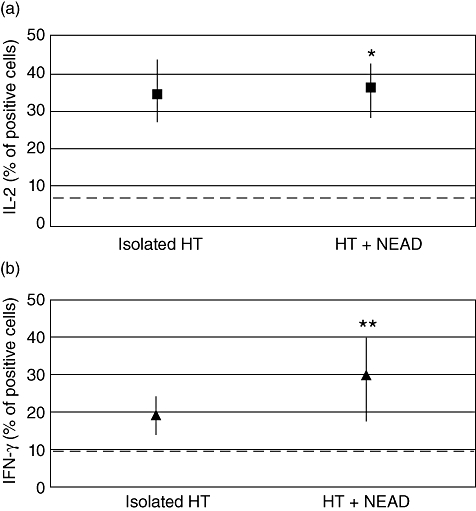
Percentages of interleukin (IL)-2 (a) and interferon (IFN)-γ (b) positive peripheral blood lymphocytes (median values) in patients with Hashimoto's thyroiditis, isolated and associated with non-endocrine autoimmune diseases. Dotted line = laboratory reference (upper value). *Mann–Whitney U-test: P = not significant. **Mann–Whitney U-test: P = 0·0082.
Fig. 2.
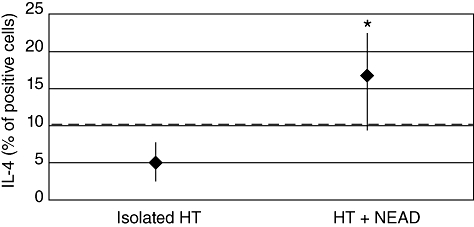
Percentages of interleukin (IL)-4-positive cells (median values) in patients with isolated Hashimoto's thyroiditis and associated with non-endocrine autoimmune diseases. Dotted line = laboratory reference (upper value). *Mann–Whitney U-test: P < 0·0001.
Table 2.
Comparison of cytokines-positive cells among different non-endocrine autoimmune diseases (NEAD) associated with autoimmune Hashimoto's thyroiditis (HT)
| IL-2 | IFN-γ | IL-4 | ||
|---|---|---|---|---|
| Patients with HT plus NEAD | No. of patients | Median % of positive cells (IQ1–IQ3) | ||
| Chronic atrophic gastritis (CAG) | 18 | 36·7 | 33·0‡ | 15·9 |
| (32·8–45·3) | (25·53–42·3) | (7·8–21·8) | ||
| Coeliac disease | 7 | 40·1 | 29·9 | 22·6 |
| (28·7–42) | (22·3–40·3) | (15·3–39·8) | ||
| Vitiligo | 6 | 23·7 | 13·5‡ | 20·6 |
| (22·7–34·8) | (12·2–16·9) | (15·6–21·3) | ||
| Sjögren's syndrome | 4 | 38·9 | 26 | 14·9 |
| (30·8–45) | (11·2–22·2) | (18–33·4) | ||
| P† | 0·3678 | 0·0051 | 0·3438 | |
Analysis of variance (anova) test was used as statistical analysis.
Kruskal–Wallis post-test: CAG versus vitiligo P < 0·01.
IQ, interquartile.
To validate these data further, we analysed whether some of the patients' characteristics represented a bias for the results. Patients' sex, age, thyroid function and autoantibody levels (anti-thyroperoxidase and anti-thyroglobulin) have been correlated with the percentage of positive cells for each cytokine. In the whole sample, no sex-related differences were observed in all cytokines studied (Table 3). The same table shows that there was no significant correlation between cytokine distribution and concentrations of TPOAb and TgAb. In contrast, linear regression revealed a positive correlation between increasing age and IFN-γ+ (P = 0·0003) (Fig. 3a). This finding was due mainly to the positive correlation between these variables observed in patients with isolated HT (Table 3). The number of IL-4-positive cells was not age-related (Fig. 3b). Euthyroid and subclinical hypothyroid patients showed similar median values of IL-4+ and IFN-γ+ cells (Table 3), even when subdivided by the presence or not of non-endocrine autoimmune disorders, making unlike an autonomous effect of thyroid function on these cytokines.
Table 3.
Statistical analysis† and mathematical correlation‡ between cytokine-positive cells and patients' characteristics
| IL-2 | IL-4 | IFN-γ | |
|---|---|---|---|
| †Sex (male versus female) | P = 0·0835 | P = 0·5852 | P = 0·1344 |
| †Function (euthyroidism versus subclinical hypothyroidism) | P = 0·3910 | P = 0·1784 | P = 0·6047 |
| ‡Age (years) | |||
| Isolated HT | P = 0·0943 | P = 0·0781 | P = 0·0151§ |
| HT+ NEAD | P = 0·8884 | P = 0·1887 | P = 0·1236 |
| ‡AbTPO (serum concentrations) | P = 0·8757 | P = 0·2584 | P = 0·9917 |
| ‡AbTg (serum concentrations) | P = 0·3631 | P = 0·2262 | P = 0·9545 |
Non-parametric Mann–Whitney U-test was used as statistical analysis.
Non-parametric correlation (Spearman's r test) was used for statistical analysis
r = 0·4194.
AbTPO, anti-thyroperoxidase autoantibody; AbTg, autoantibody thyroglobulin; HT, Hashimoto's thyroiditis; NEAD, non-endocrine autoimmune diseases; IFN, interferon; IL, interleukin.
Fig. 3.
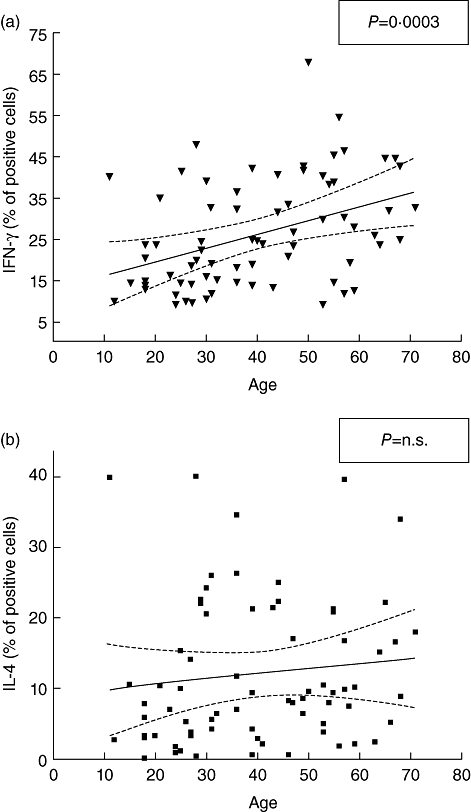
Correlation of interferon (IFN)-γ (a) and interleukin (IL)-4 (b) positive cells (%) with patients' age. Spearman's r correlation has been used to evaluate linear regression.
Based on these results, the positive predictive value of an increased percentage of IL-4+ cells as marker of association between thyroiditis and NEAD was 91%, whereas the negative predictive value was 71%. Sensitivity was 75%, specificity was 89% and the likelihood ratio was 7·000.
Discussion
The association of autoimmune thyroiditis and non-endocrine autoimmune disorders is ill-defined, although one of five patients with thyroiditis is likely to have some additional autoaggressive phenomenon [6,29]. In fact, despite thyroiditis being prototypical of organ specific autoimmune diseases, there is evidence that other non-endocrine autoimmune disorders may be associated and pathogenetically related [1,2,11,30]. A prevalent Th1 cytokine profile is usually observed in patients with organ-specific autoimmunity, whereas a prevalent Th2 profile has been associated with systemic autoimmunity [31]. Our results showed an increased percentage of IFN-γ+ and IL-2+ cells and a normal to low percentage of IL-4+ cells in all patients with isolated thyroiditis. These data confirmed the prevalent Th1 polarization in isolated thyroiditis, as reported in previous studies [19–21]. However, in our patients who were associated with more than one organ-specific autoimmune disease we observed a significant increase in the percentage of IL-4-positive cells, independently from the NEAD involved, as observed in systemic autoimmune disorders [31]. Hence, a characteristic Th2 cytokine co-exists with the described Th1 subset in these patients [31]. On these grounds it has been suggested that Th1 responses, when severe and/or chronic, may shift towards a less polarized profile (Th0) or even to responses characterized by the prevalent production of Th2 cytokines [31,32]. This phenomenon is known as immune deviation [31–33], and is in keeping with the relevant increase of IL-4-positive cells observed in our patients with NEAD. A protective Th2 activation may thus suggest that the simultaneous presence of HT and NEAD triggers a different immunological response than in isolated HT [31,32,34]. Based on the mutual inhibitory role of IFN-γ on Th2-cell differentiation and IL-4 on Th1-cell differentiation, we expected a reduction of IFN-γ+ cells [31,35]. Instead, IFN-γ+ cells were even increased along with IL-4+ cells in patients with HT and NEAD, in contrast to the expected shift of polarization towards Th2 profile [31,35]. It is notable that abundant IFN-γ-producing cells have also been described in mouse lung eosinophilia, a condition characterized by a Th1 to Th2 switch and the production of IL-4 and IL-5 [17]. These same authors speculated that IFN-γ has relatively weak effects locally and that this weakness is corrected for by its abundance, while IL-4 is very potent and needs to be produced by fewer cells to characterize the immunopathological process [17].
A previous report [19] described a small but significant increase of IL-4+ cells in euthyroid patients with isolated HT, which disappeared in hypothyroid patients. They suggested a different immunological status for euthyroid and hypothyroid HT patients [19]. In contrast, an elevated Th1/Th2 ratio (i.e. high IFN-γ+ and low IL-4+ cells) has been reported in severe HT compared with the mild form [36]. In our study, some possible sources of bias were checked but none of them affected the expression of IL-4 in PBL. In particular, the percentage of IL-4+ cells was similar and the Th1/Th2 ratio was comparable in euthyroid and hypothyroid HT patients. However, in most of our patients only mild or preclinical hypothyroidism was recognized.
We conclude that a clear-cut, unbiased increase of IL-4+ lymphocytes characterizes patients with autoimmune thyroiditis with associated non-endocrine autoimmune disorders. These findings may represent an initial tool to detect patients with autoimmune thyroiditis in which additional autoimmune disorders may be awaited, although the recognition of a specific marker of this association requires further investigation.
Acknowledgments
This study was supported by ‘Sapienza’ University of Rome (university grants – prot.0006345).
Disclosure
The authors have nothing to declare.
References
- 1.Eisenbarth GS, Gottlieb PA. Medical progress: autoimmune polyendocrine syndromes. N Engl J Med. 2004;350:2068–79. doi: 10.1056/NEJMra030158. [DOI] [PubMed] [Google Scholar]
- 2.Kahaly GJ. Polyglandular autoimmune syndromes. Eur J Endocrinol. 2009;161:11–20. doi: 10.1530/EJE-09-0044. [DOI] [PubMed] [Google Scholar]
- 3.Betterle C, Lazzarotto F, Presotto F. Autoimmune polyglandular syndrome Type 2: the tip of an iceberg? Clin Exp Immunol. 2004;137:225–33. doi: 10.1111/j.1365-2249.2004.02561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weetman AP. Association of autoimmune thyroiditis with other autoimmune diseases. Hot Thyroidology.com. 2004 n.1. [Google Scholar]
- 5.Betterle C, Dal Pra C, Mantero F, Zanchetta R. Autoimmune adrenal insufficiency and autoimmune polyendocrine syndromes: autoantibodies, autoantigens, and their applicability in diagnosis and disease prediction. Endocr Rev. 2002;23:327–64. doi: 10.1210/edrv.23.3.0466. [DOI] [PubMed] [Google Scholar]
- 6.Centanni M, Marignani M, Gargano L, et al. Atrophic body gastritis in patients with autoimmune thyroid disease: an underdiagnosed association. Arch Intern Med. 1999;159:1726–30. doi: 10.1001/archinte.159.15.1726. [DOI] [PubMed] [Google Scholar]
- 7.Centanni M, Gargano L, Canettieri G, et al. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med. 2006;354:1789–95. doi: 10.1056/NEJMoa043903. [DOI] [PubMed] [Google Scholar]
- 8.Spadaccino AC, Basso D, Chiarelli S, et al. Celiac disease in North Italian patients with autoimmune thyroid diseases. Autoimmunity. 2008;41:116–21. doi: 10.1080/08916930701620209. [DOI] [PubMed] [Google Scholar]
- 9.Gopal KV, Rama Rao GR, Kumar YH, Appa Rao MV, Vasudev P, Srikant B. Vitiligo: a part of a systemic autoimmune disease. Ind J Dermatol Venereol Leprol. 2007;73:162–5. doi: 10.4103/0378-6323.32710. [DOI] [PubMed] [Google Scholar]
- 10.Cervera R, Balasch J. Bidirectional effects on autoimmunity and reproduction. Hum Reprod Update. 2008;14:359–66. doi: 10.1093/humupd/dmn013. [DOI] [PubMed] [Google Scholar]
- 11.Wallaschofski H, Meyer A, Tuschy U, Lohmann T. HLA-DQA1*0301-associated susceptibility for autoimmune polyglandular syndrome type II and III. Horm Metab Res. 2003;35:120–4. doi: 10.1055/s-2003-39059. [DOI] [PubMed] [Google Scholar]
- 12.Weetman AP. Autoimmune thyroid disease: propagation and progression. Eur J Endocrinol. 2003;148:1–9. doi: 10.1530/eje.0.1480001. [DOI] [PubMed] [Google Scholar]
- 13.Weetman AP. Cellular immune responses in autoimmune thyroid disease. Clin Endocrinol (Oxf) 2004;61:405–13. doi: 10.1111/j.1365-2265.2004.02085.x. [DOI] [PubMed] [Google Scholar]
- 14.Castellanos-Rubio A, Santin I, Irastorza I, Castaño L, Carlos Vitoria J, Ramon Bilbao J. TH17 (and TH1) signatures of intestinal biopsies of CD patients in response to gliadin. Autoimmunity. 2009;42:69–73. doi: 10.1080/08916930802350789. [DOI] [PubMed] [Google Scholar]
- 15.D'Elios M, Appelmelk BJ, Amedei A, Bergman MP, Del Prete G. Gastric autoimmunity: the role of Helicobacter pylori and molecular mimicry. Trends Mol Med. 2004;10:316–23. doi: 10.1016/j.molmed.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Rabinovitch A, Suarez-Pinzon WL. Roles of cytokines in the pathogenesis and therapy of type 1 diabetes. Cell Biochem Biophys. 2007;48:159–63. doi: 10.1007/s12013-007-0029-2. [DOI] [PubMed] [Google Scholar]
- 17.Pala P, Hussel T, Openshaw PJ. Flow cytometric measurement of intracellular cytokines. J Immunol Methods. 2000;243:107–24. doi: 10.1016/s0022-1759(00)00230-1. [DOI] [PubMed] [Google Scholar]
- 18.Foster B, Prussin C, Liu F, Whitmire JK, Whitton JL. Detection of intracellular cytokines by flow cytometry. Curr Protoc Immunol. 2007;6:1–21. doi: 10.1002/0471142735.im0624s78. Unit 6.24. [DOI] [PubMed] [Google Scholar]
- 19.Mazziotti G, Sorvillo F, Naclerio C, et al. Type 1 response in peripheral CD4+ and CD8+ cells from patients with Hashimoto's thyroiditis. Eur J Endocrinol. 2003;148:383–8. doi: 10.1530/eje.0.1480383. [DOI] [PubMed] [Google Scholar]
- 20.Phenekos C, Vryonidou A, Gritzapis AD, Baxevanis CN, Goula M, Papamichail M. Th1 and Th2 serum cytokine profiles characterize patients with Hashimoto's thyroiditis (Th1) and Graves' disease (Th2) Neuroimmunomodulation. 2004;11:209–13. doi: 10.1159/000078438. [DOI] [PubMed] [Google Scholar]
- 21.Fisfalen ME, Palmer EM, Van Seventer GA, et al. Thyrotropin-receptor and thyroid peroxidase-specific T cell clones and their cytokine profile in autoimmune thyroid disease. J Clin Endocrinol Metab. 1997;82:3655–63. doi: 10.1210/jcem.82.11.4336. [DOI] [PubMed] [Google Scholar]
- 22.Figueroa-Vega N, Alfonso-Pèrez M, Benedicto I, Sànchez-Madrid F, Gonzàlez-Amaro R, Marazuela M. Increased circulating pro-inflammatory cytokines and Th17 lymphocytes in Hashimoto's thyroiditis. J Clin Endocrinol Metab. 2010;95:953–62. doi: 10.1210/jc.2009-1719. [DOI] [PubMed] [Google Scholar]
- 23.Petersen CM, Christensen EI, Andresen BS, Møller BK. Internalization, lysosomal degradation and new synthesis of surface membrane CD4 in phorbol ester-activated T-lymphocytes and U-937 cells. Exp Cell Res. 1992;201:160–73. doi: 10.1016/0014-4827(92)90360-k. [DOI] [PubMed] [Google Scholar]
- 24.Vitti P, Rago T. Thyroid ultrasound as a predicator of thyroid disease. J Endocrinol Invest. 2003;26:686–9. doi: 10.1007/BF03347031. [DOI] [PubMed] [Google Scholar]
- 25.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis: the updated Sydney System. Am J Surg Pathol. 1996;20:1161–81. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Green PH, Rostami K, Marsh MN. Diagnosis of coeliac disease. Best Pract Res Clin Gastroenterol. 2005;19:389–400. doi: 10.1016/j.bpg.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Taïeb A, Picardo M. Clinical practice.Vitiligo. N Engl J Med. 2009;360:160–9. doi: 10.1056/NEJMcp0804388. [DOI] [PubMed] [Google Scholar]
- 28.Vitali C, Bombardieri S, Jonsson R, et al. the European Study Group on Classification Criteria for Sjögren's Syndrome Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American–European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weetman AP. Non-thyroid autoantibodies in autoimmune thyroid disease. Best Pract Res Clin Endocrinol Metab. 2005;19:17–32. doi: 10.1016/j.beem.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Ohsako S, Elkon KB. Apoptosis in the effector phase of autoimmune diabetes, multiple sclerosis and thyroiditis. Cell Death Differ. 1999;6:13–21. doi: 10.1038/sj.cdd.4400459. [DOI] [PubMed] [Google Scholar]
- 31.Romagnani S. Regulation of T cell response. Clin Exp Allergy. 2006;36:1357–66. doi: 10.1111/j.1365-2222.2006.02606.x. [DOI] [PubMed] [Google Scholar]
- 32.Rotondi M, Chiovato L, Romagnani S, Serio M, Romagnani P. Role of chemokines in endocrine autoimmune diseases. Endocr Rev. 2007;28:492–520. doi: 10.1210/er.2006-0044. [DOI] [PubMed] [Google Scholar]
- 33.Romagnani S. Regulatory T cells: which role in the pathogenesis and treatment of allergic disorders? Allergy. 2006;61:3–14. doi: 10.1111/j.1398-9995.2006.01005.x. [DOI] [PubMed] [Google Scholar]
- 34.Marazuela M. Lymphocyte traffic and homing in autoimmune thyroid disorders. Eur J Endocrinol. 1999;140:287–90. doi: 10.1530/eje.0.1400287. [DOI] [PubMed] [Google Scholar]
- 35.Paludan SR. Interleukin-4 and interferon-γ: the quintessence of a mutual antagonistic relationship. Scand J Immunol. 1998;48:459–68. doi: 10.1046/j.1365-3083.1998.00435.x. [DOI] [PubMed] [Google Scholar]
- 36.Nanba T, Watanabe M, Inoue N, Iwatani Y. Increases of the Th1/Th2 cell ratio in severe Hashimoto's disease and in the proportion of Th17 cells in intractable Graves' disease. Thyroid. 2009;19:495–501. doi: 10.1089/thy.2008.0423. [DOI] [PubMed] [Google Scholar]


