Abstract
Improving dendritic cell (DC) functions is highly promising for therapeutic intervention of diverse diseases, including cancer. Immunosuppressive cytokines such as interleukin (IL)-10 produced by DCs themselves (autocrine) and other regulatory immune cells (paracrine) down-regulate functional profiles of DCs through specific cell surface receptors such as IL-10R. Here, we tried to improve DC functions using small interfering RNA (siRNA) technology to block an IL-10R-mediated immunosuppressive axis. DCs modified with siRNA targeting against IL-10R or IL-10 (DC/siIL-10R or DC/siIL-10) led to up-regulation of major histocompatibility complex (MHC) class II, CD40 co-stimulatory molecule, and IL-12 proinflammatory cytokine after lipopolysacharide (LPS) stimulation compared to DC/siGFP. Notably, the LPS-induced functional profiles of DC/siIL-10R were strongly resistant to the addition of recombinant IL-10, which mimicked paracrine IL-10. In contrast, those of DC/siIL-10 were reversed by adding exogenous IL-10. Consistently, DC/siIL-10R generated more human papilloma virus (HPV) E7-specific CD8+ T cells and stronger anti-tumour effects against E7-expressing TC-1 tumour cells in vaccinated mice than DC/siGFP, as well as DC/siIL-10. Taken together, these results provide the groundwork for future clinical translation of siRNA-mediated strategy targeting IL-10R to enhance DC-based vaccine potency.
Keywords: dendritic cell, IL-10 receptor, immunosuppression, immunotherapy, siRNA
Introduction
Dendritic cells (DCs) are considered to be the most potent antigen-presenting cells (APCs) because they have a distinct capacity to prime immune responses including anti-tumour immunity [1]. For this reason, there has been much interest in the use of these cells for clinical cancer immunotherapy. However, clinical trial results have not been promising. One limitation of the clinical applications of DC-based vaccines is the initiation of immunosuppressive processes by tumour cells or various immune cells, including DCs themselves. In particular, one of the most immunosuppressive of these mechanisms is immunosuppressive cytokine production.
Many cytokines secreted by either tumour or various immune cells have immunosuppressive effects. Among these, the negative effects of interleukin (IL)-10 on the host immune system have been well characterized, including the inhibition of T lymphocyte proliferation, lymphokine-activated killer cell cytotoxicity, proinflammatory cytokine production, down-regulation of both the initiation and the effector phase of inflammatory responses and delayed-type hypersensitivity responses in vitro and in vivo[2–4]. Furthermore, elevated levels of IL-10 have been found in patients with various solid tumours and may have prognostic significance in a variety of cancers [2,3,5]. Thus, IL-10 might play a central role in one of the mechanisms responsible for immune deregulation in cancer patients. In particular, IL-10 production by DCs themselves after stimulation with Toll-like receptor (TLR) ligands such as lipopolysaccharide (LPS) and other immune cells such as regulatory T cells can serve as a potent mechanism in DC malfunction such as limitation of maturation and the capacity to initiate T helper type 1 (Th1) responses [6].
The biological effects of cytokines are mediated through their specific surface receptors. These receptors transduce the binding of their cognate cytokines into cytoplasmic signals that eventually trigger a cascade of intracellular responses. The functional receptor complex of IL-10 consists of at least two subunits: IL-10R1 and IL-10R2. IL-10R1 is a cell surface receptor with a single transmembrane domain and is a member of the class II cytokine receptor family [6,7]. IL-10R2 utilizes an accessory subunit for signalling. Therefore, IL-10R1 represents a potentially ideal target of the IL-10/IL-10R immunosuppressive axis for preventing IL-10-related malfunction in DCs stimulated with TLR ligands, including LPS.
RNA interference (RNAi) by small interfering RNA (siRNA) is a mechanism for post-transcriptional gene silencing of messenger RNA (mRNA) in a sequence-specific manner [8]. This technology has recently yielded several solid insights into the immune system by helping to elucidate numerous mechanisms that regulate the development, activation and function of immune cells, including DCs. Due to its ability to suppress target gene expression effectively, this technology has also been applied to regulate DC functions for therapeutic purposes [9]. In our previous studies, we demonstrated that ex vivo manipulation of DCs using siRNAs targeting to pro-apoptotic molecules could prolong the DC lifespan and protect DCs from in vivo cytotoxic T lymphocyte (CTL)-mediated clearing [10–14].
In this study, siRNA technology was used to inhibit the IL-10/IL-10R immunosuppressive axis in DCs and enhance their vaccine potency. DC modification with IL-10R siRNA resulted in an augmented Th1 response, and in turn generated strong antigen-specific CD8+ T cell immune responses and anti-tumour effects in vaccinated mice compared to DCs with green fluorescent protein (GFP) siRNA or even IL-10 siRNA. These encouraging results suggest a potential clinical translation of siRNA strategies for targeting immunosuppressive cytokine receptors to enhance DC-based vaccine potency.
Materials and methods
Mice
Six- to 8-week-old female C57BL/6 mice were purchased from Daehan Biolink (Chungbuk, South Korea). All animal procedures were performed in accordance with National Institutes of Health (NIH) guidelines for the proper use and care of laboratory animals [Public Health Service (PHS)-approved animal welfare assurance number, Korea University A5806-01].
Tumour cell culture
TC-1 cells were generated as described previously [10]. In brief, HPV-16 E6, E7, and H-Ras oncogenes were used to transform primary C57BL/6 mice lung epithelial cells. TC-1 cells were grown in RPMI-1640 medium containing 5% fetal bovine serum (FBS), 2 mM l-glutamine, 1 mM sodium pyruvate, 100 units/ml penicillin, 100 units/ml streptomycin and 100 mM non-essential amino acids at 37°C in 5% CO2.
Preparation of DCs
Bone marrow-derived dendritic cells (BM-DCs) were generated from bone marrow progenitor cells as described [10] with some modifications. Briefly, bone marrow cells were flushed from the femurs and tibiae of 5–8-week-old C57BL/6 mice. The cells were washed twice with RPMI-1640 and then the red blood cells were lysed and resuspended at a density of 1 × 106 cells/ml in RPMI-1640 supplemented with 2 mM glutamine, 1 mM sodium pyruvate, 100 µM non-essential amino acids, 5 × 10−5 M β-mercaptoethanol, 100 IU/ml penicillin, 100 µg/ml streptomycin, 10% FBS and 20 ng/ml recombinant murine granulocyte–macrophage colony-stimulating factor (GM-CSF) (PeproTech, Rocky Hill, NJ, USA). The cells were then cultured in a 24-well plate (1 ml/well) at 37°C in 5% CO2. The wells were replenished with fresh medium supplemented with 2 mM glutamine, 1 mM sodium pyruvate, 100 µM non-essential amino acids, 5 × 10−5 M β-mercaptoethanol, 100 IU/ml penicillin, 100 µg/ml streptomycin, 10% FBS and 20 ng/ml recombinant murine GM-CSF on days 2 and 4. The average percentage of CD11C+ obtained after BM-DC differentiation was greater than 90%. The BM-DCs were harvested after 6 days and used for siRNA transfection. We also used retrovirally transduced DC2·4 cells endogenously expressing Sig/E7/lysosome-associated membrane protein 1 (LAMP-1) (DC2·4-Sig/E7/LAMP-1) for siRNA transfection to measure E7-specific CD4+ and CD8+ T cell immune responses [15]. Linking E7 with the sorting signal of the lysosome-associated membrane protein 1 (Sig/LAMP-1) allows E7 to target endosomal and lysosomal compartments, thereby increasing MHC class II presentation as well as MHC class I presentation of E7 antigen.
Preparation of siRNA and transfection
siRNA was synthesized using 2′-O-ACE-RNA phosphoramides (Dharmacon, Lafayette, CO, USA). The sense and anti-sense strands of the siRNA were: IL-10, 5′P-ACAUACUGCUAACCGACUCdTdT-3′ (sense), 5′P- GAGUCGGUUAGCAGUA GUdTdT-3′ (anti-sense); IL-10R1 5′P-CCUGAGCAUCUUAGUCAUAdTdT-3′ (sense), 5′P- UAUGACUAAGAUGCUCAGGdTdT-3′ (anti-sense); P represents the 5′ phosphate. The RNA molecules were deprotected and annealed according to the manufacturer's instructions. Control siRNA targeting an irrelevant GFP (GFP siRNA; target: 5′-GCAUCAAGGUGAACUUCAAdTdT-3′ (sense), 5′UUGAAGUUCACCUUGAUGCdTdT-3′ (anti-sense)-3′ was also acquired from Dharmacon. A total of 200 000 DCs in a six-well vessel were transfected with 300 pmol of the synthesized siRNA using Welfect EX-PLUS (Welgene, Seoul, South Korea), according to the manufacturer's instructions. We used fluorescein isothiocyanate (FITC)-labelled siRNA to document transfection efficiency of the DCs with flow cytometry analysis. Virtually 95% of the DCs were transfected successfully with siRNA (data not shown). These transfected DC cells with each of siRNAs were named DC/siIL-10, DC/siIL-10R and DC/siGFP, respectively. The expression of IL-10 or IL-10R in the DCs was confirmed by flow cytometry analysis.
Flow cytometry analysis
Flow cytometry analysis was performed to evaluate the expression of DC-associated markers in the siRNA-transfected DCs after LPS stimulation with or without recombinant IL-10 (PeproTech). The expression level of IL-10 or IL-10R on the surface of the DCs was also measured by flow cytometry analysis. For this, the DCs were incubated with phycoerythrin (PE)-labelled anti-mouse antibodies for 30 min at 4°C and analysed on a fluorescence activated cell sorter (FACScan) flow cytometer (Becton Dickson, Sunnyvale, CA, USA). PE anti-mouse I-Ab (AF6-120·1), CD40 (3/23), IL-10 and IL-10R antibodies were purchased from Pharmingen (San Diego, CA, USA) [10,12,15]. Staining for intracellular IL-12 (Becton Dickson) and flow cytometry analysis were performed similarly, as described above.
Immunization with DCs
DC2·4-Sig/E7/LAMP-1 cells or BM-DCs were transfected with the synthesized IL-10 siRNA, IL-10R1 siRNA or GFP siRNA used for vaccination. BM-DCs modified with the siRNA were pulsed with HPV-16 E7 aa49-57 peptide (aa49-57, RA YNIVTF) (10 µg/ml) at 37°C for 2 h. The peptide-pulsed BM-DCs were then washed with RPMI-1640, supplemented with 5% FBS and Hanks's balanced salt solution (HBSS) and resuspended in HBSS at a final concentration of 1 × 107/ml. The BM-DCs were injected into mice footpads at 5 × 105 cells/mouse for immunization. The DC2·4-Sig/E7/LAMP-1 cells transfected with the siRNAs were also injected into mice footpads at 1 × 106 cells/mouse for immunization. One week later, the mice were boosted once with the same immunization regimen and dose.
Intracellular cytokine staining
Prior to intracellular cytokine staining, 4 × 106 splenocytes from each mouse in each vaccination group were incubated overnight with 1 µg/ml of E7 (RAHYNIVTF) peptide containing an MHC class I epitope (aa 49–57) to detect E7-specific CD8+ T cell precursors or 10 µg/ml of E7 peptide (aa 30–67) containing an MHC class II epitope to measure E7-specific CD4+ T cell precursors in the presence of GolgiPlug (BD Pharmingen, San Diego, CA, USA). Cell surface marker staining of CD8 or CD4 and intracellular cytokine staining for IFN-γ or IL-4 were performed using conditions described previously (ref. staining for intracellular Il-12 and IFN-γ and flow cytometry analysis were performed as described previously [12,15]. Analysis was performed on a Becton Dickinson FACScan with CellQuest™ software (Becton Dickinson Immunocytometry System, Mountain View, CA, USA).
Cytokine analysis using a cytometric bead array (CBA)
Cytokine levels were determined using a CBA. The procedure was carried out according to the manufacturer's instructions (CBA™; BD Biosciences, San Jose, CA, USA). Briefly, 10 µl of each mouse capture bead suspension were mixed in 50 µl of each supernatant sample, and the mixed bead suspensions were transferred to the assay tubes. Standard dilutions or test samples were added to the appropriate tubes (50 µl/tube), and PE detection reagent (50 µl) was added. After a 2-h incubation in the dark at room temperature, the samples were washed and analysed on a FACSCalibur (BD Biosciences) using the supplied cytometer setup beads and CellQuest™ Software.
Reverse transcription–polymerase chain reaction (RT–PCR) analysis
Total RNA was isolated using a Trizol Kit (Invitrogen Life Technologies) according to the manufacturer's protocol, and cDNA was generated using the ThermoScript™ RT–PCR System for RT–PCR (Invitrogen Life Technologies, Carlsbad, CA, USA), according to the manufacturer's protocol. cDNA was used as a template for quantitative RT–PCR using SYBR Green Master Mix (Applied Biosystems Foster City, CA, USA); the gene-specific primers are listed below. RT–PCR and analysis were performed using a 9700 GeneAmp Sequence Detector (Applied Biosystems). Relative expression was normalized to levels of glucose-6-phosphate dehydrogase (G6PDH): for G6PDH; 5′-AGAACCACCTCCTGCAGATG-3′ and 5′-TCCCACCGTTCATTCTC CAC-3′; for IL-12, 5′-AAGACCCTGACCATCACTGTC-3′ and 5′-CAGAGACACCA TTCCACATGT-3′. To correlate the threshold (Ct) values from IL-12 cDNA amplification plots with copy number, a standard curve was generated using a plasmid incorporating murine IL-12 cDNA.
In vivo tumour treatments
For the in vivo tumour treatment experiment, mice (five per group) were challenged by subcutaneously injecting 5 × 105 TC-1 tumour cells/mouse into the right leg. Mice were treated with DCs 3 days after the tumour challenge. Mice were monitored twice a week for 19 days after the tumour challenge. To determine the tumour volume, each individual tumour size was measured with calipers and the tumour volume was calculated using the following equation:
Using a TC-1 lung metastasis model, we further examined the therapeutic potential of the DCs modified with IL-10R siRNA. For this, mice were challenged with 1 × 105 TC-1 tumour cells/mouse injected into the tail vein to simulate haematogenous tumour spread [11]. Mice were treated with the DCs 3 days after the tumour challenge. Mice were monitored twice a week and killed on day 42 after the vaccination. The mean number of pulmonary nodules in each mouse was determined by experimenters blinded to sample identities.
Statistical analysis
The data presented in this study are from one representative experiment of three experiments and are expressed as the mean ± standard deviation (s.d.). The number of samples in each group for any given experiment was > 3. Results for the intracellular cytokine staining with flow cytometry analysis and the tumour treatment experiments were evaluated by an analysis of variance (one-way anova) and the Turkey–Kramer multiple comparison test. Comparisons between individual data points were performed using Student's t-test. All P-values < 0·05 were considered significant.
Results
Transfection of DCs with IL-10 or IL-10R siRNA down-regulates IL-10 and IL-10R1 expression
It has been reported that the expression of IL-10 and IL-10R increases after LPS treatment [16]. These data suggest that the IL-10/IL-10R axis may provide negative feedback regulation of DC maturation in an autocrine manner. Given this assumption, we decided to block the IL-10/IL-10R axis by using siRNA technology in order to reverse the immunosuppressive functions of LPS-stimulated DCs. For this, siRNA targeting IL-10R (siIL-10R) was introduced into BM-DCs, subsequently named DC/siIL-10R. siIL-10 was also transfected into BM-DCs (DC/siIL-10) for comparison. DC/siGFP, BM-DCs transfected with a siRNA targeting an irrelevant protein, GFP, was used as a negative control. To confirm the down-regulation of IL-10 or IL-10R in each BM-DC, flow cytometry analysis was performed. As shown in Fig. 1, the expression of IL-10 or IL-10R in the LPS-treated DCs was significantly reduced 40 h after transfection of DCs with siIL-10 or siIL-10R in terms of mean fluorescence intensity (MFI) values (P < 0·05). The down-regulation of IL-10 and IL-10R was maintained by days 7–9, and expression returned to normal levels by days 9–11 after transfection (unpublished observation). Similar expression profiles of target proteins were also observed after transfection of DCs with siBAK and siBAX [11].
Fig. 1.
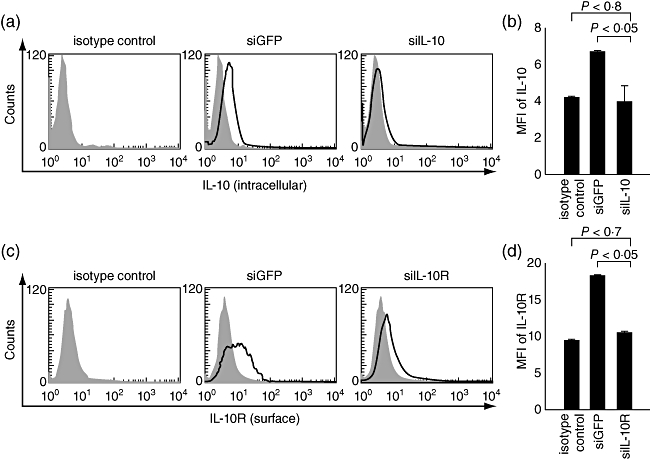
Down-regulation of interleukin (IL)-10 and IL-10R expression in bone marrow-derived dendritic cells (BM-DCs) transfected with small interfering RNA (siRNA) targeting IL-10 or IL-10R. siRNA targeting IL-10 or IL-10R1 was introduced into BM-DCs subsequently named DC/siIL-10 or DC/siIL-10R, respectively. The expression levels of IL-10 (a) or IL-10R (c) protein were measured by staining the cells with anti-IL-10 or IL-10R antibody and analysing by flow cytometry. The shaded area represents an isotype control which was BM-DC-stained with an isotype immunoglobulin (Ig)G antibody. (b,d) Bar graphs depicting the mean fluorescence intensity (MFI) indicating IL-10 or IL-10R expression, respectively. The data presented in this figure are from one representative experiment of three.
DCs treated with IL-10R siRNA highly express MHC II and CD40, which are not influenced by exogenous IL-10
It has been reported that IL-10 produced by matured DCs themselves (autocrine) or other regulatory immune cells (paracrine) induces the down-regulation of MHC class II and co-stimulatory molecules, such as CD40, on the DC surface [16]. Therefore, we investigated the expression level of MHC II and CD40 in DC/siILl-10 and DC/siIL-10R after LPS stimulation with or without exogenous recombinant IL-10. DC/siGFP was used as a negative control. As shown in Fig. 2, the expression of both MHC II and CD40 were increased significantly in the DC/siIL-10 and DC/siIL-10R after LPS treatment compared to the untreated cells. Notably, the expression levels of both MHC II and CD40 on DC/siIL-10R were slightly higher than those on DC/siIL-10.
Fig. 2.
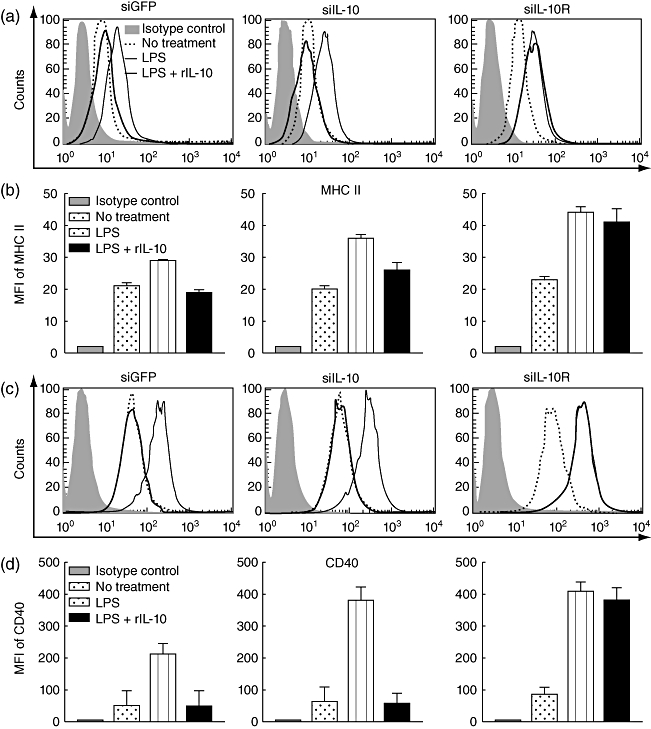
Surface expression of CD40 and major histocompatibility complex (MHC) II on bone marrow-derived dendritic cells (BM-DCs) transfected with small interfering RNA (siRNA) green fluorescent protein (siGFP), siRNA interleukin (siIL)-10 or siIL-10R. BM-DCs transfected with siGFP, siIL-10 or siIL-10R were treated with or without 1 µg/ml lipopolysaccharide (LPS) for 18 h and then collected for analysis of MHC II and CD40 expression to characterize the maturation phenotypes. In order to investigate the changes in the DC phenotypes in the presence of exogenous IL-10, the transfected DCs were also treated with both 1 µg/ml LPS and 30 ng/ml recombinant IL-10 (rIL-10) for 18 h and then collected for flow cytometry analysis (a,c). The shaded area represents background staining controls which was BM-DC-stained with corresponding isotype immunoglobulin (Ig)G antibodies. (b,d) Bar graphs depicting the mean fluorescence intensity (MFI) indicating MHC II and CD40 expression, respectively. The data presented in this figure are from one representative experiment of three.
In order to mimic paracrine IL-10-mediated suppression, exogenous rIL-10 protein was added concomitantly during LPS stimulation of each DC line (LPS + rIL-10 group). Interestingly, the addition of rIL-10 drastically down-regulated the expression level of both MHC II and CD40 in DC/siIL-10. In contrast, this was not observed in DC/siIL-10R. Thus, the DC/siIL-10R phenotype is not affected by an autocrine IL-10 or paracrine IL-10. Taken together, these data demonstrate that silencing the IL-10R can render DCs resistant to IL-10-mediated immunosuppression.
Blocking the IL-10/IL-10R axis significantly increases IL-12 release from the modified DCs
One of the regulatory functions of the IL-10/IL-10R axis is the inhibition of IL-12 production in DCs [17]. IL-12 is a prototypical DC-derived cytokine which was shown to be a key factor for the development of Th1 responses [18]. In addition, the Th1 response is known to be critical for priming antigen-specific CD8+ T cells. We therefore examined whether silencing IL-10R1 affected the production of IL-12 from LPS-treated BM-DCs. For this, DC/siGFP, DC/siIL-10 and DC/siIL-10R were treated with 1 µg/ml LPS for 18 h and, in turn, were collected to analyse the expression levels of IL-12 mRNA and IL-12 protein by RT–PCR and flow cytometry. IL-12 mRNA expression in both DC/siILl-10 and DC/siIL-10R was increased more than 15-fold and 90-fold, respectively, compared to DC/siGFP (Fig. 3a). Protein levels of intracellular IL-12 in DC/siIL-10 and DC/siIL-10R were increased more than 1·4-fold and 6·9-fold, respectively, compared to DC/siGFP (Fig. 3b and c). Concentration of IL-12 and IL-10 in supernatants form DC/siGFP, DC/siIL-10 or DC/siIL-10R was also measured with a cytometric bead array. The IL-12p70 concentration in both DC/siIL-10 and DC/siIL-10R was increased more than 1·3-fold and 1·8-fold, respectively, compared to that in DC/siGFP (Fig. 3d). In contrast, IL-10 concentration in both DC/siIL-10 and DC/siIL-10R was decreased more than 1·6-fold and 1·5-fold, respectively, compared to DC/siGFP (Fig. 3e). Notably, IL-12 expression in DC/siIL-10R was much higher than that in DC/siIL-10 at both the mRNA and protein levels. In contrast, IL-10 expression in DC/siIL-10R was not. These data suggest that the knock-down of IL-10R on DCs might be more effective in eliciting a Th1 immune response than that of IL-10 knock-down by increasing IL-12 production.
Fig. 3.
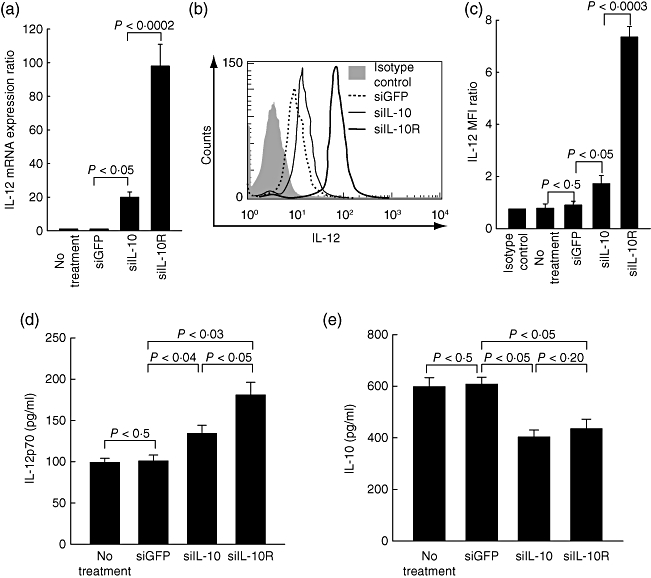
Expression of interleukin (IL)-12 in bone marrow-derived dendritic cells (BM-DCs) transfected with small interfering RNA (siRNA) green fluorescent protein (siGFP), siRNA siIL-10 or siIL-10R. BM-DCs transfected with siGFP, siIL-10 or siIL-10R were stimulated with lipopolysaccharide (LPS) for 18 h and then collected for reverse transcription–polymerase chain reaction (RT–PCR), real-time PCR and flow cytometry analysis. Total RNA was extracted from the LPS-stimulated cells. IL-12 mRNA levels were determined by real-time PCR. Non-tranfected BM-DCs were also analysed for comparison (no treatment group) (a). To measure the protein expression levels of IL-12, each cell line was treated with 1 µg/ml LPS for 18 h and then analysed by flow cytometry. The shaded area represents an isotype control which was BM-DC-stained with an isotype immunpglobulin (Ig)G antibody (b). Bar graphs depicts the mean fluorescence intensity (MFI) for IL-12 (c). Real-time PCR data and fluorescence activated cell sorter (FACS) data are represented by the average ± standard deviation (s.d.) of three independent experiments. BM-DCs transfected with siGFP, siIL-10 or siIL-10R were stimulated with LPS for 18 h and then the culture medium was collected for cytometric bead array (CBA) analysis. IL-12p70 (d) and IL-10 (e) concentrations in the medium from BM-DCs transfected with siGFP, siIL-10 or siIL-10R were evaluated by CBA analysis. The FACS data are represented by the average ± s.d. of three independent experiments.
Vaccination with E7-expressing DCs transfected with IL-10R siRNA leads to an augmented E7-specific CD4+ Th1 response
To determine whether Th1-favoured effects of IL-10/IL-10R siRNA observed in the LPS-stimulated DCs in vitro can be translated into an in vivo model, we used the retrovirally transduced DC2·4 endogenously expressing Sig/E7/LAMP-1 (DC2·4-Sig/E7/LAMP-1) [15]. Linking E7 with the sorting signal of the lysosome-associated membrane protein 1 (Sig/LAMP-1) allow E7 to target endosomal and lysosomal compartment and increase class II presentation of E7 antigen. Therefore, DC2·4-Sig/E7/LAMP-1 is an appropriate DC model to measure E7-specific CD4+ T cell immune responses. Before immunization, DC2·4-Sig/E7/LAMP-1 was modified by transfection with siRNA targeting IL-10, IL-10R1 or GFP. DC2·4-Sig/E7/LAMP-1 cells treated with siIL-10 or siIL-10R displayed similar functional phenotypes which were observed in the siRNAs-transfected BM-DCs (data not shown). Each of the transfected DCs were injected into mouse footpads twice at 1-week intervals for immunization. DC2·4-no insert transfected with IL-10R siRNA was used as a negative control. To determine the nature of the E7-specific CD4+ T cell response, we performed intracellular cytokine staining for IFN-γ (secreted by Th1 cells) or IL-4 (secreted by Th2 cells) with flow cytometry analysis using mouse splenocytes taken from immunized mice 1 week after the last vaccination and cultured overnight with or without MHC class II E7 peptide (aa 30–67). As shown in Fig. 4a, mice vaccinated with DC2·4-Sig/E7/LAMP-1 cells that treated with IL-10R siRNA generated the highest number of E7-specific Th1 CD4+ T cells, more than 3·5-fold greater than mice with DCs transfected with GFP siRNA (69·8 ± 12·7 versus 25·8 ± 8·9/3 × 105 splenocytes; P < 0·003). However, there was no difference in the number of Th1 CD4+ T cells without MHC class II E7 peptide stimulation between these two groups, meaning that Th1 CD4+ T cell responses induced by siIL-10R were E7-specific. Similarly, vaccination with DCs transfected with siIL-10 also led to a significant increase in the number of E7-specific Th1 CD4+ T cells [40·3 ± 11·2 versus 18·2 ± 2·4/3 × 105 splenocytes (P < 0·05)], although this increase was not higher than that observed in mice immunized with the DCs transfected with siIL-10R (P < 0·05). In contrast, DC2·4-Sig/E7/LAMP-1 transfected with siIL-10R failed to generate more Th2 CD4+ T cells after immunization than DCs transfected with IL-10 siRNA or GFP siRNA, as shown in Fig. 4c. These results suggest that the down-regulation of IL-10R in DCs can promote increases of antigen-specific Th1 CD4+ T cells in vaccinated mice.
Fig. 4.
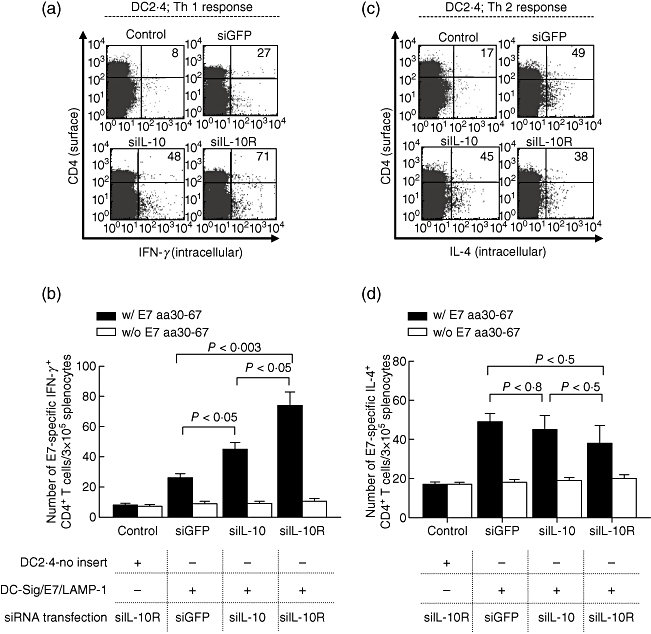
Vaccination with E7-expressing DCs transfected with small interfering RNA (siRNA) interleukin (siIL)-10 or siIL-10R enhances E7-specific CD4+ T helper type 1 (Th1) responses. C57BL/6 mice (five per group) were vaccinated twice with DC2·4-Sig-E7-lysosome-associated membrane protein 1 (LAMP-1) transfected with siIL-10, siIL-10R or siRNA green fluorescent protein (siGFP) at 1-week intervals. Splenocytes from mice vaccinated with DC2·4-no insert transfected with siIL-10R were used as a control to demonstrate non-specific background generated by siRNA transfection. Splenocytes were harvested at 1 week after the last vaccination. To detect E7-specific CD4+ T cell precursors, the splenocytes were incubated with 5 µg/ml of E7 peptide containing a major histocompatibility complex (MHC) class II epitope (aa 30–67). Intracellular IL-4 and interferon (IFN)-γ staining and flow cytometry analysis were performed (a,c). Numbers on (a) and (c) present a number of CD4+ cells that secret IFN-γ or IL-4, respectively. (b,d) Bar graphs depicting the number of E7-specific IFN-γ and IL-4-secreting CD4+ T cell precursors per 3 × 105 splenocytes, respectively. The data presented in this figure are from one representative experiment of three.
Vaccination with DCs transfected with siIL-10R leads to a significant increase in the number of E7-specific CD8+ T cells
To determine if the immune adjuvant effects of IL-10/IL-10R siRNA observed in DCs in vitro and in vivo can be translated into an increase of CD8+ T cell responses in vivo, we used DC2·4-Sig/E7/LAMP-1 for immunization. DC2·4-Sig/E7/LAMP-1 cells were only transfected with siRNA targeting IL-10, IL-10R or GFP without E7 peptide pulsing. DC2·4-no insert cells transfected with Il-10R siRNA were used as a negative control. BM-DCs were also used to determine whether the IL-10R siRNA technology is applicable to clinical settings as BM-DCs are thought to be a more appropriate DC type for clinical applications than immortalized DC cell lines such as DC2·4. In the case of BM-DCs, the cells were first transfected with the relevant siRNAs and then pulsed with MHC class I E7 peptide (aa 49–57) for 2 h prior to immunization. BM-DCs that were transfected IL-10R siRNA but not pulsed with the E7 peptide were used as a negative control. For immunization, each group of the DCs was injected into the mouse footpad twice at 1-week intervals. Using flow cytometry, the number of E7-specific IFN-γ-secreting CD8+ T cell was counted as described previously after overnight incubation of splenocytes with or without E7 peptide (aa 49–57) [9]. As shown in Fig. 5, IL-10R siRNA transfection drastically increased the number of E7-specific CD8+ T cells in mice immunized with all types of DCs we tested compared with GFP siRNA: DC2·4-Sig/E7/LAMP-1 (162·3 ± 23·1 versus 47·3 ± 3·7/3 × 105 splenocytes; P < 0·005); BM-DCs (526·3 ± 42·8 versus 60·4 ± 12·5/3 × 105 splenocytes (P < 0·005). However, there was no difference in the number of IFN-γ-secreting CD8+ T cell without the E7 peptide stimulation between these groups, meaning that CD8+ T cell responses induced by siIL-10R were E7-specific. Similarly, IL-10 siRNA also increased the number of E7-specific CD8+ T cells in all types of DCs we tested compared to GFP siRNA. However, DCs transfected with IL-10R siRNA generated more E7-specific CD8+ T cells after immunization than did those transfected with IL-10 siRNA. Thus, our data indicate that blocking the IL-10/IL-10R axis in DCs using siRNA technology can significantly enhance antigen-specific CD8+ T cell immune responses. Furthermore, IL-10R targeting can be more effective than IL-10 targeting for generating antigen-specific CD8+ T cells using DC-mediated vaccine systems.
Fig. 5.
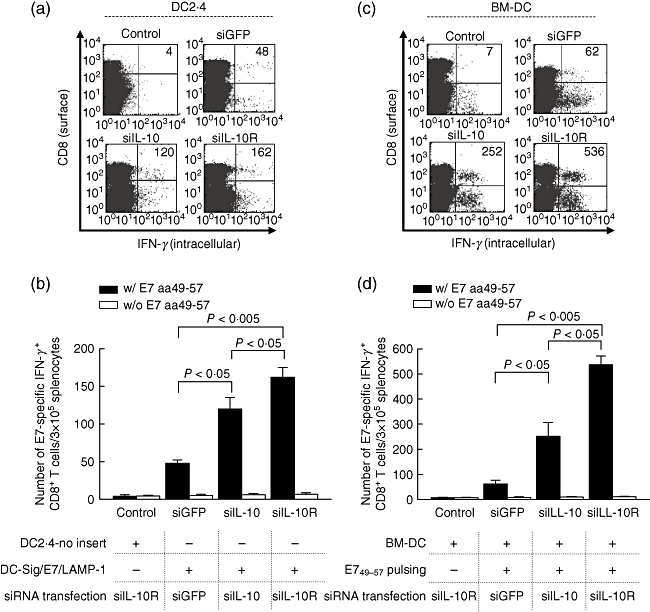
Vaccination with dendritic cells (DCs) transfected with small interfering RNA (siRNA) interleukin (siIL)-10R enhances an antigen-specific CD8+ T cell immune response. Intracellular cytokine staining and flow cytometry analysis were used to determine the number of interferon (IFN)-γ-producing E7-specific CD8+ T cells in mice after immunization with DC2·4-Sig/E7/lysosome-associated membrane protein 1 (LAMP-1) (a,b) and E7 peptide-pulsed bone marrow (BM)-DCs (c,d) transfected with siIL-10, siIL-10R or siRNA green fluorescent protein (siGFP). The transfected DCs were injected into mice via the footpad. DC2·4-no insert cells and no-peptide pulsed BM-DCs transfected with siIL-10R were used controls, respectively. One week later, the mice were boosted once with the same immunization regimen and dose. One week later, splenocytes were cultured overnight with E7 peptide (aa 49–57) and stained for both CD8 and intracellular IFN-γ. The number of CD8+ T cell precursors secreting IFN-γ was determined using flow cytometry (a,c). (a,c) Numbers present a number of IFN-γ-secreting CD8+ cells. Bar graphs depict the number of E7-specific DC8+ T cells (b,d). The data presented in this figure are from one representative experiment of three.
Vaccination with DC/siIL-10 or DC/siIL-10R inhibits tumour growth and eradicates established tumours
To determine if the observed enhancement in E7-specific CD8+ T cell responses leads to an increase in E7-specific anti-tumour effects, we performed an in vivo tumour treatment experiment using a previously described E7-expressing tumour model, TC-1. For this, mice first received 5 × 105 TC-1 cells/mouse and were immunized 3 days later with the transfected BM-DCs, as described in Fig. 5. BM-DCs that were transfected with IL-10R siRNA but not pulsed with E7 peptide were used as a negative control. As shown in Fig. 6a, mice immunized with DC/siIL-10R exhibited the smallest tumour volumes, although mice vaccinated with DC/siIL-10 also had significantly suppressed tumour growth compared to the control group. In an independent and identical experimental setting, 80% of the mice treated with DC/siIL-10R remained tumour-free 30 days after tumour challenge, whereas all of the other groups of mice developed tumours (data not shown).
Fig. 6.
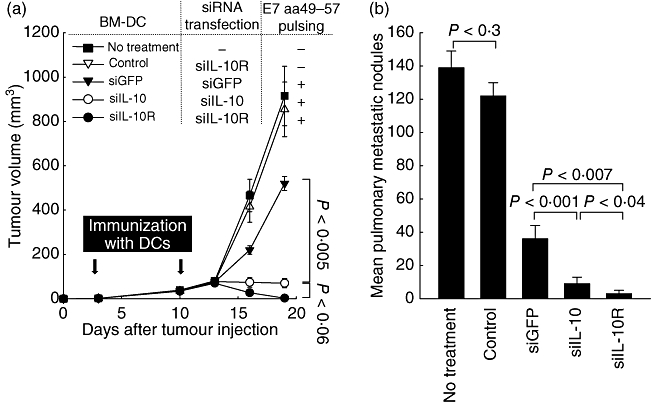
In vivo tumour treatment experiments. E7 peptide-pulsed bone marrow-derived dendritic cells (BM-DCs) transfected with small interfering RNA (siRNA) interleukin (siIL)-10, siIL-10R or siRNA green fluorescent protein (siGFP) were administered 3 days after the TC-1 tumour challenge (5 × 105 TC-1 cells/mouse) via subcutaneous injection (a) or (1 × 105 TC-1 cells/mouse) via intravenous injection (b). No-peptide pulsed BM-DCs transfected with siIL-10R were used as control. Mice were boosted with the same dose and regimen of E7 peptide-loaded DCs 1 week later. Data are expressed as the tumour volumes (mm3) from each group recorded for 19 days after tumour inoculation (a) and the number of metastatic lung nodules in mice 42 days after the last vaccination (b). Error bars represent the mean ± standard deviation (s.d.). The data presented in this figure are from one representative experiment of three.
Using a TC-1 lung metastasis model, we further examined the therapeutic potential of BM-DCs modified with IL-10R siRNA. Mice received 1 × 105 TC-1 cells/mouse and were immunized as described in Fig. 5. As shown in Fig. 6b, mice immunized with DC/siIL-10R had the lowest mean number of pulmonary nodules (1·3 ± 1·5) compared to mice immunized with DC/siIL-10 (7·4 ± 1·6; P < 0·04) or DC/siGFP (32·5 ± 3·5; P < 0·007). Notably, 60% of the mice treated with DC/siIL-10R remained tumour-free after the tumour challenge, whereas all the other groups of mice developed tumours. Taken together, these data indicate that immunization with DCs transfected with IL-10R siRNA leads to a significant increase of therapeutic effects against established tumour models in vivo.
Discussion
IL-10 is a cytokine with pleiotropic activities associated with anti-inflammatory and immunosuppressive properties [6,7]. In particular, IL-10 serves as a potent factor in the malfunction of DC, such as a limitation of maturation and diminished capacity to initiate Th1 responses [19]. Previous studies have shown that IL-10-treated DCs induce antigen-specific anergy in cytotoxic CD8+ T cells, a process that might be a tumour-related mechanism that inhibits immune surveillance by converting DCs into tolerogenic antigen-presenting cells [20–22]. Noticeably, elevated levels of IL-10 have been found in patients with various solid tumours as well as haematological malignancies [23,24]. Thus, increased levels of this cytokine have been shown to underlie the malfunctions of ex vivo-manipulated DCs in both animal models and human patients.
IL-10 exerts its actions through the heterodimeric membrane receptor formed by a binding chain (IL-10R1) and transducing chain (IL-10R2), whose mutual interaction activates a series of intracellular signalling molecules, including signal transducer and activator of transcription (STAT) proteins [25,26]. IL-10R1 binds IL-10 with high affinity in co-operation with IL-R2, but IL-10R2 contributes little to IL-10-binding affinity [26]. The expression level of IL-10R1 in BM-DCs increased after LPS stimulation (data not shown). This up-regulation of IL-10R1 was highly associated with IL-10-mediated immunosuppression, as shown in Fig. 2. The expression of IL-10R is well correlated with the sensitivity of DCs to exogenous recombinant IL-10-mediated suppression of MHC II and CD 40 expression. Thus, knock-down of IL-10R in DCs could be a fundamental solution for blocking an IL-10-mediated immunosuppression. In contrast, reducing IL-10 production could not rescue immunosuppressive phenotypes in DCs caused by exogenous IL-10, as shown in Fig. 2.
We also successfully demonstrated that the DCs transfected with IL-10R siRNA generates robust therapeutic anti-tumour effects in mice bearing TC-1 tumours which produce significant levels of IL-10 (data not shown) compared to DCs transfected with control siRNA or even IL-10 siRNA (Fig. 6). This promising adjuvant effect of IL-10R siRNA seems to be closely related to the increased expression of co-stimulatory molecules and IL-12 cytokines in our experimental settings, as shown in Fig. 3. Thus, blocking the IL-10/IL-10R immunosuppressive axis appears to be a plausible target for enhancing DC-mediated cancer vaccine potency.
One key finding of this study is the demonstration of a promising siRNA application as an immune adjuvant of DCs that blocks IL-10R signalling. Previously, anti-IL-10 antibodies were used to up-regulate anti-cancer T cell responses by inhibiting the suppressive effects of IL-10 [27]. However, systemic administration of IL-10 antibodies could non-specifically activate autoreactive T cells and, as a result, raises significant concerns regarding the potential for autoimmune diseases similar to IL-10−/− mice that develop enterocolitis [28]. In contrast to antibody-mediated immune modulation, our siRNA-based immunization strategy allows selective activation of tumour antigen-specific T cells by ex vivo genetic manipulation of tumour antigen-loading DCs prior to immune priming. Thus, our data suggest that modifying the function of DCs using siRNA technology targeting receptors of key immunosuppressive proteins, such as IL-4, IL-6 or transforming growth factor (TGF)-β, could lead to enhanced DC-based vaccine potency without inducing severe autoreactive immune responses. Thus, considering the number of well-known immune-suppressing receptors on DCs, it will be interesting to determine whether DCs modified by these adjuvant siRNA molecules could enhance therapeutic immune responses against tumours.
Acknowledgments
This research was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A062260); a grant from the Korea Health 21 R&D Project funded by the Ministry of Health & Welfare (A080816); a grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare (070355); a grant from the Korea Food and Drug Administration in 2008 (08122-KFDA-314); and a grant from the Basic Research Program of the Korea Science and Engineering Foundation (R1-2006-000-10565-0).
Disclosure
No conflict of interest reported.
References
- 1.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–47. doi: 10.1016/s0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 2.Huang M, Sharma S, Mao JT, Dubinett SM. Non-small cell lung cancer-derived soluble mediators and prostaglandin E2 enhance peripheral blood lymphocyte IL-10 transcription and protein production. J Immunol. 1996;157:5512–20. [PubMed] [Google Scholar]
- 3.Huang M, Stolina M, Sharma S, et al. Non-small cell lung cancer cyclooxygenase-2-dependent regulation of cytokine balance in lymphocytes and macrophages: up-regulation of interleukin 10 and down-regulation of interleukin 12 production. Cancer Res. 1998;58:1208–16. [PubMed] [Google Scholar]
- 4.Sharma S, Stolina M, Lin Y, et al. T cell-derived IL-10 promotes lung cancer growth by suppressing both T cell and APC function. J Immunol. 1999;163:5020–8. [PubMed] [Google Scholar]
- 5.Seo N, Hayakawa S, Takigawa M, Tokura Y. Interleukin-10 expressed at early tumour sites induces subsequent generation of CD4(+) T-regulatory cells and systemic collapse of antitumour immunity. Immunology. 2001;103:449–57. doi: 10.1046/j.1365-2567.2001.01279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 7.Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–79. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 8.Kim VN. Small RNAs: classification, biogenesis, and function. Mol Cells. 2005;19:1–15. [PubMed] [Google Scholar]
- 9.Pai SI, Lin YY, Macaes B, Meneshian A, Hung CF, Wu TC. Prospects of RNA interference therapy for cancer. Gene Ther. 2006;13:464–77. doi: 10.1038/sj.gt.3302694. [DOI] [PubMed] [Google Scholar]
- 10.Kim JH, Kang TH, Noh KH, et al. Enhancement of dendritic cell-based vaccine potency by anti-apoptotic siRNAs targeting key pro-apoptotic proteins in cytotoxic CD8(+) T cell-mediated cell death. Immunol Lett. 2009;122:58–67. doi: 10.1016/j.imlet.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Kim TW, Lee JH, He L, et al. Modification of professional antigen-presenting cells with small interfering RNA in vivo to enhance cancer vaccine potency. Cancer Res. 2005;65:309–16. [PubMed] [Google Scholar]
- 12.Kang TH, Lee JH, Noh KH, et al. Enhancing dendritic cell vaccine potency by combining a BAK/BAX siRNA-mediated antiapoptotic strategy to prolong dendritic cell life with an intracellular strategy to target antigen to lysosomal compartments. Int J Cancer. 2007;120:1696–703. doi: 10.1002/ijc.22377. [DOI] [PubMed] [Google Scholar]
- 13.Peng S, Kim TW, Lee JH, et al. Vaccination with dendritic cells transfected with BAK and BAX siRNA enhances antigen-specific immune responses by prolonging dendritic cell life. Hum Gene Ther. 2005;16:584–93. doi: 10.1089/hum.2005.16.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JH, Kang TH, Noh KH, et al. Enhancement of DC vaccine potency by activating the PI3K/AKT pathway with a small interfering RNA targeting PTEN. Immunol Lett. 2010;134:47–54. doi: 10.1016/j.imlet.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Ji H, Wang TL, Chen CH, et al. Enhancement of dendritic cell-based vaccine potency by targeting antigen to endosomal/lysosomal compartments. Immunol Lett. 2006;106:126–34. doi: 10.1016/j.imlet.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Samarasinghe R, Tailor P, Tamura T, Kaisho T, Akira S, Ozato K. Induction of an anti-inflammatory cytokine, IL-10, in dendritic cells after toll-like receptor signaling. J Interferon Cytokine Res. 2006;26:893–900. doi: 10.1089/jir.2006.26.893. [DOI] [PubMed] [Google Scholar]
- 17.Zhou L, Nazarian AA, Smale ST. Interleukin-10 inhibits interleukin-12 p40 gene transcription by targeting a late event in the activation pathway. Mol Cell Biol. 2004;24:2385–96. doi: 10.1128/MCB.24.6.2385-2396.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Garra A, Hosken N, Macatonia S, Wenner CA, Murphy K. The role of macrophage- and dendritic cell-derived IL12 in Th1 phenotype development. Res Immunol. 1995;146:466. doi: 10.1016/0923-2494(96)83017-3. [DOI] [PubMed] [Google Scholar]
- 19.Corinti S, Albanesi C, la Sala A, Pastore S, Girolomoni G. Regulatory activity of autocrine IL-10 on dendritic cell functions. J Immunol. 2001;166:4312–18. doi: 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- 20.Enk AH, Angeloni VL, Udey MC, Katz SI. Inhibition of Langerhans cell antigen-presenting function by IL-10. A role for IL-10 in induction of tolerance. J Immunol. 1993;151:2390–8. [PubMed] [Google Scholar]
- 21.Bellinghausen I, Sudowe S, König B, Reske-Kunz AB, Knop J, Saloga J. Interleukin-10-treated dendritic cells do not inhibit Th2 immune responses in ovalbumin/alum-sensitized mice. Int Arch Allergy Immunol. 2006;141:61–9. doi: 10.1159/000094255. [DOI] [PubMed] [Google Scholar]
- 22.Enk AH, Saloga J, Becker D, Mohamadzadeh M, Knop J. Induction of hapten-specific tolerance by interleukin 10 in vivo. J Exp Med. 1994;179:1397–402. doi: 10.1084/jem.179.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pawelec G. Tumour escape: antitumour effectors too much of a good thing? Cancer Immunol Immunother. 2004;53:262–74. doi: 10.1007/s00262-003-0469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haase C, Jorgensen TN, Michelsen BK. Both exogenous and endogenous interleukin-10 affects the maturation of bone-marrow-derived dendritic cells in vitro and strongly influences T-cell priming in vivo. Immunology. 2002;107:489–99. doi: 10.1046/j.1365-2567.2002.01529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray PJ. STAT3-mediated anti-inflammatory signalling. Biochem Soc Trans. 2006;34:1028–31. doi: 10.1042/BST0341028. [DOI] [PubMed] [Google Scholar]
- 26.Ding Y, Qin L, Zamarin D, et al. Differential IL-10R1 expression plays a critical role in IL-10-mediated immune regulation. J Immunol. 2001;167:6884–92. doi: 10.4049/jimmunol.167.12.6884. [DOI] [PubMed] [Google Scholar]
- 27.Mocellin S, Marincola FM, Young HA. Interleukin-10 and the immune response against cancer: a counterpoint. J Leukoc Biol. 2005;78:1043–51. doi: 10.1189/jlb.0705358. [DOI] [PubMed] [Google Scholar]
- 28.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]


