Abstract
Studies have indicated that interleukin (IL)-10 has a pathogenic role in systemic lupus erythematosus (SLE); however, a protective effect of IL-10 in SLE was also observed. Because the exact mechanism of IL-10 signalling in the pathogenesis of SLE is unclear, this study sought to assess the expression and signalling of interleukin-10 receptor (IL-10R) in peripheral leucocytes from patients with SLE. We used flow cytometry to examine the expression of IL-10R1 on different peripheral leucocytes from 28 SLE patients, of whom 14 had lupus nephritis (LN) and 14 were healthy controls. We also examined the effects of IL-10 on phosphorylation of signal transducer and activator of transcription (STAT)-3 and STAT-1 in peripheral blood mononuclear cells (PBMCs) obtained from 13 SLE patients and seven healthy controls. Plasma cytokines were detected by flow cytometric bead array (CBA) techniques. Although IL-10R1 expression levels on each peripheral leucocyte subset from 28 SLE patients and 14 healthy controls were similar, the expression levels on CD4+ T cells from LN patients were significantly lower than on CD4+ T cells from controls and SLE patients without nephritis (P < 0·01). IL-10R1 expression levels on CD4+ and CD8+ T cells were correlated negatively with the SLE disease activity index (P < 0·01). Additionally, the phosphorylation of STAT-3 was delayed and reduced in PBMCs from LN patients and active SLE patients. Plasma IL-10 levels were significantly higher in LN patients than controls. IL-10R1 expression on CD4+ T cells and signalling in PBMCs were down-regulated in LN patients, indicating that IL-10 and its receptor may have a special role in LN pathogenesis.
Keywords: interleukin-10 receptor, lupus nephritis, signal transduction, STAT-3 transcription factor, systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by production of numerous autoantibodies and damage to multiple organ systems. As seen commonly in autoimmune diseases, genetic and (or) environmental factors damage the immune system and result in the development of SLE [1].
Interleukin (IL)-10 is pleiotropic in its abilities to stimulate B lymphocyte proliferation, immunoglobulin secretion, inhibit T helper type 1 (Th1) responses, promote Th2 responses and to induce the differentiation of regulatory CD4+ T cells (Tr1) [2]. Because of its potential ability for inducing autoantibody production, IL-10 was presumed to play an important role in the pathogenesis of SLE. Indeed, a series of studies have indicated that IL-10 may play a central role in the pathogenesis of SLE. Llorente and co-workers published the first paper describing IL-10 overproduction by peripheral blood mononuclear cells (PBMCs) from SLE patients [3]. Several subsequent studies also confirmed this observation [4–7]. Furthermore, correlation of serum IL-10 levels with disease activity has been demonstrated in almost all related studies [8–10]. However, the exact contribution of IL-10 to the pathogenesis of SLE is undefined, and the origin of IL-10 overproduction is unclear. There was also a report showing that IL-10 can down-modulate murine lupus through inhibition of pathogenic Th1 cytokine responses [11]. Additionally, recent studies have identified some types of regulatory B cells, including B10 cells, whose regulatory effects are mediated by IL-10 [12–15], suggesting that IL-10 has a protective effect during lupus progression. These contradictory results suggest that IL-10 signalling has multiple and complex effects on the development of SLE. As the IL-10 receptor (IL-10R) is an indispensable component of the IL-10 signalling pathway and is expressed differentially on immune cells, we hypothesized that IL-10R might be involved in the development of human or animal lupus.
Functional IL-10R is a tetrameric complex composed of two ligand-binding alpha chains (IL-10R1) and two accessory-signalling beta chains (IL-10R2). IL-10R1 expression is critical for IL-10-mediated immune regulation [16]. Our previous study showed that lupus-prone New Zealand white (NZW) and Murphy Roths large (MRL) mice share the same genetic polymorphisms on the coding sequence (CDS) of the IL-10R1 gene, and this finding implies that an IL-10R1 abnormality may be related to the susceptibility to murine lupus [17]. However, few studies have focused on the potential correlation between IL-10R1 and human SLE. Two studies have shown no difference in the IL-10R1 expression levels between SLE patients and healthy controls [18,19]; however, the later study also showed that the gene expression pattern was aberrant in immune cells from SLE patients when induced through IL-10R [19].
The major signal transduction pathway for IL-10 is the Janus kinase/signal transducer and activator of transcription (JAK/STAT) system. Binding of IL-10 to the extracellular domain of IL-10R1 activates phosphorylation of the receptor-associated Janus tyrosine kinases – JAK1 and Tyk2. These kinases then induce the phosphorylation and activation of the transcription factors, mainly signal transducer and activator of transcription 3 (STAT-3) and STAT-1, which translocate to the nucleus, modifying gene expression [16]. In this paper, we investigated the involvement of IL-10R1 in human SLE by examining its expression and signal transduction in different PBMC subsets from SLE patients and healthy controls, and showed that IL-10R1 expression and signalling were down-regulated in CD4+ cells from lupus nephritis (LN) patients.
Materials and methods
Patients and healthy controls
Twenty-eight SLE patients, 24 females and four males, from Shengjing Hospital of China Medical University in Shenyang (China) and fulfilling the American College of Rheumatology revised classification criteria for lupus [20], were included in the study. Fourteen of the 28 patients were categorized as having lupus nephritis, based on the urine protein and sediment. The mean age was 36 years (range 17–56 years). Lupus disease activities were assessed using the SLE disease activity index (SLEDAI) [21]. A patient was defined as having active SLE when the SLEDAI score was ≥ 10·0, and was defined otherwise as inactive. The data from SLE patients and healthy controls are shown in Table 1.
Table 1.
Clinical characteristics of SLE patients and controls
| LN | Non-LN | HC | |
|---|---|---|---|
| Number | 14 | 14 | 14 |
| Gender (male/female) | 3/11 | 4/10 | 2/12 |
| Age (years) (range, median) | 22–53, 32 | 17–56, 36 | 19–55, 35 |
| Disease duration (years) | 0–20 | 0–30 | |
| Newly diagnosed/treated | 3/11 | 5/9 | |
| SLEDAI (range, median) | 4–31, 14 | 0–22, 7 |
LN: lupus nephritis patients; non-LN: systemic lupus erythematosus (SLE) patients without nephritis; HC: healthy controls; SLEDAI: SLE disease activity index.
Fourteen age- and gender-matched healthy hospital employees (mean age 35 years; age range 19–55) were studied in parallel as controls. This study was approved by the ethics committee of China Medical University, and all participating subjects provided their informed consent.
Immunostaining and flow cytometric analysis
The following monoclonal antibodies were used for the detection of IL-10R1 expression on the surface of different peripheral leucocytes: phycoerythrin (PE)-IL-10R1 [clone 3F9, rat immunoglobulin (Ig)G2aκ], PE-isotype (R35-95, rat IgG2aκ), fluorescein isothiocyanate (FITC)-anti-CD4 (SK3, mouse IgG1), peridinin chlorophyll protein (PerCP)-anti-CD8 (SK1, mouse IgG1), FITC-anti-CD14 (M5E2, mouse IgG2aκ) and FITC-anti-CD19 (HIB19, mouse IgG1κ). All monoclonal antibodies were purchased from BD PharMingen (San Diego, CA, USA). Briefly, fresh whole blood samples were incubated for 30 min at room temperature with monoclonal antibodies. The red blood cells were lysed for 15 min, and the remaining cells were washed twice with phosphate-buffered saline (PBS) and detected within 30 min using a BD fluorescence activated cell sorter (FACS)Calibur cytometer (BD Biosciences, San Jose, CA, USA). Flow cytometry data were collected and analysed using CellQuest software. As IL-10R1 labelling displays with monophasic distribution, the data are presented as the mean fluorescence intensity (MFI) within each cell subset.
Cells culture and IL-10 stimulation
For the detection of IL-10R signals after IL-10 stimulation, PBMCs were isolated from 10 ml of venous blood by Ficoll-Hypaque (TianJin Hao Yang Biological Manufacture Co., TianJin, China) density gradient centrifugation. Cell viability was determined, and cells were adjusted to 5 × 105 cells/ml in HyClone RPMI-1640 culture medium with l-glutamine (Thermo Fisher Scientific, Waltham, MA, USA), supplemented with 10% heat-inactivated fetal calf serum (TianJin Hao Yang Biological Manufacture Co.). After culture at 37°C in a humidified 5% CO2 atmosphere for 1 h, cells were stimulated with recombinant human IL-10 (Spodoptera frugiperda, Sf 21-derived; R&D Systems, Minneapolis, MN, USA), followed by phosphorylation analysis by flow cytometry.
Phosphorylation analysis by flow cytometry
For dose–response experiments, cells were stimulated with increasing doses of recombinant human IL-10 (rhIL-10) (2, 5, 10, 20 and 40 ng/ml). For time–courses, PBMCs were stimulated with rhIL-10 (10 ng/ml) or left unstimulated and collected at different times (5, 15 or 30 min). Phosphorylation of STAT-1 and STAT-3 was detected by flow cytometry according to the manufacturer's protocol (BD™ Phosflow protocol III for human PBMC). The following antibodies were used: AlexaFluor 488 mouse anti-pSTAT1 (pY701), clone: 4a; AlexaFluor 647 mouse anti-pSTAT3 (pY705), clone: 4/P-STAT3; and mouse IgG2a, mouse IgG1 isotype. Flow cytometry analysis was performed using a BD FACSCalibur cytometer (BD Biosciences). Flow cytometry data were collected in list mode and analysed using CellQuest software.
Cytokine detection
To determine the cytokine profiles of SLE patients and controls, we detected several Th1/Th2 [interferon (IFN)-γ, IL-2, IL-6 and IL-10] cytokines simultaneously in the plasma of SLE patients and healthy controls using flow cytometric bead array (CBA) techniques. The human enhanced sensitivity Flex Set system (BD Biosciences) was used. Briefly, following the preparation of standards and dilution of the individual plasma samples, mixed capture beads were incubated with the standards or plasma for 2 h, and then with added detection reagent for another 2 h. After washing the tubes, the enhanced sensitivity detection reagent was added and incubation was continued for an additional 1 h. After washing the tubes again, samples were analysed by a FACSAria cytometer (BD Biosciences) and data were analysed using FCAP Array software.
Statistical analysis
Statistical analysis was performed using spss version 13·0 software. The MFIs of IL-10R1 expression levels were expressed as mean ± standard deviation (s.d.), and the differences were examined using two-tailed t-tests or one-way analysis of variance (anova) and least significant difference (LSD) multiple comparisons, because the data were distributed normally. The differences of plasma cytokine levels were examined using a non-parametric Kruskal–Wallis test and the Mann–Whitney U-test. Correlations were assessed using Spearman's rank correlation test. Statistical analyses of time–courses and levels of phosphorylation for STAT-3 and STAT-1 between groups were performed using two-way anova. In all tests, statistical significance was defined as a P-value < 0·05.
Results
No difference in PBMC IL-10R1 expression was found between total SLE patients and healthy controls
To determine if IL-10R1 was expressed aberrantly in SLE patients, we examined the IL-10R1 expression on PBMC subsets from SLE patients by flow cytometry. Figure 1a shows the representative flow cytometric histograms of IL-10R1 expression on the different leucocyte subsets. We found that the expression intensities varied among peripheral CD4+ T lymphocytes, CD8+ T lymphocytes, CD14+ monocytes and CD19+ B lymphocytes. The highest levels of IL-10R1 were consistently on monocytes, the next highest levels were on CD8+ cells and CD4+ cells, and the lowest levels were on CD19+ cells. The MFIs of IL-10R1 on CD14, CD8, CD4 and CD19 cells from healthy control subjects were 34·4 ± 8·3, 19·1 ± 3·8, 15·7 ± 3·9 and 10·0 ± 3·4, respectively.
Fig. 1.
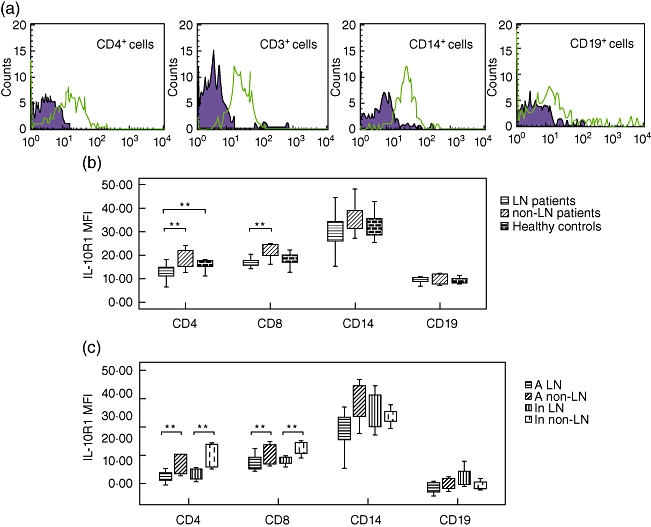
The expression of interleukin-10 receptor alpha chains (IL-10R1) on different leucocyte subsets. (a) Representative flow cytometric histograms show the levels of IL-10R1 expression (solid line) on the surface of different leucocyte subsets. Black lines filled with shadow represent isotype controls. (b) Comparison of IL-10R1 expression on leucocyte subsets from lupus nephritis (LN) patients, systemic lupus erythematosus (SLE) patients without nephritis (non-LN patients) and healthy controls. (c) Comparison of IL-10R1 expression on leucocyte subsets from active LN patients, active non-LN patients, inactive LN patients and inactive non-LN patients. A LN: active LN patients; A non-LN: active non-LN patients; In LN: inactive LN patients; In non-LN: inactive non-LN patients. Data are depicted as box-plots. Means are noted by inside bars and the boxes span the 25th and 75th percentiles, while whiskers represent the 5th and 95th percentiles. **P < 0·01.
No significant differences in IL-10R1 intensity on total leucocytes or leucocyte subsets were observed between 28 SLE patients and 14 healthy controls. In addition, no differences were observed among eight newly diagnosed SLE patients, 20 treated patients and 14 healthy controls, or between any two groups. These results indicated that IL-10R1 was not commonly involved in SLE pathogenesis.
Patients with LN have less IL-10R1 expressed on CD4+ cells than healthy controls or SLE patients without LN
As SLE patients developed various clinical manifestations of their disease, we looked for the association of IL-10R1 abnormalities with specified clinical subtypes and found that the expression intensity of IL-10R1 was lower in PBMCs from patients with LN. As shown in Fig. 1b, the IL-10R1 expression intensity on CD4+ cells from LN patients was significantly lower compared to cells from healthy controls and SLE patients without LN (non-LN patients); the MFIs were 12·8 ± 2·9 versus 15·9 ± 2·4 and 21·7 ± 4·2, P < 0·01. In addition, we observed that the IL-10R1 expression intensity on CD8+ cells from LN patients was significantly lower than on CD8+ cells from non-LN patients (MFIs were 16·9 ± 3·2 versus 21·8 ± 4·1, P < 0·01), but only slightly (not significantly) lower than on cells from controls. Although we observed that non-LN patients also expressed slightly higher levels of IL-10R1 on CD14+ and CD19+ cell subsets, no significant differences were observed among controls, LN and non-LN patients, or between any two groups.
IL-10R1 expression levels on CD4+ and CD8+ T cells were correlated negatively with SLEDAI scores
We assessed the correlation between IL-10R1 expression levels and SLEDAI scores using Spearman's rank correlation test. As shown in Fig. 2a, a strong negative correlation was observed between the expression intensity of IL-10R1 on CD4+ cells and the SLEDAI scores. A similar negative correlation was observed for CD8+ cells (Fig. 2b). No correlation was observed between IL-10R1 expression on CD14+ cells or CD19+ cells and the SLEDAI scores.
Fig. 2.
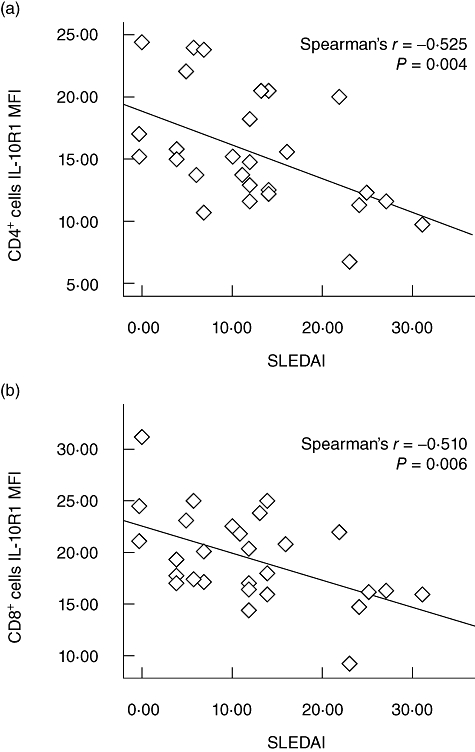
Correlation of interleukin-10 receptor alpha chains (IL-10R1) expression levels with systemic lupus erythematosus (SLE) disease activity index (SLEDAI). (a) Regression of the IL-10R1 expression on CD4+ cells (28 patients) to the SLEDAI of all SLE patients. (b) Regression of the IL-10R1 expression on CD8+ cells (28 patients) to the SLEDAI of all SLE patients.
Because some active SLE patients also have nephritis, the differences between active versus inactive patients and LN versus non-LN patients may be affected by each other. To diminish the interactions, we compared the IL-10R1 expression levels of LN versus non-LN patients in active patients (SLEDAI ≥ 10) and inactive patients (SLEDAI < 10) separately by subdividing the patients into the following groups: active LN group (11 patients), active non-LN group (five patients), inactive LN group (five patients) and inactive non-LN group (seven patients). As shown in Fig. 1c, we found that LN patients still expressed significantly lower levels of IL-10R1 on CD4+ and CD8+ cells compared with non-LN patients, P < 0·01, regardless of whether they were in an active or an inactive patient group. However, the IL-10R1 expression levels of active versus inactive patients were not significantly different in the LN group or in the non-LN group. This result emphasized that the expression of IL-10R1 on CD4+ and CD8+ T cells was down-regulated in LN, a particular subtype of SLE, and this may contribute to the pathogenesis of LN.
IL-10R signalling was impaired in LN and active SLE patients
The reduced expression of IL-10R1 may affect the downstream signalling of IL-10. To identify whether the IL-10R signalling in SLE patients is abnormal, we evaluated in vitro the phosphorylation of STAT-1 and STAT-3, two critical transcription factors in IL-10 signalling, in PBMCs from 13 SLE patients and seven healthy controls by flow cytometry. Because 10 ng/ml IL-10 was usually used to elicit STAT-3 activation in macrophages and was proved to produce efficient suppression of tumour necrosis factor (TNF)-α release [22,23], we selected several concentrations (0, 5, 10, 20 and 40 ng/ml) around 10 ng/ml to perform the titration of rhIL-10 for stimulation (PBMCs were collected at 15 min after stimulation). After demonstrating several cases of detection, we concluded that a concentration of 10 ng/ml rhIL-10 was sufficient to elicit STAT-3 and STAT-1 activation (Fig. 3). Therefore, in the following detection, addition of 10 ng/ml rhIL-10 was used for stimulation of PBMCs, and the phosphorylations of STAT-1 and STAT-3 were detected at 0 min, 5 min, 15 min and 30 min after rhIL-10 stimulation. We found that the phosphorylation of STAT-3 was induced more strongly by rhIL-10 than was phosphorylation of STAT-1 in both SLE patients and healthy controls, suggesting that STAT-3 is the main transcription factor in IL-10 signalling.
Fig. 3.
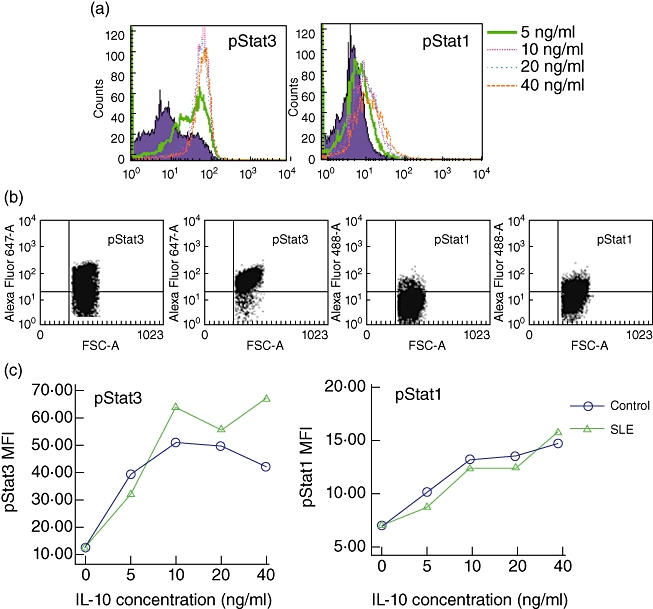
Phosphorylation of signal transducer and activator of transcription (STAT)-3 and STAT-1 in peripheral blood mononuclear cells (PBMCs) after increasing doses of recombinant human interleukin (rhIL)-10 stimulation. After 1 h of culture, PBMCs were stimulated with rhIL-10 (5, 10, 20 or 40 ng/ml) or left unstimulated, collected at 15 min, and then phosphorylation of STAT-3 and STAT-1 was detected by flow cytometry. (a) Representative flow cytometric histograms show the phosphorylation of STAT-3 and STAT-1 with different concentrations of IL-10 stimulation. Shaded histograms represent phosphorylation in unstimulated PBMCs. (b) Representative flow cytometric dot plots show positive cells for phosphorylation of STAT-3 and STAT-1 before (left) and after (right) rhIL-10 (10 ng/ml) stimulation. Dots in upper right quadrant represent positive cells. (c) Phosphorylation of STAT-3 and STAT-1 with different concentrations of IL-10 stimulation in two samples; y-axes represent mean fluorescence intensity (MFI) and x-axes represent different doses of rhIL-10. pSTAT3: phosphorylation of STAT-3. pSTAT1: phosphorylation of STAT-1.
As shown in Fig. 4a, in healthy controls, the phosphorylation of STAT-3 in PBMCs reached a peak value at 15 min after IL-10 stimulation. However, in SLE patients phosphorylation of STAT-3 was delayed, taking up to 30 min to reach the peak value. Also, although the phosphorylation levels of STAT-3 upon rhIL-10 stimulation were similar between 13 SLE patients and seven healthy controls, they were significantly lower in four active SLE patients when compared with seven healthy controls and nine inactive patients. As shown in Fig. 4c, the P-values determined by two-way anova were both < 0·001. In addition, we analysed the phosphorylation levels of STAT-3 and STAT-1 after rhIL-10 stimulation in six LN patients, seven non-LN patients and seven healthy controls. As shown in Fig. 4d, the phosphorylation levels of STAT-3 after rhIL-10 stimulation in LN patients were significantly lower than in non-LN patients and healthy controls, P < 0·05. Figure 4d also shows the average phosphorylation levels of STAT-3 in the subdivided groups according to the SLEDAI and LN. Although the patients with simultaneously active SLE and LN disease manifested the lowest phosphorylation levels of STAT-3, the sample number was too small to perform a statistical analysis. There were no differences in the phosphorylation levels of STAT-3 in newly diagnosed SLE patients, treated patients and healthy controls.
Fig. 4.
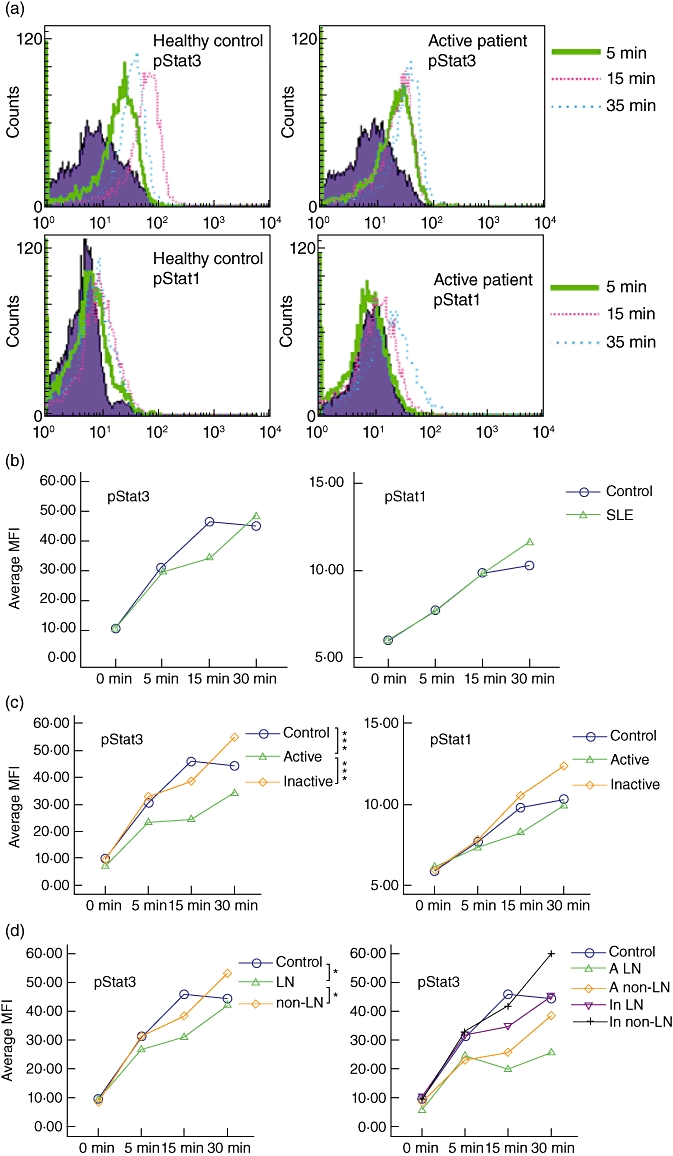
Phosphorylation of signal transducer and activator of transcription (STAT)-3 and STAT-1 in peripheral blood mononuclear cells (PBMCs) at different times following recombinant human interleukin (rhIL)-10 stimulation. PBMCs were stimulated with rhIL-10 (10 ng/ml) or left unstimulated (0 min), and collected at 5 min, 15 min and 30 min; phosphorylation of STAT-3 and STAT-1 was detected by flow cytometry. (a) Representative flow cytometric histograms for phosphorylation of STAT-3 and STAT-1 from an active systemic lupus erythematosus (SLE) patient and a healthy control. Shaded histograms represent unstimulated PBMCs (0 min). (b) Analysis of time–courses for phosphorylation of STAT-3 and STAT-1 in controls and SLE patients. (c) Analysis of time–courses for phosphorylation of STAT-3 and STAT-1 in controls, active patients, and inactive patients. (d) Analysis of time courses for phosphorylation of STAT-3 in controls, LN, and non-LN group (left), and in different subgroups (right); y-axes represent average mean fluorescence intensity (MFI) and x-axes represent time–courses after rhIL-10 stimulation. pSTAT3: phosphorylation of STAT-3. pSTAT1: phosphorylation of STAT-1. A LN: active LN patients; A non-LN: active non-LN patients; In LN: inactive LN patients; In non-LN: inactive non-LN patients. *P < 0·05; ***P < 0·001.
For STAT-1, we also observed delayed phosphorylation in SLE patients; however, the phosphorylation levels were similar among controls, active patients, inactive patients, LN patients and non-LN patients. In summary, our data suggest that IL-10 signalling is defective in patients with LN and in active SLE patients.
Plasma cytokine levels in SLE patients and healthy controls
We observed significantly higher plasma IL-6 and lower plasma IL-2 levels in all SLE patients than in healthy controls, but observed similar levels of IL-6 and IL-2 in LN and non-LN patients. Plasma IL-10 levels were significantly higher in LN patients than in controls, but not in non-LN patients. The plasma IFN-γ concentrations of patients and controls were all close to the lowest detection limit of the assay, and were not taken into consideration. The results are displayed in Table 2. There was a negative correlation between plasma IL-10 levels and IL-10R1 levels on CD4+ and CD8+ T cells, indicating that IL-10 and its receptor on T cells may have some regulatory effect on each other. Plasma IL-6 and IL-2 levels were not correlated with IL-10R1 expression. Plasma IL-10, IL-6 and IL-2 levels were not correlated with SLEDAI.
Table 2.
Plasma cytokines concentration in different SLE subgroups and controls
| Cytokine | Subgroup | Median (IQR) (pg/ml) |
|---|---|---|
| IL-2 | LN | 0·03 (0·00, 0·19)* |
| Non-LN | 0·05 (0·00, 0·21)* | |
| HC | 0·12 (0·05, 0·49) | |
| IL-6 | LN | 2·47 (1·03, 16·28)* |
| Non-LN | 2·65 (0·94, 4·41)* | |
| HC | 0·61 (0·37, 1·51) | |
| IL-10 | LN | 1·01 (0·12, 4·06)* |
| Non-LN | 0·00 (0·00, 0·59) | |
| HC | 0·00 (0·00, 0·25) |
P < 0·05 versus controls.
IL: interleukin; IQR: interquartile range; LN: lupus nephritis patients; non-LN: systemic lupus erythematosus (SLE) patients without nephritis; HC: healthy controls.
Discussion
SLE is clinically heterogeneous, and individual cytokine patterns will be more or less important to different disease manifestations and subtypes of patients [24]. In this study we investigated the expression and signalling of IL-10R1 in SLE patients to elucidate the role of the IL-10 signalling pathway in the pathogenesis of SLE. We found that the patients with LN expressed lower levels of IL-10R1 on CD4+ cells than controls and non-LN patients. The patients with LN also expressed lower levels of IL-10R1 on CD8+ cells than non-LN patients, but not lower than controls. Moreover, the expression levels of IL-10R1 on CD4+ and CD8+ T cells were correlated negatively with SLE disease activity. In addition to the IL-10R1 expression difference, we found that plasma IL-10 levels were higher in the LN group than in controls, but not in the non-LN group, which was also observed by Lit et al. [25]. Our results indicated that dysregulation of IL-10 and its receptor in CD4+ and CD8+ T cells may play an important role in the pathogenesis and development of LN, a particular subtype of SLE, but not in all SLE patients.
T cells are thought to play a central role in the regulation of the immune system. They activate B cell functions, including the production of autoantibodies, and initiate renal disease by increasing intrarenal nephritogenic cytokines [26–28]. Simultaneous blockading of the B7/CD28 and CD40/gp39 co-stimulation pathways could produce beneficial effects in murine lupus [29]. With regard to the effects of IL-10 on T cells, studies have proved that IL-10 administration results in the direct and indirect inhibition of T cell functions [30–33]. IL-10 administration was also reported to convert responder T cells into IL-10 producers, acting to suppress inflammatory responses [34]. In addition, some studies have demonstrated that IL-10R1 expression plays a critical role in determining whether cells respond to IL-10 [35–37]. Because we found that IL-10R1 expression levels on CD4+ T cells and CD8+ T cells were correlated negatively with SLE disease activity, and the STAT-3 phosphorylation of PBMCs upon IL-10 stimulation were delayed and down-regulated in LN and active patients, we hypothesized that IL-10R expression and signalling down-regulation may lead to a poorer response of effector T cells to the inhibitory signals of IL-10. These effects could result in T cell activation, followed by initiation or enhancement of autoimmune pathogenesis in LN patients.
However, the mechanisms of IL-10R1 expression and signalling down-regulation in CD4+ and CD8+ cells are not yet clear. In this study, we found a negative correlation between plasma IL-10 and IL-10R1 levels on CD4+ and CD8+ T cells. A previous study has shown that the expression of IL-10R1 mRNA was down-regulated after activation in some human T cell clones [38]. These results indicated that circulatory IL-10 and its receptor on T cells may have some regulatory effect on each other. In Caucasian populations, IL-10R1 sense polymorphisms S138G and G330R were proved to be loss-of-function alleles, which could influence IL-10-induced STAT-1 and STAT-3 activation, and G330R may possibly contribute to RA or SLE disease susceptibility [39,40]. However, in the Han populations of China, we have detected IL-10R1 sense polymorphism within exon, but found no contribution to SLE susceptibility (data not shown). Therefore, further research is required to elucidate the mechanism of IL-10R1 expression and signalling down-regulation in CD4+ and CD8+ T cells in LN patients, and to elucidate whether the down-regulation of IL-10R1 expression is a pathogenic factor or a result of an abnormal phenotype.
Regrettably, in this study, we could not verify the positive correlation between circulatory IL-10 levels and disease activity which had been demonstrated earlier [8–10]. We believe that several factors may have interfered with the results. First, the limited sample size in this study may have reduced the statistical power. Secondly, the detection sensitivity may be lower when plasma rather than serum is used for detection of circulatory cytokines, and in fact the IL-10 levels in nearly 50% of the cases in this study were below the lowest detection limit. However, our results may still be of significance because half of our study subjects were non-LN patients, in which both we and Lit et al. [25] observed no higher levels of IL-10, and the lower levels of IL-10 in this subgroup may decrease the correlation. Our observation that IL-10R1 expression levels on CD8+ cells from LN patients were not significantly lower than from controls could also be attributed to the limited sample size. Therefore, a larger study including more clinical cases and more subgroups is necessary. Although we found no differences of IL-10R1 between newly diagnosed SLE patients and treated patients, a paired control study before and after therapies was not included in our study, so it is not clear whether the steroids or other therapies had an effect on IL-10R1 expression.
In summary, we found dysregulation of IL-10R1 expression and signalling in CD4+ cells from LN patients, indicating that IL-10R1 may play a partial role in the pathogenesis of LN. However, elucidation of the exact mechanism for IL-10R1 in LN requires further studies.
Acknowledgments
We thank Yang Chen, Department of Central Laboratory, the First Affiliated Hospital of China Medical University, for technical assistance. This work was sponsored by the grants from the National Nature Science Foundation of China (no. 30600541, 30571701).
Disclosure
The authors have no financial conflict of interest.
References
- 1.Crow MK. Collaboration, genetic associations, and lupus erythematosus. N Engl J Med. 2008;358:956–61. doi: 10.1056/NEJMe0800096. [DOI] [PubMed] [Google Scholar]
- 2.Groux H, Cottrez F. The complex role of interleukin-10 in autoimmunity. J Autoimmun. 2003;20:281–5. doi: 10.1016/s0896-8411(03)00044-1. [DOI] [PubMed] [Google Scholar]
- 3.Llorente L, Richaud-Patin Y, Wijdenes J, et al. Spontaneous production of interleukin-10 by B lymphocytes and monocytes in systemic lupus erythematosus. Eur Cytokine Netw. 1993;4:421–7. [PubMed] [Google Scholar]
- 4.Llorente L, Richaud-Patin Y, Fior R, et al. In vivo production of interleukin-10 by non-T cells in rheumatoid arthritis, Sjögren's syndrome, and systemic lupus erythematosus. A potential mechanism of B lymphocyte hyperactivity and autoimmunity. Arthritis Rheum. 1994;37:1647–55. doi: 10.1002/art.1780371114. [DOI] [PubMed] [Google Scholar]
- 5.Llorente L, Zou W, Levy Y, et al. Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J Exp Med. 1995;181:839–44. doi: 10.1084/jem.181.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gelati M, Lamperti E, Dufour A, et al. IL-10 production in multiple sclerosis patients, SLE patients and healthy controls: preliminary findings. Ital J Neurol Sci. 1997;18:191–4. doi: 10.1007/BF02080463. [DOI] [PubMed] [Google Scholar]
- 7.Gröndal G, Kristjansdottir H, Gunnlaugsdottir B, et al. Increased number of interleukin-10-producing cells in systemic lupus erythematosus patients and their first-degree relatives and spouses in Icelandic multicase families. Arthritis Rheum. 1999;42:1649–54. doi: 10.1002/1529-0131(199908)42:8<1649::AID-ANR13>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 8.Houssiau F, Lefebvre C, Vanden Berghe M, Lambert M, Devogelaer J, Renauld J. Serum interleukin 10 titers in systemic lupus erythematosus reflect disease activity. Lupus. 1995;4:393–5. doi: 10.1177/096120339500400510. [DOI] [PubMed] [Google Scholar]
- 9.Park YB, Lee SK, Kim DS, Lee J, Lee CH, Song CH. Elevated interleukin-10 levels correlated with disease activity in systemic lupus erythematosus. Clin Exp Rheumatol. 1998;16:283–8. [PubMed] [Google Scholar]
- 10.Miret C, Font J, Molina R, et al. Relationship of oncogenes (sFas, Bcl-2) and cytokines (IL-10, alfa-TNF) with the activity of systemic lupus erythematosus. Anticancer Res. 2001;21:3053–9. [PubMed] [Google Scholar]
- 11.Yin Z, Bahtiyar G, Zhang N, et al. IL-10 regulates murine lupus. J Immunol. 2002;169:2148–55. doi: 10.4049/jimmunol.169.4.2148. [DOI] [PubMed] [Google Scholar]
- 12.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–50. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Blair PA, Noreña LY, Flores-Borja F, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity. 2010;32:129–40. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Haas KM, Watanabe R, Matsushita T, et al. Protective and pathogenic roles for B cells during systemic autoimmunity in NZB/W F1 mice. J Immunol. 2010;184:4789–800. doi: 10.4049/jimmunol.0902391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe R, Ishiura N, Nakashima H, et al. Regulatory B cells (B10 cells) have a suppressive role in murine lupus: CD19 and B10 cell deficiency exacerbates systemic autoimmunity. J Immunol. 2010;184:4801–9. doi: 10.4049/jimmunol.0902385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donnelly RP, Dickensheets H, Finbloom DS. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J Interferon Cytokine Res. 1999;19:563–73. doi: 10.1089/107999099313695. [DOI] [PubMed] [Google Scholar]
- 17.Qi ZM, Wang J, Sun ZR, et al. Polymorphism of the mouse gene for the interleukin 10 receptor alpha chain (IL-10ra) and its association with the autoimmune phenotype. Immunogenetics. 2005;57:697–702. doi: 10.1007/s00251-005-0036-7. [DOI] [PubMed] [Google Scholar]
- 18.Cairns AP, Crockard AD, Bell AL. Interleukin-10 receptor expression in systemic lupus erythematosus and rheumatoid arthritis. Clin Exp Rheumatol. 2003;21:83–6. [PubMed] [Google Scholar]
- 19.Valencia-Pacheco G, Layseca-Espinosa E, Niño-Moreno P, et al. Expression and function of IL-10R in mononuclear cells from patients with systemic lupus erythematosus. Scand J Rheumatol. 2006;35:368–78. doi: 10.1080/03009740600709840. [DOI] [PubMed] [Google Scholar]
- 20.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 21.Bombardier C, Gladman D, Urowitz M, Caron D, Chang C. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 22.Niemand C, Nimmesgern A, Haan S, et al. Activation of STAT3 by IL-6 and IL-10 in primary human macrophages is differentially modulated by suppressor of cytokine signaling 3. J Immunol. 2003;170:3263–72. doi: 10.4049/jimmunol.170.6.3263. [DOI] [PubMed] [Google Scholar]
- 23.Williams LM, Sarma U, Willets K, Smallie T, Brennan F, Foxwell BM. Expression of constitutively active STAT3 can replicate the cytokine-suppressive activity of interleukin-10 in human primary macrophages. J Biol Chem. 2007;282:6965–75. doi: 10.1074/jbc.M609101200. [DOI] [PubMed] [Google Scholar]
- 24.Kariuki SN, Niewold TB. Genetic regulation of serum cytokines in systemic lupus erythematosus. Transl Res. 2010;155:109–17. doi: 10.1016/j.trsl.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lit LC, Wong CK, Tam LS, Li EK, Lam CW. Raised plasma concentration and ex vivo production of inflammatory chemokines in patients with systemic lupus erythematosus. Ann Rheum Dis. 2006;65:209–15. doi: 10.1136/ard.2005.038315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inghirami G, Simon J, Balow JE, Tsokos GC. Activated T lymphocytes in the peripheral blood of patients with systemic lupus erythematosus induce B cells to produce immunoglobulin. Clin Exp Rheumatol. 1988;6:269–76. [PubMed] [Google Scholar]
- 27.Wada T, Schwarting A, Chesnutt MS, Wofsy D, Rubin Kelley V. Nephritogenic cytokines and disease in MRL-Fas(lpr) kidneys are dependent on multiple T-cell subsets. Kidney Int. 2001;59:565–78. doi: 10.1046/j.1523-1755.2001.059002565.x. [DOI] [PubMed] [Google Scholar]
- 28.Tucci M, Stucci S, Strippoli S, Silvestris F. Cytokine overproduction, T-cell activation, and defective T-regulatory functions promote nephritis in systemic lupus erythematosus. J Biomed Biotechnol. 2010;2010:457146. doi: 10.1155/2010/457146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daikh DI, Finck BK, Linsley PS, Hollenbaugh D, Wofsy D. Long-term inhibition of murine lupus by brief simultaneous blockade of the B7/CD28 and CD40/gp39 costimulation pathways. J Immunol. 1997;159:3104–8. [PubMed] [Google Scholar]
- 30.Fiorentino D, Zlotnik A, Mosmann T, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–22. [PubMed] [Google Scholar]
- 31.Fiorentino D, Zlotnik A, Vieira P, et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–51. [PubMed] [Google Scholar]
- 32.Lentsch AB, Shanley TP, Sarma V, Ward PA. In vivo suppression of NF-kappa B and preservation of I kappa B alpha by interleukin-10 and interleukin-13. J Clin Invest. 1997;100:2443–8. doi: 10.1172/JCI119786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eo SK, Chun S, Lee S, Rouse BT. On the mechanisms of T cell silencing by IL-10 DNA: direct and indirect inhibition of T cell functions. Cell Immunol. 2000;206:59–69. doi: 10.1006/cimm.2000.1731. [DOI] [PubMed] [Google Scholar]
- 34.Buer J, Lanoue A, Franzke A, Garcia C, von Boehmer H, Sarukhan A. Interleukin 10 secretion and impaired effector function of major histocompatibility complex class II-restricted T cells anergized in vivo. J Exp Med. 1998;187:177–83. doi: 10.1084/jem.187.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding Y, Qin L, Zamarin D, et al. Differential IL-10R1 expression plays a critical role in IL-10-mediated immune regulation. J Immunol. 2001;167:6884–92. doi: 10.4049/jimmunol.167.12.6884. [DOI] [PubMed] [Google Scholar]
- 36.Cassatella MA, Gasperini S, Bovolenta C, et al. Interleukin-10 (IL-10) selectively enhances CIS3/SOCS3 mRNA expression in human neutrophils: evidence for an IL-10-induced pathway that is independent of STAT protein activation. Blood. 1999;94:2880–9. [PubMed] [Google Scholar]
- 37.Crepaldi L, Gasperini S, Lapinet JA, et al. Up-regulation of IL-10R1 expression is required to render human neutrophils fully responsive to IL-10. J Immunol. 2001;167:2312–22. doi: 10.4049/jimmunol.167.4.2312. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Wei S, Ho A, de Waal Malefyt R, Moore K. Expression cloning and characterization of a human IL-10 receptor. J Immunol. 1994;152:1821–9. [PubMed] [Google Scholar]
- 39.Gasche C, Grundtner P, Zwirn P, et al. Novel variants of the IL-10 receptor 1 affect inhibition of monocyte TNF-alpha production. J Immunol. 2003;170:5578–82. doi: 10.4049/jimmunol.170.11.5578. [DOI] [PubMed] [Google Scholar]
- 40.Hermann J, Gruber S, Neufeld JB, et al. IL10R1 loss-of-function alleles in rheumatoid arthritis and systemic lupus erythematosus. Clin Exp Rheumatol. 2009;27:603–8. [PubMed] [Google Scholar]


