Abstract
Apoptosis is known as a major mechanism which contributes to beta cell decay in type 1 diabetes. Commitment to this pathway generally involves caspase-mediated protein cleavage and was found to induce cross-presentation of a specific antigen repertoire under certain inflammatory conditions. We aimed to assess the significance of the CD8 T cell population reactive against such caspase-cleaved apoptotic self-antigens in pancreatic islets of prediabetic human leucocyte antigen (HLA)-A2 transgenic non-obese diabetic chimeric monochain transgene construct (NOD.HHD) mice. We have reproduced a unique peptide library consisting of human CD8 T cell-derived apoptosis-specific antigens, all of which belong to structural proteins expressed ubiquitously in human islets. Pancreatic islets from prediabetic NOD.HHD mice, harbouring humanized major histocompatibilty complex (MHC) class I, were isolated and handpicked at various ages, and islet-infiltrating CD8 T cells were expanded in vitro and used as responders in an interferon (IFN)-γ enzyme-linked immunospot (ELISPOT) assay. Human T2 cells were used as antigen-presenting cells (APC) to avoid endogenous antigen presentation. Analogous to the interindividual variability found with peptides from known islet autoantigens such as islet-specific glucose-6-phosphatase catalytic subunit related protein (IGRP) and insulin, some mice showed variable, low-degree CD8 T cell reactivity against caspase-cleaved self-antigens. Because reactivity was predominantly minor and often undetectable, we conclude that beta cell apoptosis does not routinely provoke the development of dominant cytotoxic T lymphocyte (CTL) reactive against caspase-cleaved self-antigens in the NOD.HHD model.
Keywords: apoptosis, CD8 T cells, diabetes, epitope spreading, humanized mice
Introduction
Type 1 diabetes (T1D) is characterized by the autoimmune destruction of pancreatic islets [1,2]. A prime pathogenic role for CD8 T cells has been theorized based on their predominance within islet lesions around onset and the detection of islet antigen-reactive species in peripheral blood from T1D patients [3]. CD8 T cell specificities associated with T1D include insulin [4], preproinsulin[5], islet-specific glucose-6-phosphatase catalytic subunit related protein (IGRP; [6,7]), glutamic acid decarboxylase (GAD65; [8]), insulinoma-associated antigen-2 (I-A2; [9]) and preproislet amyloid polypeptide (ppIAPP; [10]). A unique study by Skowera et al. demonstrated that preproinsulin-reactive CD8 T cells from human T1D patients exert cytotoxicity against islets in vitro, which serves as a direct indication of their pathological relevance [5]. In prediabetic non-obese diabetic (NOD) mice, it was shown that up to 60% of CD8 T cells obtained from inflamed islets recognize either insulin, IGRP or dystrophia myotonica kinase (DMK; [11]). Whereas these disease-specific reactivities are generally believed to initiate and drive disease progression, it remains unknown to what extent secondary neo-antigens are involved in driving the later stages of beta cell decay.
Apoptosis is the principal pathway leading to degeneration of beta cell function and viability in T1D. This highly conserved process is orchestrated by the enzymatic cleavage of proteins by caspases, resulting in controlled phagocytosis of cellular debris. The outcome of this cascade is typically tolerogenic and does not give rise to immunity against components of the dying cell. Consequently, induction of a limited degree of apoptosis in prediabetic NOD mice or transfer of dendritic cells loaded with apoptotic bodies prevents the progression to hyperglycaemia and stimulates tolerization against islet antigens [12,13]. In some occasions autoantigen overexpression itself may lead to beta cell apoptosis in the absence of primary inflammation [14]. Other studies, however, have found that caspase activation, similar to oxidative modifications [15], may selectively modify common cellular components, which in turn can become ‘neo-antigens’ responsible for activating autoreactive lymphocytes [16]. Apoptotic cells are thus considered to be an important source of self-antigens that, depending on their surrounding milieu, may induce tolerance or invigorate ongoing autoimmune responses.
In the current study we wished to assess the relative contribution of CD8 T cells specific for caspase-cleaved apoptotic self-antigens to islet lesions in the human leucocyte antigen (HLA)-A0201-transgenic non-obese diabetic chimeric monochain transgene construct (NOD.HHD) mouse. This mouse strain develops autoimmune diabetes analogous to the conventional NOD mouse, but has a CD8 T cell repertoire that recognizes islet epitopes similar to the ones found in T1D patients carrying the HLA-A0201 haplotype [7,17]. We have utilized a unique set of HLA-A0201-restricted peptides derived from ubiquitous structural cell proteins that are generated specifically under conditions of caspase activity in humans [18]. While Rawson and co-workers originally derived this peptide library from apoptotic CD8 T cells, all belong to ubiquitously expressed ‘housekeeping genes’ and can thus be found consistently within pancreatic islet cells [19,20]. In using this library we therefore assumed that these common proteins are processed similarly through caspase activity in apoptotic beta cells. While Rawson et al. demonstrated that caspases 3 and 8 are responsible for generation of the peptides studied here, data from a variety of mouse and human assays indeed show that both executioner caspases are the principal effectors in beta cell apoptosis [21]. We therefore expanded the T cell infiltrates from isolated islets obtained from prediabetic NOD.HHD mice and probed this population for reactivity against apoptosis-associated peptides by enzyme-linked immunospot (ELISPOT).
We demonstrate here that apoptosis-related neo-antigen formation in the NOD.HHD mouse appears to be an incidental phenomenon in most animals.
Materials and methods
Mice
Female NOD.β2mnull.HHD mice [7] were purchased from The Jackson Laboratory (Bar Harbor, MI, USA). NOD.β2mnull.HHD mice express a monochain chimeric HLA-A*0201 molecule consisting of human β2-microglobulin covalently linked to the α1 and α2 domains of HLA-A*0201, followed by the α3 transmembrane and cytoplasmic portions of H-2Db. Mice were euthanized humanely before each experiment and all animal procedures were approved by the Ethics Committee at The La Jolla Institute for Allergy and Immunology.
Peptides
All peptides used were described previously by Rawson et al. We selected the set of peptides that had significant binding capacity [half maximal inhibitory concentration (IC50) < 500 nm] for HLA-A0201, the HLA class I molecule that is transgenically expressed by the NOD.β2mnull.HHD strain. A total of 123 peptides (Table S1) were reproduced by Mimotopes (Clayton, Victoria, Australia) and dissolved in dimethylsulphoxide (DMSO) (Sigma, St Louis, MO, USA).
Islet isolation
The procedure for islet isolation and CD8 T cell expansion is described in detail by Jarchum et al. [37]. Briefly, islets were isolated from female NOD.β2mnull.HHD mice by collagenase (collagenase P; Roche, Mannheim, Germany) perfusion of the common bile duct. After pancreas homogenization, islets were purified over a Ficoll PM400 gradient (Sigma). Islets were stained with dithizone (Sigma), handpicked under a dissecting microscope and cultured for 7 days in 24-well tissue culture plates (∼50 islets/well) in RPMI-1640 medium (Invitrogen, San Diego, CA, USA) supplemented with 10% fetal bovine serum (FBS), 50 units/ml recombinant human interleukin (IL)-2 (Roche), glutamax, penicillin, streptamycin and fungizone (all from Invitrogen). The entire study describes the screening of a 123-peptide library in triplicate. Each peptide was screened twice at 10 weeks and once at 16 weeks using pooled islet samples of two mice, for a total of 14 female NOD.HHD mice accounting for approximately 1400 handpicked islets.
IFN-γ ELISPOT assay
IFN-γ ELISPOT assay was performed as described previously [37]. ELISPOT plates (Multiscreen from Millipore, Billerica, MA, USA) were precoated with anti-mouse IFN-γ monoclonal antibody (mAb) (BD Biosciences, San Diego, CA, USA) and blocked with 1% bovine serum albumin (BSA). Irradiated T2 cells (American Type Culture Collection no. CRl-1992™) were added at 2 × 104 cells/well and pulsed with 1 µm peptide. Cultured islet-infiltrating T cells were added at 2 × 104 cells/well, and plates were incubated at 37°C for 40 h. IFN-γ secretion was detected with a second, biotinylated anti-mouse IFN-γ mAb (BD Biosciences) and spots were developed using streptavidin–horseradish peroxidase (Vector Laboratories, Burlingame, CA, USA) and 3-amino-9-ethylcarbazole substrate (Sigma-Aldrich). Spots were counted manually regardless of size and the counts from the wells stimulated with medium only were subtracted.
Flow cytometry
After culturing, the content of the wells was filtered over a 40 micron strainer and conventional antibody staining was performed. All antibodies used were purchased from BD Biosciences and analysis was performed using an LSR-II flow cytometer (BD Biosciences). The brightfield image in Fig. 1b was taken with an upright Nikon Eclipse microscope equipped with a 4× air objective.
Fig. 1.
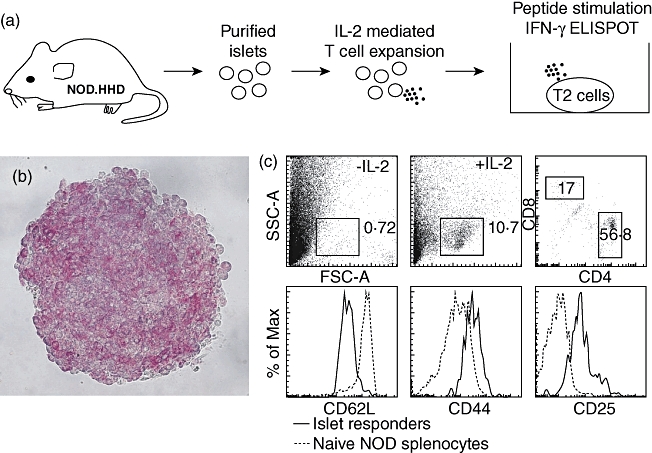
Experimental approach. (a) Pancreatic islets were isolated and handpicked from two NOD.β2mnull.HHD mice, expressing human leucocyte antigen (HLA)-A*0201, but no murine class I molecules, yielding a population of highly pure islets (b, stained with dithizone). These islets were cultured for 7 days in the presence of interleukin (IL)-2, harvested and used as responders in an interferon (IFN)-γ enzyme-linked immunospot ELISPOT (assay). Class II major histocompatibility complex (MHC) antigen-negative, transporter antigen processing (TAP)-deficient T2 cells were used as antigen-presenting cells (APC). These cells express low levels of class I MHC molecules, which are increased and stabilized upon binding of exogenous peptides. (c) Flow cytometry reveals that a subset of highly activated CD8 T cells was expanded. Data are from a single preparatory experiment performed prior to and independent of the seven experimental runs to screen the peptide library.
Results
Experimental approach for the analysis of apoptosis-associated CD8 T cell responses in the humanized NOD.HHD mouse
The caspase-cleaved apoptotic self-antigens used in this study have been reported previously by Rawson et al. and were generated by apoptosis induction in HLA-A2-restricted, human CD95+CD8+ T cell clones by treatment with a monoclonal antibody to CD95 [18]. The apoptotic cells were lysed and their proteome was compared to control cells using subtractive analysis by two-dimensional electrophoresis to identify modified proteins. Most of the spots found only in apoptotic cells corresponded to fragments derived from cytoskeletal proteins [non-muscle myosin, cytoplasmic actin, vimentin and lamin B1), proteins involved in the regulation of the cytoskeleton (Rho guanine nucleotide dissociation inhibitor-2 (Rho GDPI2)] or nuclear lamina and matrix proteins [heterogeneous nuclear ribonucleoprotein K (hnRNPK)]. Several of these proteins have been reported to be targets of autoantibodies, thus supporting the interaction between apoptosis and autoimmunity. Peptides containing HLA binding motifs were then identified, synthesized and tested for their class I binding capacity, as described previously [22]. For our study we selected the peptides associated with moderate to high binding capacity to HLA-A0201 (Table S1).
Pancreatic islets were isolated and handpicked from NOD.β2mnull.HHD mice (Fig. 1a,b), which exclusively express HLA-A0201 and harbour an HLA-A0201-restricted diabetogenic CD8 T cell population that infiltrates the islets. Previous analysis of 8-week-old NOD.β2mnull.HHD and NOD female mice indicated similar levels of leucocytic infiltration [7]. The T cell population within the cultured islets was then expanded for 7 days by addition of exogenous IL-2, and these cells were finally used as responders in an IFN-γ ELISPOT with MHC II-negative, transporter antigen processing (TAP)-deficient T2 cells as antigen-presenting cells (APC). Figure 1c shows the expansion of a population of highly activated CD8 T cells from the islets after culturing.
Unique CD8 T cell reactivity profiles within islet infiltrates from NOD.β2mnull.HHD mice
Taking into account that all NOD.β2mnull.HHD mice used here had been inbred for many generations and are thus genetically virtually identical, one would expect similar T cell reactivity profiles and hierarchies compared to regular NOD mice as diabetes development progresses. Takaki and co-workers demonstrated earlier that individual NOD.β2mnull.HHD mice exhibit distinct patterns of CD8+ T cell reactivity to IGRP peptides and that peptide 228–236 is the immunodominant HLA-A0201-binding epitope of IGRP [7]. Another study identified the immunodominant insulin epitope Ins1/2 A2–10 and showed that most NOD.β2mnull.HHD mice responded to both Ins1/2 A2-10 and IGRP 228–236 with minor subsets of animals responding to only one of both or to none [23]. We chose to use both peptides as positive controls in our assays for reactivity against apoptosis-related reactivity. Our results here confirm the results by Jarchum et al. in showing that individual animals develop distinct diabetogenic CD8 T cell profiles within insulitic lesions at 10 and 16 weeks of age (Fig. 2). Our data also indicate that at 10 weeks of age reactivity can be directed against one of both peptides (compare rectangle 1 versus 2 in Fig. 2a), whereas in the late prediabetic phase at 16 weeks reactivity against IGRP 228–236 is generally dominant. In conclusion, these data confirm the observation that insulitis in the NOD.β2mnull.HHD is associated with unique patterns of CD8 T cell reactivity against diabetes-associated islet autoantigens.
Fig. 2.
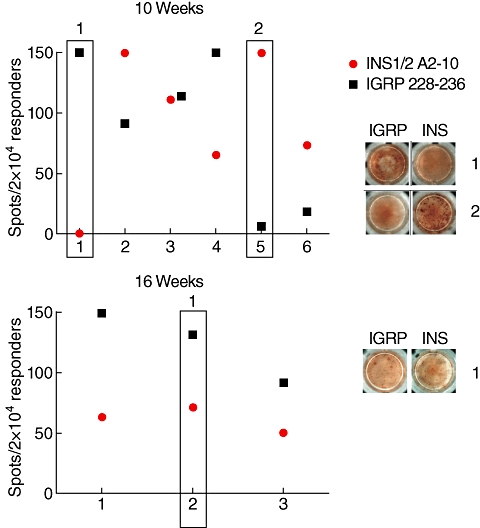
Variability in CD8 T cell islet specificities among individual mice. (a) Significant variability in reactivity against two immunodominant CD8 T cell epitopes within six individual experiments using 10-week-old mice. Each experiment represents pooled expanded islet CD8 T cell populations from two mice. (b) At 16 weeks of age, variability remains, although reactivity can always be found against both epitopes. The response against islet-specific glucose-6-phosphatase catalytic subunit related protein (IGRP) 228–236 is dominant at this stage. Rectangles correspond with images on right taken from individual wells. These data are derived from positive controls included in seven experiments performed to screen the peptide library and two independent preparatory experiments using 10-week-old mice. Values equal to 150 represent spot counts ≥ 150 due to limitations on counting accuracy above that value.
Secondary CD8 T cell reactivity against apoptosis-related epitopes is incidental
All 123 apoptosis-related peptides listed in Table S1 were screened for the ability to induce IFN-γ production in expanded islet-infiltrating CD8 T cells. As the number of cells that can be expanded from a single animal is limited, a series of four experiments using pooled populations from two individual mice was performed in order to screen each peptide in duplicate at 10 weeks of age. Representative experiments are shown in Fig. 3 and show that such reactivity, although often detectable, is limited in comparison to both Ins1/2 A2-10 and IGRP 228–236. Analogous to the variable reactivity pattern against both control islet epitopes, different mice showed distinct reactivity profiles against apoptosis-related peptides (compare Fig. 3a versus b). Similar results were obtained at 16 weeks of age (Fig. 4), at which stage the mice were still normoglycaemic in our hands, but higher numbers of T cells could be expanded from the harvested islets. The latter observation indicated that this stage represents the late prediabetic phase and allowed us to complete screening of the entire library in three experiments. We did not see a consistent correlation between the magnitude of the IFN-γ response and the affinity of the stimulating peptide for HLA-A0201. For instance, peptide 63 derived from myosin type A, which exhibits the strongest stimulatory capacity in Fig. 4b, was classified as only an intermediate binder. An excellent binder from the same molecule such as peptide 57 did not yield any response. Collectively, these results indicate that reactivity against apoptosis-related epitopes in the prediabetic NOD.β2mnull.HHD mouse constitutes a minor subset among islet-infiltrating cells.
Fig. 3.
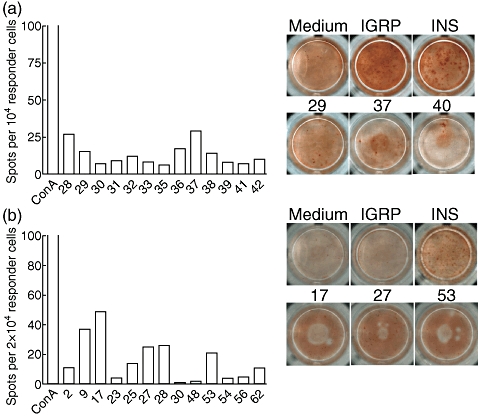
Low and variable responses against apoptosis-specific epitopes at 10 weeks of age. (a) Peptides 28–42 were screened, all of which are derived from myosin type A, except 28 (lamin B1). Background using medium only was seven spots. (b) Peptides 1–90 were screened, derived from actin cyto beta (1–8), hnRNP K (9–16), lamin B1 (17–28), myosin type A (29–88) and Rho GDI 2 (89–90). Only peptides associated with above-background detection are plotted. Concanavalin A stimulation was used as an additional positive control. Background using medium only was 57 spots in this experiment. Each experiment represents pooled expanded islet CD8 T cell populations from two mice. The entire peptide library was screened twice in four individual experiments using mice of the same age.
Fig. 4.
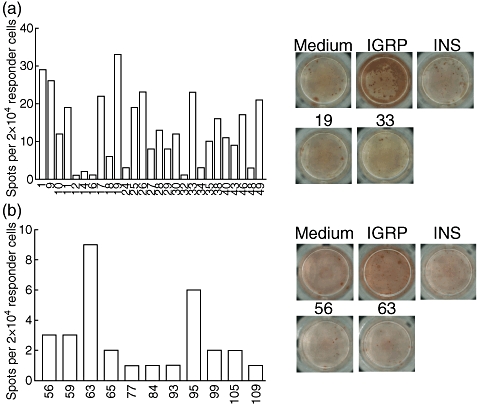
The same low variable responses against apoptosis-related epitopes can be found at 16 weeks of age. (a) Peptides 1–49 were screened derived from actin cyto beta (1–8), hnRNP K (9–16), lamin B1 (17–28), myosin type A (29–49). Background was 30 spots. (b) Peptides 50–123 were screened, derived from myosin type A (50–88), Rho GDI 2 (89–92), vimentin (93–114), 60S acidic ribosomal protein P2 (115–118) and proteasome component C2 (119–123). Only peptides associated with above-background detection are plotted. Background was 14 spots in this experiment. Each experiment represents pooled expanded islet CD8 T cell populations from two mice. The entire peptide library was screened once in three individual experiments using mice of the same age.
Dominant reactivity against a vimentin epitope was encountered in a single occasion (Fig. 5). In this experimental run, the anti-vimentin response surpassed the response against Ins1/2 A2-10. In order to ascertain that the vimentin sequence is not found in any known diabetes-related proteins we performed a Basic Local Alignment Search Tool (BLAST) search which returned no such molecules. The vimentin peptide did not particularly bind more strongly to HLA-A0201 compared with other peptides examined and was classified as having intermediate affinity. Finally, in an unsuccessful attempt to reproduce this strong response, we performed additional experiments at 10 and 16 weeks of age, but none showed this dominant response. We conclude that the development of a dominant secondary response against apoptosis-related antigens during diabetes development in the NOD.β2mnull.HHD mouse is a rare phenomenon.
Fig. 5.
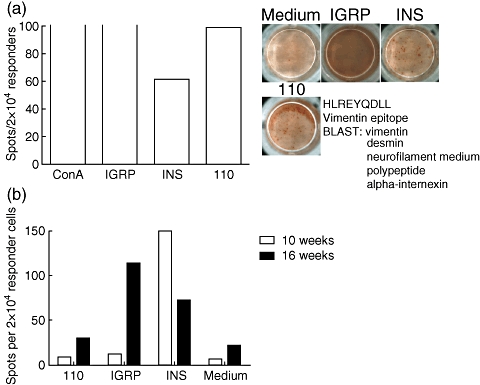
Some mice develop dominant responses against apoptosis-related epitopes at 10 weeks. (a) In one experiment we found strong reactivity against a vimentin-derived epitope at 10 weeks of age. A Basic Local Alignment Search Tool (BLAST) returned no sequence relation to known islet autoantigens. Background using medium only was six spots. (b) This reactivity appears to be incidental, as no reactivity was found in two pairs of other mice aged 10 and 16 weeks. Concanavalin A stimulation was used as positive control. Open bars indicate that amount of spots precluded accurate counting. Background using medium only was five spots (10 weeks) and 22 spots (16 weeks). Each experiment represents pooled expanded islet CD8 T cell populations from two mice, aged 10 weeks in (a) and (b) and 16 weeks in (b).
Discussion
In this present study we investigated the relative contribution of CD8 T cells reactive against apoptosis-related epitopes within the total population of islet-infiltrating lymphocytes from prediabetic NOD.β2mnull.HHD mice. We found that only a small subset reacts against these epitopes, with different reactivity patterns among individual mice.
The rationale for this study was built on the premise that the set of peptides generated upon caspase activation in human apoptotic cells is derived from structural proteins with highly conserved sequences between man and mouse. For instance, vimentin shares 97% of its amino acid sequence between both species [24,25]. Whereas some of the 123 epitopes tested may not share complete sequence similarity, conserved sequences can be assumed for the majority of included peptides. The original study by Rawson and co-workers generated a more extensive list of peptides that are expressed preferentially under conditions of apoptosis, with a focus on CD8 T cell reactivity profiles in human immunodeficiency virus (HIV) patients [18]. We selected the subset of peptides that was capable of efficient binding to HLA-A201 and asked whether islet-infiltrating CD8 T cells recognize these neo-epitopes during the course of autoimmune diabetes.
The potential differences in expression between the structural proteins investigated here from human CD8 T cells (from which Rawson et al. obtained the peptide library) and islet cells can be expected to be minimal. Indeed, comprehensive proteomic studies of human islets demonstrate that these ‘housekeeping genes’ are, without exception, expressed in islet cells [19,20]. In conjunction with the shared predominance of executioner caspases 3 and 8 during apoptosis induction in both cell types [18,21], we reasoned that similar peptide fragments are probably generated under apoptotic conditions.
Because the islet-infiltrating T cell population in patients is inaccessible, we resorted to the ‘humanized’ NOD.HHD mouse, which has been shown previously to exhibit a close resemblance to T1D patients with regard to CD8 T cell reactivity profiles [7]. The CD8 T cells in this animal are diabetogenic in vivo and are restricted to the HLA-A0201 sequence, which is one of the most prevalent class I alleles, with a frequency of > 60% in T1D patients. Moreover, its expression has been shown to confer additional risk to the development of T1D in patients possessing the high-risk class II DR3/4-DQ8 haplotype [26,27]. The choice for this humanized model may thus allow for better interpretation in the context of human disease.
Aberrant autoimmunity to common structural cell proteins has been described on multiple occasions. Early studies found that the immunological outcome of apoptosis depends critically on the local milieu and in particular the concurrent release of ‘danger’ signals. Systemic exposure to apoptotic cells or apoptosis induction in the presence of viral particles was shown to shift the balance from tolerance to autoimmunity[28,29]. Similarly, the induction of efficient anti-tumour responses requires signals provided by ongoing necrosis to clear apoptotic cells [30]. Collectively, these data suggest that apoptotic cells are captured consistently by dendritic cells, but induce dendritic-cell maturation and T cell cross-priming only in the presence of exogenous inflammatory mediators or CD4+ T cell help; otherwise, tolerance is induced [31]. Apoptosis is an important pathological component in many autoimmune diseases, including type 1 diabetes [32], and the requirement for additional ‘danger’ signals is often fulfilled in affected patients. Under these conditions, ubiquitous structural proteins can become the target of a secondary immune response, as exemplified by cytotoxic T lymphocyte (CTL) priming against vinculin from apoptotic cells [33]. Another example is the detection of autoantibodies against vimentin in lupus patients or CD8 T cell reactivity against that protein in cardiac transplant patients [34–36].
From the results presented here, we conclude that the contribution of neo-antigen formation and CTL priming as a result of caspase activation in apoptotic cells is limited in the prediabetic NOD.β2mnull.HHD mouse. Furthermore, the latter responses vary substantially between genetically identical animals, in line with the interindividual variability observed in CD8 T cell responses against bona fide islet autoantigens. Only on one occasion was a significant response against a vimentin epitope detected, but this result could not be confirmed in other animals. Finally, the possibility cannot be excluded that the low activation levels observed are related to the secondary priming of cells responsive to the self-peptides.
In summary, this study indicates that while a unique reactivity pattern develops against islet-specific epitopes in islets from NOD.HHD mice, we found no evidence of any significant secondary repertoire directed against apoptosis-related epitopes.
Acknowledgments
We thank Vincenzo Barnaba and Alessandro Sette for help with the production of the peptides. This work was funded by a U-01 Prevention Centers pilot grant from the NIH. We thank the Brehm coalition for their continued support.
Disclosure
The authors have nothing to disclose.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Table S1. List of peptides used in this study.
*IC50 indicates binding affinity for A*0201 and it is expressed as 50% inhibitory nanomolar concentration; peptides with IC50 <50 nM are classified as high affinity peptides, peptides with IC50 >50 nM are classified as intermediate affinity peptides, peptides with IC50 >500 nM were classified as low affinity peptides in the original study and were not resynthesized [18].
hnRNP K, heterogeneous nuclear ribonucleoprotein K; Rho GDI 2, Rho guanine nucleotide dissociation inhibitor-2. References on islet expression pertain to studies on isolated human islets or human beta cell lines.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Staeva-Vieira T, Peakman M, von Herrath M. Translational mini-review series on type 1 diabetes: immune-based therapeutic approaches for type 1 diabetes. Clin Exp Immunol. 2007;148:17–31. doi: 10.1111/j.1365-2249.2007.03328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol. 2009;155:173–81. doi: 10.1111/j.1365-2249.2008.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coppieters KT, von Herrath MG. Histopathology of type 1 diabetes: old paradigms and new insights. Rev Diabet Stud. 2009;6:85–96. doi: 10.1900/RDS.2009.6.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinkse GG, Tysma OH, Bergen CA, et al. Autoreactive CD8 T cells associated with beta cell destruction in type 1 diabetes. Proc Natl Acad Sci USA. 2005;102:18425–30. doi: 10.1073/pnas.0508621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skowera A, Ellis RJ, Varela-Calvino R, et al. CTLs are targeted to kill beta cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope. J Clin Invest. 2008;118:3390–402. doi: 10.1172/JCI35449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unger WW, Pinkse GG, Mulder-van der Kracht S, et al. Human clonal CD8 autoreactivity to an IGRP islet epitope shared between mice and men. Ann NY Acad Sci. 2007;1103:192–5. doi: 10.1196/annals.1394.024. [DOI] [PubMed] [Google Scholar]
- 7.Takaki T, Marron MP, Mathews CE, et al. HLA-A*0201-restricted T cells from humanized NOD mice recognize autoantigens of potential clinical relevance to type 1 diabetes. J Immunol. 2006;176:3257–65. doi: 10.4049/jimmunol.176.5.3257. [DOI] [PubMed] [Google Scholar]
- 8.Panina-Bordignon P, Lang R, van Endert PM, et al. Cytotoxic T cells specific for glutamic acid decarboxylase in autoimmune diabetes. J Exp Med. 1995;181:1923–7. doi: 10.1084/jem.181.5.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi K, Honeyman MC, Harrison LC. Cytotoxic T cells to an epitope in the islet autoantigen IA-2 are not disease-specific. Clin Immunol. 2001;99:360–4. doi: 10.1006/clim.2001.5031. [DOI] [PubMed] [Google Scholar]
- 10.Panagiotopoulos C, Qin H, Tan R, Verchere CB. Identification of a beta-cell-specific HLA class I restricted epitope in type 1 diabetes. Diabetes. 2003;52:2647–51. doi: 10.2337/diabetes.52.11.2647. [DOI] [PubMed] [Google Scholar]
- 11.Lieberman SM, Takaki T, Han B, Santamaria P, Serreze DV, DiLorenzo TP. Individual nonobese diabetic mice exhibit unique patterns of CD8+ T cell reactivity to three islet antigens, including the newly identified widely expressed dystrophia myotonica kinase. J Immunol. 2004;173:6727–34. doi: 10.4049/jimmunol.173.11.6727. [DOI] [PubMed] [Google Scholar]
- 12.Marin-Gallen S, Clemente-Casares X, Planas R, et al. Dendritic cells pulsed with antigen-specific apoptotic bodies prevent experimental type 1 diabetes. Clin Exp Immunol. 2010;160:207–14. doi: 10.1111/j.1365-2249.2009.04082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hugues S, Mougneau E, Ferlin W, et al. Tolerance to islet antigens and prevention from diabetes induced by limited apoptosis of pancreatic beta cells. Immunity. 2002;16:169–81. doi: 10.1016/s1074-7613(02)00273-x. [DOI] [PubMed] [Google Scholar]
- 14.Harashima SI, Harashima C, Nishimura T, Hu Y, Notkins AL. Overexpression of the autoantigen IA-2 puts beta cells into a pre-apoptotic state: autoantigen-induced, but non-autoimmune-mediated, tissue destruction. Clin Exp Immunol. 2007;150:49–60. doi: 10.1111/j.1365-2249.2007.03455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trigwell SM, Radford PM, Page SR, et al. Islet glutamic acid decarboxylase modified by reactive oxygen species is recognized by antibodies from patients with type 1 diabetes mellitus. Clin Exp Immunol. 2001;126:242–9. doi: 10.1046/j.1365-2249.2001.01653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahoney JA, Rosen A. Apoptosis and autoimmunity. Curr Opin Immunol. 2005;17:583–8. doi: 10.1016/j.coi.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Marron MP, Graser RT, Chapman HD, Serreze DV. Functional evidence for the mediation of diabetogenic T cell responses by HLA-A2.1 MHC class I molecules through transgenic expression in NOD mice. Proc Natl Acad Sci USA. 2002;99:13753–8. doi: 10.1073/pnas.212221199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rawson PM, Molette C, Videtta M, et al. Cross-presentation of caspase-cleaved apoptotic self antigens in HIV infection. Nat Med. 2007;13:1431–9. doi: 10.1038/nm1679. [DOI] [PubMed] [Google Scholar]
- 19.Kutlu B, Burdick D, Baxter D, et al. Detailed transcriptome atlas of the pancreatic beta cell. BMC Med Genomics. 2009;2:3–14. doi: 10.1186/1755-8794-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metz TO, Jacobs JM, Gritsenko MA, et al. Characterization of the human pancreatic islet proteome by two-dimensional LC/MS/MS. J Proteome Res. 2006;5:3345–54. doi: 10.1021/pr060322n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi D, Woo M. Executioners of apoptosis in pancreatic {beta}-cells: not just for cell death. Am J Physiol Endocrinol Metab. 2010;298:E735–41. doi: 10.1152/ajpendo.00696.2009. [DOI] [PubMed] [Google Scholar]
- 22.Sidney J, Southwood S, Mann DL, Fernandez-Vina MA, Newman MJ, Sette A. Majority of peptides binding HLA-A*0201 with high affinity crossreact with other A2-supertype molecules. Hum Immunol. 2001;62:1200–16. doi: 10.1016/s0198-8859(01)00319-6. [DOI] [PubMed] [Google Scholar]
- 23.Jarchum I, Baker JC, Yamada T, et al. In vivo cytotoxicity of insulin-specific CD8+ T-cells in HLA-A*0201 transgenic NOD mice. Diabetes. 2007;56:2551–60. doi: 10.2337/db07-0332. [DOI] [PubMed] [Google Scholar]
- 24.Wood L, Theriault N, Vogeli G. Vimentin cDNA clones covering the complete intermediate-filament protein are found in an EHS tumor cDNA library. Gene. 1989;76:171–5. doi: 10.1016/0378-1119(89)90020-6. [DOI] [PubMed] [Google Scholar]
- 25.Hennekes H, Kuhn S, Traub P. Coding sequence and flanking regions of the mouse vimentin gene. Mol Gen Genet. 1990;221:33–6. doi: 10.1007/BF00280364. [DOI] [PubMed] [Google Scholar]
- 26.Fennessy M, Metcalfe K, Hitman GA, et al. A gene in the HLA class I region contributes to susceptibility to IDDM in the Finnish population. Childhood Diabetes in Finland (DiMe) Study Group. Diabetologia. 1994;37:937–44. doi: 10.1007/BF00400951. [DOI] [PubMed] [Google Scholar]
- 27.Robles DT, Eisenbarth GS, Wang T, et al. Millennium award recipient contribution. Identification of children with early onset and high incidence of anti-islet autoantibodies. Clin Immunol. 2002;102:217–24. doi: 10.1006/clim.2001.5171. [DOI] [PubMed] [Google Scholar]
- 28.Mevorach D, Zhou JL, Song X, Elkon KB. Systemic exposure to irradiated apoptotic cells induces autoantibody production. J Exp Med. 1998;188:387–92. doi: 10.1084/jem.188.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosen A, Casciola-Rosen L, Ahearn J. Novel packages of viral and self-antigens are generated during apoptosis. J Exp Med. 1995;181:1557–61. doi: 10.1084/jem.181.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–34. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rovere-Querini P, Manfredi AA, Sabbadini MG. Environmental adjuvants, apoptosis and the censorship over autoimmunity. Autoimmun Rev. 2005;4:555–60. doi: 10.1016/j.autrev.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 32.Thomas HE, McKenzie MD, Angstetra E, Campbell PD, Kay TW. Beta cell apoptosis in diabetes. Apoptosis. 2009;14:1389–404. doi: 10.1007/s10495-009-0339-5. [DOI] [PubMed] [Google Scholar]
- 33.Propato A, Cutrona G, Francavilla V, et al. Apoptotic cells overexpress vinculin and induce vinculin-specific cytotoxic T-cell cross-priming. Nat Med. 2001;7:807–13. doi: 10.1038/89930. [DOI] [PubMed] [Google Scholar]
- 34.Senecal JL, Rothfield NF, Oliver JM. Immunoglobulin M autoantibody to vimentin intermediate filaments. J Clin Invest. 1982;69:716–21. doi: 10.1172/JCI110500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barber LD, Whitelegg A, Madrigal JA, Banner NR, Rose ML. Detection of vimentin-specific autoreactive CD8+ T cells in cardiac transplant patients. Transplantation. 2004;77:1604–9. doi: 10.1097/01.tp.0000129068.03900.25. [DOI] [PubMed] [Google Scholar]
- 36.Alcover A, Molano J, Renart J, Gil-Aguado A, Nieto A, Avila J. Antibodies to vimentin intermediate filaments in sera from patients with systemic lupus erythematosus. Arthritis Rheum. 1984;27:922–8. doi: 10.1002/art.1780270812. [DOI] [PubMed] [Google Scholar]
- 37.Jarchum I, Takaki T, DiLorenzo TP. Efficient culture of CD8(+) T cells from the islets of NOD mice and their use for the study of autoreactive specificities. J Immunol Methods. 2008;339:66–73. doi: 10.1016/j.jim.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fred RG, Adams CM, Welsh N. Affinity binding analysis shows that hnRNP K and hnRNP E, in cytosolic extracts from human islets, binds specifically to the stabilizing segment of insulin mRNA. Diabetologia. 2007;50(Suppl 1):S207. [Google Scholar]
- 39.Moss SF, Krivosheyev V, de Souza A, et al. Decreased and aberrant nuclear lamin expression in gastrointestinal tract neoplasms. Gut. 1999;45:723–9. doi: 10.1136/gut.45.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elrick LJ, Docherty K. Phosphorylation-dependent nucleocytoplasmic shuttling of pancreatic duodenal homeobox-1. Diabetes. 2001;50:2244–52. doi: 10.2337/diabetes.50.10.2244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


