Abstract
α-Fetoprotein (AFP) is a tumour-associated antigen in hepatocellular carcinoma (HCC). The biological properties of AFP have been identified in its regulatory effects on immune responses of T cells and B cells. However, AFP effects on natural killer (NK) cells are still unclear. In this study, we examined the immunoregulation of AFP on NK activity. The cytolytic activity against K562 cells and Huh7 cells of NK cells co-cultured with AFP-treated dendritic cells (DCs) (AFP-DCs) was lower than that with albumin-treated DCs (Alb-DCs). Direct addition of AFP to NK cells did not alter the cytolytic activity of NK cells. Adding AFP inhibited the interleukin (IL)-12 production of DCs after stimulation with lipopolysaccharide (LPS) [Toll-like receptor (TLR)-4 ligand], or Poly(I:C) (TLR-3 ligand), but not IL-18 production. The mRNAs of IL-12p35 and IL-12p40 were significantly inhibited in AFP-DCs compared with Alb-DCs, but those of TLR-4 or TLR-3 were not. Transwell experiments revealed that soluble factors derived from DCs played roles in inhibition of the ability of activating NK cells by AFP-DCs. Adding the neutralizing antibody of IL-12 to NK cells co-cultured with Alb-DCs resulted in a decrease of cytolytic activity to the levels of NK cells co-cultured with AFP-DCs. Adding IL-12 to NK cells co-cultured with AFP-DCs resulted in an increase of cytolytic activity to the levels of NK cells co-cultured with Alb-DCs. These demonstrated that the impairment of IL-12 production from AFP-DCs resulted in inhibition of the ability of the activation of NK cells by DCs, and thus suggests a role of AFP in HCC development.
Keywords: α-fetoprotein, dendritic cells, hepatocellular carcinoma, NK cells
Introduction
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer deaths worldwide. Chronic liver disease caused by hepatitis virus infection and non-alcoholic steatohepatitis leads to a predisposition for HCC, with liver cirrhosis, in particular, being considered a premalignant condition [1,2]. With regard to treatment, surgical resection or percutaneous techniques such as ethanol injection and radiofrequency ablation are considered to be choices for the curable treatment of localized HCC, whereas transarterial chemo-embolization is a well-established technique for more advanced HCC [3]. Recently the Sorafenib Hepatocellular carcinoma Assessment Randomized Protocol (SHARP) trial has demonstrated that sorafenib, a multi-targeting kinase molecule that inhibits receptor tyrosine kinases [vascular endothelial growth factor receptor (VEFGR)-2, VEGFR-3, Flt ligand (Flt)-3, platelet-derived growth factor receptor beta (PDGFR) and fibroblast growth factor receptors (FGFR)-1] as well as Raf serine–threonine kinase in the signal transduction, is effective for prolonging median survival and time-to-progression in patients with advanced HCC [4]. The liver contains a large compartment of innate immune cells [natural killer (NK) cells and NK T cells] and acquired immune cells (T cells) [5,6]. However, what remain unclear are the details of the activation of these immune cells in the process of HCC development. If the mechanism of tumour surveillance by immune cells in HCC development can be elucidated, this could lead to the establishment of new strategies for HCC treatment.
α-Fetoprotein (AFP), a glycoprotein of molecular mass 68–72 kDa, is a tumour-associated antigen in HCC and a target for immunotherapy [7]. Measurement of serum levels of AFP is important for the diagnosis of HCC and monitoring of treatment [8]. Recently, several biological properties of AFP have been identified in its regulatory effects on immune responses [9–13]. AFP induces the suppression of cytotoxic T lymphocytes (CTLs) activity and antibody responses of B lymphocytes [9–11]. Alisa et al. demonstrated that AFP may contain specific epitopes which activate the expansion of inducible transforming growth factor (TGF)-β producing regulatory T cells, leading to evasion of tumour control [12]. Antigen-presenting cells (APCs) of HCC patients with high levels of AFP are dysfunctional, and AFP impairs dendritic cell (DC) function and induces their apoptosis [13]. However, the biological role of AFP on innate immune responses still remains unclear.
In this study, we investigated the immunoregulation of NK activity and DC function by AFP. We demonstrate that AFP impairs NK activity via inhibition of interleukin (IL)-12 production from DCs. The present study sheds light on previously unrecognized immunological effects of AFP on NK cells, and thus suggests a role of AFP in HCC development.
Materials and methods
Cell culture
Cell culture was maintained in a medium (RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin, 100 ug/ml streptomycin and 10 mM l-glutamine: all reagents from Gibco /Life Technologies, Grand Island, NY, USA) in a humidified incubator at 5% CO2 and 37°C. Purified human cord blood AFP [purity, > 98%; sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE)] and purified human serum albumin (Alb) (purity, > 97%; SDS-PAGE) were obtained from HyTest (Turku, Finland) and Sigma-Aldrich (St Louis, MO, USA), respectively.
Generation of monocyte-derived DCs from peripheral blood monocytes (PBMCs)
Monocyte-derived DCs were generated from PBMCs of healthy volunteers. PBMCs, isolated by Ficoll Hypaque density centrifugation, were washed twice in phosphate-buffered saline (PBS) and resuspended in AIM-V medium for 60 min. Non-adherent cells were removed by gentle washing, and adherent cells were cultured in DC medium (RPMI-1640 supplemented with 10% fetal calf serum) containing human granulocyte–macrophage colony-stimulating factor (GM-CSF) (50 pg/ml; PeproTech, Rocky Hill, NJ, USA) and human IL-4 (50 pg/ml; PeproTech) with either AFP (25 µg/ml) or Alb (25 µg/ml). On day 6, immature DCs were harvested. DC maturation was induced by the addition of lipopolysaccharide (LPS) (10 µg/ml; Sigma-Aldrich) or Poly(I:C) (10 µg/ml; InvivoGen, San Diego, CA, USA) to immature DCs for 24 h.
Analysis of DC surface markers
For phenotypic analysis of DCs, allophycocyanin (APC)-, peridinin chlorophyll protein complex (PerCP)- or phycoerythrin (PE)-labelled monoclonal antibodies (mAbs) [anti-human CD11c, CD40, CD80, CD83, CD86, human leucocyte antigen D-related (HLA-DR) relevant isotype controls; BD Pharmingen, San Diego, CA, USA], according to the manufacturer's instructions. Flow cytometric analysis was performed using a fluorescence activated cell sorter (FACS)Calibur (Becton Dickinson, San Jose, CA, USA) flow cytometer. We defined DCs with CD11c+ HLA-DR+ cells by flow cytometry and evaluated the expression of these antigen-presenting related molecules. Data were analysed using FlowJo software (Tree Star, Ashland, OR, USA) and reported as the mean fluorescence intensity (MFI).
Measurements of cytokine production of DCs
IL-12p70, IL-15, IL-18 and interferon (IFN)-γ of the DC culture were measured by a single solid-phase sandwich enzyme-linked immunosorbent assay (ELISA) using paired specific mAbs and recombinant cytokine standards, according to the manufacturer's instructions (IL-12p70, IL-15 and IFN-γ from BD Pharmingen, IL-18 from MBL, Woburn, MA, USA).
Real time reverse transcription–polymerase chain reaction (RT–PCR)
Total RNA was isolated using an RNeasy Mini Kit (Qiagen K.K., Tokyo, Japan), and was reverse-transcribed using the high-capacity RNA-to-cDNA Master Mix (Invitrogen, Carlsbad, CA, USA). Random hexamers were added as primers. The mRNA levels were evaluated using an ABI PRISM 7900 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). Ready-to-use assays (Applied Biosystems) were used for the quantification of Toll-like receptor (TLR)-3, TLR-4, IL-12p35, IL-12p40 and β-actin, according to the manufacturer's instructions. The thermal cycling conditions for all genes were 2 min at 50°C and 10 min at 95°C, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. β-Actin mRNA from each sample was quantified as an endogenous control of internal RNA.
DC/NK cell co-culture
NK cells were isolated from PBMCs by magnetic cell sorting using CD56 MicroBeads according to the manufacturer's instructions (Miltenyi Biotech, Auburn, CA, USA). More than 95% of the cells were CD56+CD3- lymphocytes. Enriched NK cells were co-cultured with AFP (25 µg/ml, AFP-DCs) or Alb (25 µg/ml, Alb-DCs) pretreated DCs for 24 h. The cytolytic activity of NK cells co-cultured with AFP-DCs or Alb-DCs against target cells (K562, NK sensitive cells, or Huh7, human HCC cells) was assessed by 4-h 51Cr-releasing assay with or without the presence of neutralizing antibody of IL-12 (BD Pharmingen) or recombinant IL-12p70 protein (PeproTech), as described previously [14]. In some experiments, a Transwell insert was also used to prevent direct contact of NK cells and DCs in co-culture systems, as described previously [14].
Statistical analysis
The statistical significance of differences between the two groups was determined by applying the Mann–Whitney U-test. We defined statistical significance as P < 0·05.
Results
NK activity co-cultured with AFP-DCs was lower than that with Alb-DCs
We investigated the activity of NK cells co-cultured with AFP-DCs or Alb-DCs. NK cells from the same healthy volunteers were co-cultured with AFP-DCs or Alb-DCs for 24 h, and we evaluated the cytolytic activity of NK cells co-cultured with DCs against K562 cells as target cells with the 51Cr-releasing assay. The cytotoxicity of NK cells co-cultured with AFP-DCs against K562 cells was significantly lower than those with Alb-DCs (Fig. 1a). Similarly, the cytotoxicity of NK cells co-cultured with AFP-DCs against Huh7 cells was significantly lower than that with Alb-DCs (Fig. 1b). We also evaluated the IFN-γ production from NK cells co-cultured with AFP-DCs or Alb-DCs by specific ELISA. IFN-γ production from NK cells co-cultured with AFP-DCs was significantly lower than that from NK cells co-cultured with Alb-DCs (Fig. 1c). These results demonstrated that NK activity co-cultured with AFP-DCS was lower than that with Alb-DCs. Next, NK cells were cultured with AFP (AFP-NK cells) or Alb (Alb-NK cells) for 24 h, and we evaluated the cytolytic activity of AFP-NK and Alb-NK against K562 cells with the 51Cr-releasing assay. The cytotoxicity of AFP-NK cells was almost similar to that of Alb-NK cells, and the presence of DCs could enhance the cytotoxicity of NK cells (Fig. 2a). These results suggested that AFP does not directly impair NK cell function and that DCs play a critical role in activating NK cells. To examine whether this attenuation of NK cells was caused by the cytokine from DCs or by direct contact with DCs, NK cells were co-cultured with AFP-DCs or Alb-DCs in Transwell culture for 24 h. The cytotoxicity of NK cells co-cultured with AFP-DCs was lower than that with Alb-DCs, which was similar to the results without Transwell membrane (Fig. 2b). These results suggested that soluble factors derived from DCs played a role in activating NK cells.
Fig. 1.
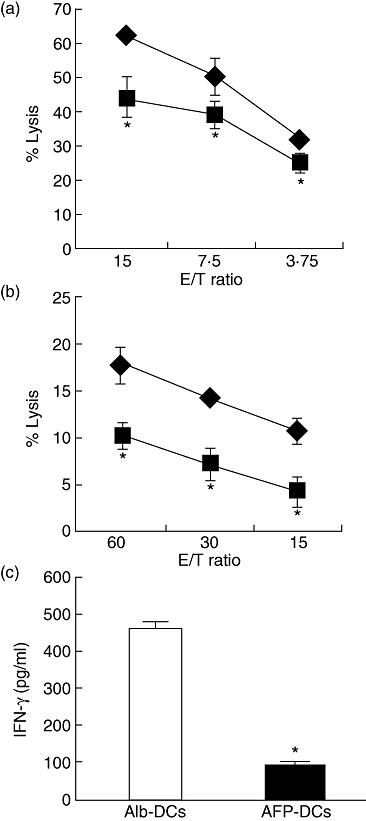
The cytolytic activity and interferon (IFN)-γ production of natural killer (NK) cells co-cultured with α-fetoprotein-dendritic cells (AFP-DCs) were impaired. (a,b) NK cells were isolated from peripheral blood mononuclear cells (PBMCs) by magnetic cell sorting using CD56 MicroBeads according to the manufacturer's instructions. Enriched NK cells were co-cultured with AFP (25 µg/ml, AFP-DCs) or albumin (Alb) (25 µg/ml, Alb-DCs) pretreated DCs for 24 h. The cytolytic activities of NK cells co-cultured with AFP-DCs ( ) or Alb-DCs (♦) against K562 cells (a) or Huh7 cells (b) were evaluated by 51Cr-releasing assay. *P < 0·05 versus the cytolytic activity of NK cells cultured with Alb-DCs. (c) NK cells were isolated from PBMCs by magnetic cell sorting using CD56 MicroBeads, according to the manufacturer's instructions. Enriched NK cells were co-cultured with AFP (25 µg/ml, AFP-DCs) or Alb (25 µg/ml, Alb-DCs) pretreated DCs for 24 h. The IFN-γ productions from NK cells were analysed by specific enzyme-linked immunosorbent assay (ELISA). To evaluate the IFN-γ production from NK cells, we also evaluated the IFN-γ production from AFP-DCs or Alb-DCs cultured without NK cells, and these values were subtracted from all experimental determinations to determine specific IFN-γ productions of NK cells (results in pg/ml; mean ± standard deviation of triplicate samples). We analysed statistically the production of IFN-γ between AFP-DCs and Alb-DCs. *P < 0·05.
) or Alb-DCs (♦) against K562 cells (a) or Huh7 cells (b) were evaluated by 51Cr-releasing assay. *P < 0·05 versus the cytolytic activity of NK cells cultured with Alb-DCs. (c) NK cells were isolated from PBMCs by magnetic cell sorting using CD56 MicroBeads, according to the manufacturer's instructions. Enriched NK cells were co-cultured with AFP (25 µg/ml, AFP-DCs) or Alb (25 µg/ml, Alb-DCs) pretreated DCs for 24 h. The IFN-γ productions from NK cells were analysed by specific enzyme-linked immunosorbent assay (ELISA). To evaluate the IFN-γ production from NK cells, we also evaluated the IFN-γ production from AFP-DCs or Alb-DCs cultured without NK cells, and these values were subtracted from all experimental determinations to determine specific IFN-γ productions of NK cells (results in pg/ml; mean ± standard deviation of triplicate samples). We analysed statistically the production of IFN-γ between AFP-DCs and Alb-DCs. *P < 0·05.
Fig. 2.
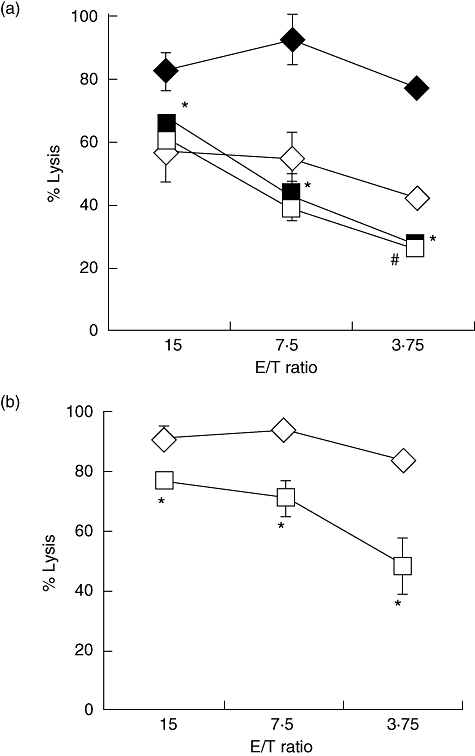
α-Fetoprotein (AFP) did not directly affect the cytolytic activity of natural killer (NK) cells and soluble factor from dendritic cells (DCs) played a role in the inhibition of NK activity. (a) NK cells were cultured with AFP (25 µg/ml, □, AFP-NK cells) or albumin (Alb) (25 µg/ml, ◊, Alb-NK cells) or cultured with AFP-DCs ( ) or Alb-DCs (♦) for 24 h. We evaluated the cytolytic activity of AFP-NK cells and Alb-NK cells or NK cells stimulated by AFP-DCs or Alb-DCs using K562 cells as target cells by 51Cr-releasing assay. We analysed statistically between the cytolytic activity of NK cells co-cultured with AFP-DCs (
) or Alb-DCs (♦) for 24 h. We evaluated the cytolytic activity of AFP-NK cells and Alb-NK cells or NK cells stimulated by AFP-DCs or Alb-DCs using K562 cells as target cells by 51Cr-releasing assay. We analysed statistically between the cytolytic activity of NK cells co-cultured with AFP-DCs ( ) and Alb-DCs (♦) or between that of AFP-NK cells (□) and Alb-NK cells (◊). *P < 0·05 versus the cytolytic activity of NK cells cultured with Alb-DCs, #P < 0·05 versus the cytolytic activity of Alb-NK cells. (b) Enriched NK cells were co-cultured with AFP-DCs (□) or Alb-DCs (◊) for 24 h in the presence of 0·4 µm of inserting membrane (Transwell). NK cells were harvested and subjected to examine the cytolytic activity against K562 cells by 51Cr-releasing assay. *P < 0·05 versus the cytolytic activity of NK cells cultured with Alb-DCs. Representative results are shown. Similar results were obtained from three independent experiments in all experiments.
) and Alb-DCs (♦) or between that of AFP-NK cells (□) and Alb-NK cells (◊). *P < 0·05 versus the cytolytic activity of NK cells cultured with Alb-DCs, #P < 0·05 versus the cytolytic activity of Alb-NK cells. (b) Enriched NK cells were co-cultured with AFP-DCs (□) or Alb-DCs (◊) for 24 h in the presence of 0·4 µm of inserting membrane (Transwell). NK cells were harvested and subjected to examine the cytolytic activity against K562 cells by 51Cr-releasing assay. *P < 0·05 versus the cytolytic activity of NK cells cultured with Alb-DCs. Representative results are shown. Similar results were obtained from three independent experiments in all experiments.
Maturation of AFP-treated DCs was impaired
We next examined the function of AFP-DCs. We obtained DCs from eight healthy volunteers and cultured the DCs for 7 days in RPMI-1640 with AFP (AFP-DCs) or Alb (Alb-DCs). On day 6, we added LPS to induce DC maturation. We identified DCs with CD11c+/HLA-DR+ cells by flow cytometry. As shown in Fig. 3a, adding LPS, the TLR-4 ligand, resulted in increasing the expression of HLA-DR in both AFP-DCs and Alb-DCs. The numbers of harvested AFP-DCs or Alb-DCs were (1·64 ± 0·62) × 106 and (1·77 ± 0·73) × 106, respectively, with no significant difference being observed between the two groups. We evaluated the expression of the antigen-presenting related molecules on AFP-DCs and Alb-DCs. The expression of CD80, CD86, CD40 and CD83 increased on both AFP-DCs and Alb-DCs after addition of LPS. The expression of these molecules was not significantly different between immature (day 6) AFP-DCs and immature (day 6) Alb-DCs (data not shown). The expression of CD83 and CD86 on LPS-treated mature AFP-DCs was inhibited significantly compared with those on LPS-treated mature Alb-DCs, although the expression of CD80 and CD40 was not (Fig. 3b), suggesting that maturation of AFP-DCs was impaired. We also examined the expression of antigen-presenting related molecules on AFP-DCs or Alb-DCs which were matured by Poly(I:C), the TLR-3 ligand. On day 6 of the DC culture, we added Poly(I:C) (10 µg/ml) to immature-DC. The results of Poly(I:C)-matured AFP-DCs was similar to those of LPS-matured AFP-DCs (data not shown).
Fig. 3.
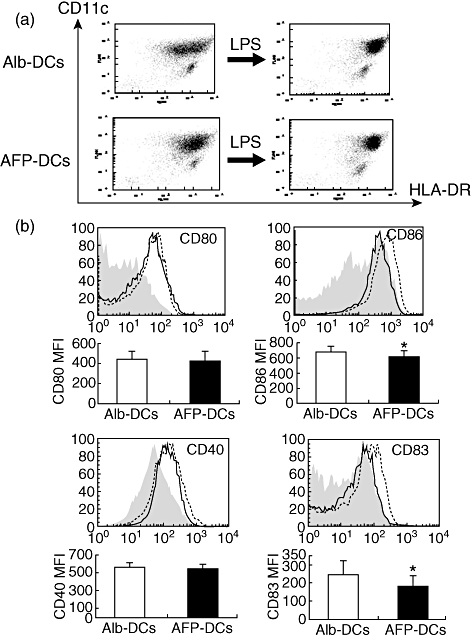
The maturation of α-fetoprotein-dendritic cells (AFP-DCs) was inhibited more than that of Alb-DCs. Monocyte-derived DCs were generated from eight healthy volunteers. DCs were cultured for 7 days in RPMI-1640 with AFP (25 µg/ml) or albumin (Alb) (25 µg/ml). On day 6, lipopolysaccharide (LPS) was added to induce DC maturation. (a) We defined DCs with CD11c+ and human leucocyte antigen D-related (HLA-DR+) cells by flow cytometry. (b) We evaluated the expression of CD80, CD86, CD40 and CD83 on AFP-DCs (black line) and Alb-DCs (dotted line). Grey histogram indicates control immunoglobulin (Ig)G staining. The expression of each molecule on AFP-DCs and Alb-DCs from seven healthy volunteers was evaluated by the mean fluorescence intensity (MFI) ± standard deviation. All experiments were performed three times independently and representative results (upper panels) as well as the statistical analysis (lower panels) are shown as the MFI of the staining cells. *P < 0·05.
IL-12 production from AFP-DCs was impaired
We examined IL-12, IL-15 and IL-18 production in the supernatant of LPS (TLR-4 ligand)-treated DC culture by specific ELISA. IL-12 was not detected in the supernatants of the non-treated immature AFP-DCs and Alb-DCs (data not shown). The production of IL-12 from mature AFP-DCs was significantly lower than that from mature Alb-DCs (Fig. 4a). When mature DCs were generated under various AFP concentrations (25 µg/ml, 12·5 µg/ml or 6·25 µg/ml), the production of IL-12 from DCs decreased in a dose-dependent manner (Fig. 4a). IL-15 was not detected from the supernatants of both LPS-treated AFP-DCs and Alb-DCs (data not shown), and IL-18 was detected equally in the supernatants of both LPS-treated mature AFP-DCs and Alb-DCs (Fig. 4b). We also examined IL-12 production of AFP-DCs or Alb-DCs which were matured by Poly(I:C). The IL-12 production of mature AFP-DCs was significantly lower than that of Alb-DCs (Fig. 4c), which is consistent with the results of LPS-treated DCs.
Fig. 4.
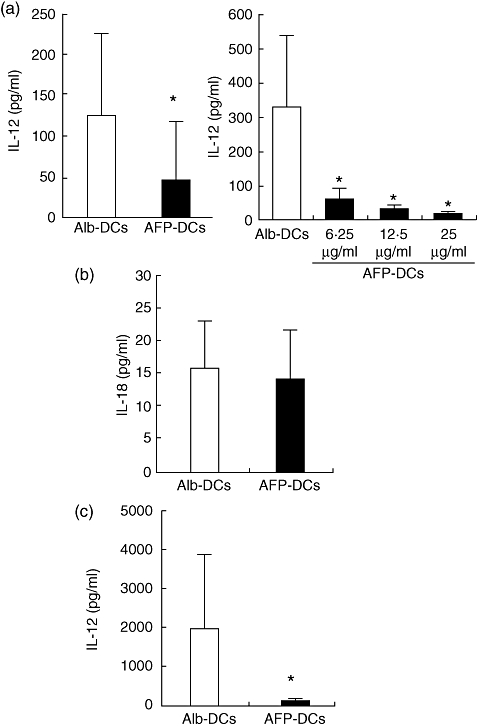
The production of interleukin (IL)-12p70 from α-fetoprotein-dendritic cells (AFP-DCs) was lower than that from Alb-DCs, but that of interleukin (IL)-18 was not. We cultured DCs for 7 days in RPMI-1640 with AFP (25 µg/ml, 12·5 µg/ml, 6·25 µg/ml) or albumin (Alb) (25 µg/ml). On day 6, we added lipopolysaccharide (LPS) (10 µg/ml, a,b) or Poly(I:C) (10 µg/ml, c) to induce DC maturation. Twenty-four hours later, IL-12p70 (a,c) or IL-18 (b) production from LPS- or Poly(I:C)-treated DCs was measured by specific enzyme-linked immunosorbent assay (ELISA) (results in pg/ml; mean ± standard deviation of triplicate samples). (a, left panel; b,c) We analysed statistically the production of both cytokines between AFP-DCs and Alb-DCs. *P < 0·05. (a, right panel) We analysed statistically the production of IL-12p70 between Alb-DCs and AFP (6·25 µg/ml)-DCs, AFP (12·5 µg/ml)-DCs or AFP(25 µg/ml)-DCs. *P < 0·05 versus IL-12p70 production of Alb-DCs.
mRNA of IL-12 in AFP-DCs was lower than that in Alb-DCs
The bioactive form of IL-12 is a 75 kDa heterodimer (IL-12p70) comprised of independently regulated disulphide-linked 40 kDa (p40) and 35 kDa (p35) subunits. Next, we examined the expression of mRNA of IL-12p35 and IL-12p40 by real-time PCR. Both IL-12p35-mRNA and IL-12p40 mRNA of AFP-DCs were significantly lower than those of Alb-DCs with both LPS and Poly(I:C) stimulation (Fig. 5a). We examined the expression of mRNA of TLR-3 and TLR-4 in the mature DCs. The expression of TLR-3-mRNA and TLR-4-mRNA of AFP-DCs were similar to those of Alb-DCs (Fig. 5b). These results suggested that AFP might cause inhibition downstream of the TLR-3 or TLR-4 signalling pathway, resulting in inhibition of translation of the IL-12 gene at the mRNA level.
Fig. 5.
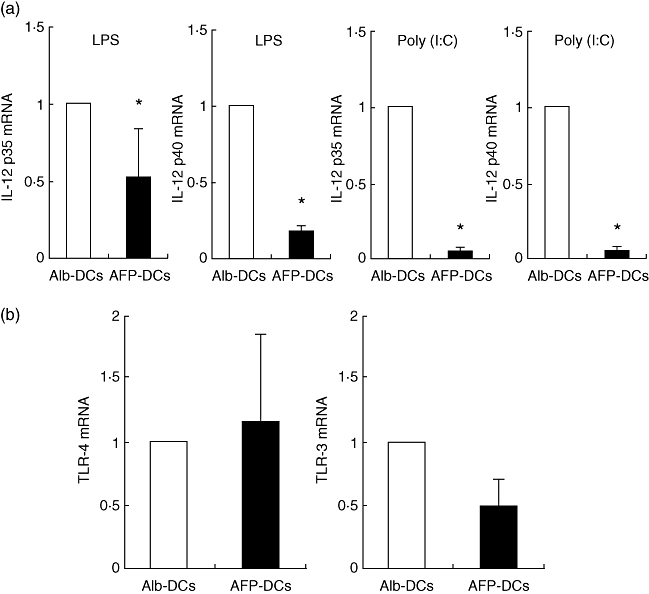
The mRNAs of interleukin (IL)-12p35 and p40 from α-fetoprotein-dendritic cells (AFP-DCs) were lower than that from albumin (Alb)-DCs, but the mRNA of Toll-like receptor (TLR)-4 and TLRL-3 were not. We cultured DCs for 7 days in RPMI-1640 with AFP (25 µg/ml) or Alb (25 µg/ml). On day 6, we added lipopolysaccharide (LPS) (10 µg/ml) or Poly(I:C) (10 µg/ml) to induce DC maturation. Twenty-four hours later, total RNA was isolated from LPS or Poly(I:C)-treated AFP-DCs or Alb-DCs and was subjected to real-time polymerase chain reaction (PCR) to detect mRNA of IL-12 (a: IL-12p35 mRNA, IL-12p40 mRNA) or mRNA of TLRs (b, TLR-4 mRNA or TLR-3 mRNA). Similar results were obtained from three independent experiments. We analysed statistically the mRNA levels of IL-12p35, IL-12p40, TLR-4 and TLR-3 between AFP-DCs and Alb-DCs. *P < 0·05.
IL-12 from DCs played critical roles in NK activation
To examine the involvement of IL-12 in the activated NK cells, NK cells were co-cultured with AFP-DCs or Alb-DCs with or without the presence of neutralizing antibody for IL-12. The cytolytic activity of NK cells co-cultured with Alb-DCs was significantly higher than that with adding anti-IL-12 neutralizing antibody, but the cytolytic activity of NK cells co-culture with AFP-DCs did not decrease significantly on addition of anti-IL-12 neutralizing antibody (Fig. 6a). Next, NK cells were co-cultured with AFP-DCs or Alb-DCs, and IL-12 was added to the NK cell/AFP-DC co-cultures. Adding IL-12 resulted in significant enhancement of the cytotoxicity of NK cells co-cultured with AFP-DCs to the levels of that with Alb-DCs (Fig. 6b). These results demonstrated that NK activity was impaired in the co-culture with AFP-DCs possibly because of less IL-12 production from AFP-DCs.
Fig. 6.
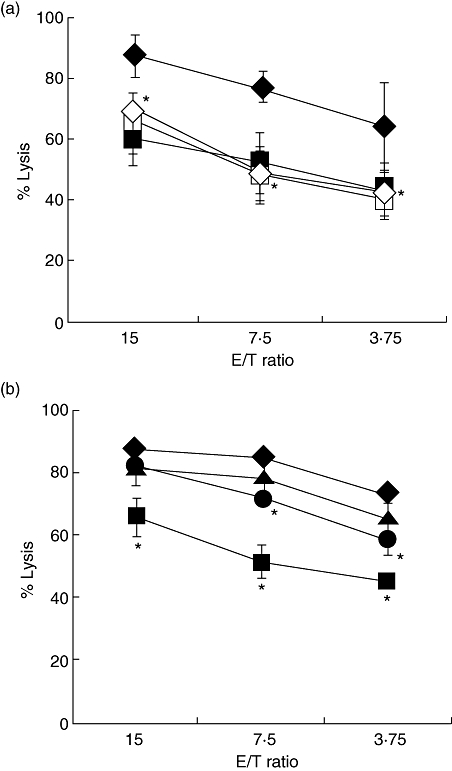
Interleukin (IL)-12 derived from dendritic cells (DCs) played a critical role in natural killer (NK) cell activation. (a) Enriched NK cells were co-cultured with α-fetoprotein (AFP)-DCs or albumin (Alb)-DCs for 24 h with or without the presence of the neutralizing antibody of IL-12. The cytolytic activity of NK cells against K562 cells were evaluated by 51Cr-releasing assay. The cytolytic activity against K562 cells of NK cells co-cultured with AFP-DCs ( ) or Alb-DCs (♦) without neutralizing antibody of IL-12 or AFP-DCs (□) or Alb-DCs (◊) with neutralizing antibody of IL-12. We analysed statistically between the antibody-adding and not-adding groups in both AFP-DC and Alb-DC cultures, respectively. *P < 0·05 versus the cytolytic activity of NK cells cultured with Alb-DCs. Significant difference was observed between the antibody-adding group and not-adding group in the cytolytic activity of NK cells cultured with Alb-DCs. In contrast, no significant difference was observed between the groups in the cytolytic activity of NK cells cultured with AFP-DCs. (b) Enriched NK cells were co-cultured with AFP-DCs or Alb-DCs for 24 h with or without recombinant IL-12p70 protein (150 pg/ml, 300 pg/ml). NK cells were harvested and subjected to examine the cytolytic activity against K562 cells by 51Cr releasing assay. The cytolytic activity co-cultured with AFP-DCs without IL-12 (
) or Alb-DCs (♦) without neutralizing antibody of IL-12 or AFP-DCs (□) or Alb-DCs (◊) with neutralizing antibody of IL-12. We analysed statistically between the antibody-adding and not-adding groups in both AFP-DC and Alb-DC cultures, respectively. *P < 0·05 versus the cytolytic activity of NK cells cultured with Alb-DCs. Significant difference was observed between the antibody-adding group and not-adding group in the cytolytic activity of NK cells cultured with Alb-DCs. In contrast, no significant difference was observed between the groups in the cytolytic activity of NK cells cultured with AFP-DCs. (b) Enriched NK cells were co-cultured with AFP-DCs or Alb-DCs for 24 h with or without recombinant IL-12p70 protein (150 pg/ml, 300 pg/ml). NK cells were harvested and subjected to examine the cytolytic activity against K562 cells by 51Cr releasing assay. The cytolytic activity co-cultured with AFP-DCs without IL-12 ( ) or with IL-12 (150 pg/ml, •; 300 pg/ml, ▴) or Alb-DCs (♦). *P < 0·05 versus the cytolytic activity of NK cells cultured with Alb-DCs. Representative results are shown. Similar results were obtained from three independent experiments in all experiments.
) or with IL-12 (150 pg/ml, •; 300 pg/ml, ▴) or Alb-DCs (♦). *P < 0·05 versus the cytolytic activity of NK cells cultured with Alb-DCs. Representative results are shown. Similar results were obtained from three independent experiments in all experiments.
Discussion
A variety of tumour-derived soluble factors have been reported to contribute to the emerging of complex local and regional immunosuppressive networks [15]. Recent study has demonstrated that innate immune system via NKG2D signals, expressed on NK cells, might play a critical role in tumour surveillance [16]. This led us to try to identify the immunosuppressive factors in innate immunity to develop a new strategy for cancer prevention. Elevation of serum AFP in cirrhosis patients is believed to be a high risk factor for HCC development [17]. AFP has already been reported to have immune regulatory function in T cells and B cells [9–11]. In this study, we hypothesized that AFP elevation might affect the immune-surveillance of innate immunity in HCC patients. We used a concentration of AFP (6·25–25 µg/ml) that is in a range similar to that detected in the sera of cirrhosis or HCC patients. Our data show that AFP inhibited DC maturation and IL-12 production from DCs which might impair NK activity. This suggested that elevated AFP might affect HCC development by inhibiting NK activity in HCC patients.
The cytolytic activities of NK cells co-cultured with AFP-DCs against K562, NK-sensitive cells as well as Huh7 hepatoma cells were lower than those co-cultured with Alb-DCs. These results suggested that the presence of AFP-stimulated DCs could alter NK cytotoxicity. We have demonstrated previously that the expression of MICA/B on DCs, NK-activating molecules, plays a critical role in the pathogenesis of chronic hepatitis and HCC [14,18]. In this study, we examined these molecules on AFP-DCs and Alb-DCs. However, the expression of MICA/B on AFP-DCs were similar to those on Alb-DCs (Yamamoto et al. unpublished data), which suggested that the soluble factor from DCs was more important in the impairment of NK cytotoxicity. In NK activation by DCs, both direct contact with these cells and soluble factors such as IL-12 from activated DCs contribute to NK activation [19]. We demonstrated that the cytolytic activities of NK cells co-cultured with AFP-DCs were also lower than those with Alb-DCs in using Transwell culture, suggesting that the soluble factor contributed to the impairment of NK cytotoxicity. To examine the involvement of IL-12 from DCs in the activation of NK cells, we co-cultured NK cells with AFP-DCs or Alb-DCs with or without the presence of neutralizing antibody for IL-12. The cytolytic activity of NK cells co-cultured with Alb-DCs decreased to the level of that with AFP-DCs on addition of anti-IL-12 neutralizing antibody. Moreover, adding IL-12 to the co-culture of AFP-DCs and NK cells resulted in enhancement of the cytolytic activity of NK cells to the levels of Alb-DCs and NK cells. Taken together, these data demonstrated that IL-12 derived from AFP-DCs plays essential roles in the impairment of NK cytotoxicity, which is consistent with the results of the production of IL-12 from AFP-DCs and Alb-DCs. Serum AFP is often high in patients with cirrhosis without HCC [8]. Oka et al. reported that the incidence of HCC development is significantly high in cirrhosis patients who had elevated serum levels of AFP [17], which suggests that high production of AFP in cirrhosis patients might also impair innate immunity, leading to HCC development. Our results might offer support for the hypothesis that elevation of AFP in cirrhosis patients impairs innate immunity which plays an essential role in the deletion of micro HCC, and thus results in promotion of HCC development.
Although the expression of antigen-presenting related molecules on AFP-DCs was not altered, the maturation of AFP-DCs was inhibited compared with Alb-DCs. This is consistent with a previous report [13] suggesting that the presence of AFP impairs DC maturation. DCs have been implicated in the activation of NK cells [19]. However, activated NK cells have been shown to recognize and lyse DCs in vitro and in vivo, but maturation of DCs results in resistance to NK lysis [19]. In HCC patients, it has been shown that impairment of DCs is associated with increased tumour progression [20] and that the levels of activated DCs are significantly low in HCC tissues [21]. High levels of AFP produced by HCC tissues may impair DC maturation, which would enhance HCC progression by removing DCs from HCC tumour areas.
IL-12 exhibits a number of immunologically important activities, including the ability to enhance NK cell and CTL functionality, to polarize CD4+ T cell responses by preferentially supporting the T helper type 1/cytotoxic T cell (Th1/Tc1)-type and to suppress Th2-type immunity [22]. We have demonstrated that the production of IL-12 protein from LPS-stimulated or Poly(I:C)-stimulated AFP-DCs was impaired significantly compared with that from Alb-DCs, which might affect immunosuppression in AFP-elevated patients. The expression of mRNA of both IL-12p35 and IL-12p40 were also inhibited significantly in AFP-DCs compared with Alb-DCs but not those of TLR-4, LPS receptor and TLR-3, Poly(I:C) receptor. The production of IL-12 is regulated strictly by positive and negative regulatory mechanisms and differential expression of TLRs affect the IL-12 production from DCs [23]. Our data revealed that adding AFP resulted in inhibition of IL-12 production at the transcriptional level, not by decreased expression of TLRs. Although the regulation of transcription of IL-12p40 and IL-12p35 has been elucidated in various studies [23,24], the detailed mechanism of inhibition of IL-12 transcription by AFP remains unclear. Further study is needed to clarify the detailed mechanism of inhibition of IL-12 by AFP. Taken together, IL-12 might play a mainly essential role in the impairment of NK activity by AFP. To evaluate the possibility of involvement of other immunosuppressive cytokines inhibiting NK activity, we examined the IL-6 and IL-10 levels in the supernatants of the co-cultures of NK cells and AFP-DCs/Alb-DCs by specific ELISAs. IL-6 levels in the supernatants of AFP-DCs were similar to those of Alb-DCs, and IL-10 levels in the supernatants of AFP-DCs were significantly lower than those of Alb-DCs (M. Yamamoto, unpublished data). These results suggest that the addition of AFP might impair the ability of cytokine production of DCs.
In a previous report, Um et al. demonstrated that AFP impairs the function of dendritic cells and induces their apoptosis [13]. In their report, they used the commercially available human cord blood AFP. Thus, we used human cord blood AFP because this is the only commercially available AFP. The carbohydrates of AFP are heterogeneous, which is reflected by differences in the binding of individual AFP molecules to lectins. Therefore, we also added the supernatants of Huh7 cells, AFP-producing HCC cells or control medium on the DCs and evaluated IL-12 production after LPS stimulation by specific ELISA. The supernatants of Huh7 cells contained AFP (1·76 µg/ml) and control medium contained no AFP. The IL-12 production of DCs co-cultured with the supernatants of Huh7 cells was significantly lower than that with control medium (M. Yamamoto, unpublished data). These results were consistent with the results using human cord blood AFP. Although we cannot deny the possibility that unknown factors, except AFP, in the supernatants of Huh7 cell might affect the IL-12 production of DCs, these results suggest that another type of AFP might also have immunoregulatory ability on DCs.
In this study, we demonstrate that AFP might down-regulate IL-12 production from DCs which inhibit NK activity. Zhang et al. demonstrated that IL-12 improves the cytotoxicity of NK cells via up-regulated expression of NKG2D on NK cells [25]. We have demonstrated previously that NKG2D expression on NK cells was down-regulated in the progression of chronic liver disease, including HCC [18], which suggested that NK activities were impaired in HCC patients. The expression of NKG2D on NK cells in HCC patients with high serum AFP was significantly lower than those in HCC patients with low serum AFP (M. Yamamoto, unpublished data). This might be the result of long-term continuous suppression of NK cells by elevated AFP.
In conclusion, this study demonstrates that AFP impair the DC ability of activation of NK cells. These findings might provide new insight into understanding the mechanisms underlying the suppression of innate immune responses in chronic liver disease patients with high serum AFP levels.
Acknowledgments
This work was supported by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan and a Grant-in-Aid for Research on Hepatitis and BSE from the Ministry of Health, Labour and Welfare of Japan.
Disclosure
The authors have no conflicts of interest.
References
- 1.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and trends. Gastroenterology. 2004;127:S35–50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Takayasu K, Arii S, Ikai I, et al. Liver Cancer Study Group of Japan Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461–9. doi: 10.1053/j.gastro.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Eng J Med. 2008;24:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 5.Doherty DG, O'Farrelly C. Innate and adaptive lymphoid cells in human liver. Immunol Rev. 2000;174:5–20. doi: 10.1034/j.1600-0528.2002.017416.x. [DOI] [PubMed] [Google Scholar]
- 6.Mehal WZ, Azzaroli F, Crispe IN. Immunology of the healthy liver: old questions and new insights. Gatsroenterology. 2001;120:250–60. doi: 10.1053/gast.2001.20947. [DOI] [PubMed] [Google Scholar]
- 7.Butterfield LH. Immunotherapeutic strategies for hepatocellular carcinoma. Gastroenterology. 2004;127:S232–41. doi: 10.1053/j.gastro.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 8.Daniele B, Bencivenga A, Megna AS, Tinessa V. α-Fetoprotein and ultrasonography screening for hepatocellular carcinoma. Gastroenterology. 2004;127:S108–12. doi: 10.1053/j.gastro.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 9.Peck AB, Murgita RA, Wigzell H. Cellular and genetic restrictions in the immunoregulatory activities of α-fetoprotein. II. α-Fetoprotein-induced suppression of cytotoxic T lymphocyte development. J Exp Med. 1978;148:360–72. doi: 10.1084/jem.148.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murgita RA, Tomasi TB., Jr Suppression of the immune response by α-fetoprotein on the primary and secondary antibody response. J Exp Med. 1975;141:269–86. doi: 10.1084/jem.141.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murgita RA, Goidl EA, Kontianen S, Wigzell H. α-Fetoprotein induces suppressor T cells in vitro. Nature. 1977;267:257–9. doi: 10.1038/267257a0. [DOI] [PubMed] [Google Scholar]
- 12.Alisa A, Boswell S, Pathan AA, Ayaru L, Williams R, Behboudi S. Human CD4+ T cells recognize an epitope within α-fetoprotein sequence and develop into TGF-β-producing CD4+ T cell. J Immunol. 2008;180:5109–17. doi: 10.4049/jimmunol.180.7.5109. [DOI] [PubMed] [Google Scholar]
- 13.Um SH, Mulhall C, Alisa A, et al. α-Fetoprotein impairs APC function and induce their apoptosis. J Immunol. 2004;173:1772–8. doi: 10.4049/jimmunol.173.3.1772. [DOI] [PubMed] [Google Scholar]
- 14.Jinushi M, Takehara T, Tatsumi T, et al. Autocrine/paracrine IL-15 that is required for type I IFN-mediated dendritic cell expression of MHC class I-related chain A and B is impaired in hepatitis C virus infection. J Immunol. 2003;171:5423–9. doi: 10.4049/jimmunol.171.10.5423. [DOI] [PubMed] [Google Scholar]
- 15.Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121:1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guerra N, Tan YX, Joncker NT, et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–80. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oka H, Tamori A, Kuroki T, Kobayashi K, Yamamoto S. Prospective study of α-fetoprotein in cirrhotic patients monitored for development of hepatocellular carcinoma. Hepatology. 1994;19:61–6. [PubMed] [Google Scholar]
- 18.Kohga K, Takehara T, Tatsumi T, et al. Serum levels of soluble major histocompatibility complex (MHC) class I-related chain A in patients with chronic liver disease and changes during transcatheter arterial embolization for hepatocellular carcinoma. Cancer Sci. 2008;99:1643–9. doi: 10.1111/j.1349-7006.2008.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferlazzo G, Munz C. NK cell compartments and their activation by dendritic cells. J Immunol. 2004;172:1333–9. doi: 10.4049/jimmunol.172.3.1333. [DOI] [PubMed] [Google Scholar]
- 20.Kunitani H, Shimizu Y, Murata H, Higuchi K, Watanabe A. Phenotypic analysis of circulating and intrahepatic dendritic cell subsets in patients with chronic liver diseases. J Hepatol. 2002;36:734–41. doi: 10.1016/s0168-8278(02)00062-4. [DOI] [PubMed] [Google Scholar]
- 21.Chen S, Akbar SM, Tanimoto K, et al. Absence of CD83-positive mature and activated dendritic cells at cancer nodules from patients with hepatocellular carcinoma: relevance to hepatocarcinogenesis. Cancer Lett. 2000;148:49–57. doi: 10.1016/s0304-3835(99)00312-2. [DOI] [PubMed] [Google Scholar]
- 22.Del Vecchio M, Bajetta E, Canova S, et al. Interkeukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13:4677–85. doi: 10.1158/1078-0432.CCR-07-0776. [DOI] [PubMed] [Google Scholar]
- 23.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 24.Pulendran B. Variegation of the immune response with dendritic cells and pathogen recognition receptors. J Immunol. 2005;173:2457–65. doi: 10.4049/jimmunol.174.5.2457. [DOI] [PubMed] [Google Scholar]
- 25.Zhang C, Zhang J, Niu J, Zhou Z, Zhang J, Tian Z. Interleukin-12 improves cytotoxicity of natural killer cells via upregulated expression of NKG2D. Human Immunol. 2008;69:490–500. doi: 10.1016/j.humimm.2008.06.004. [DOI] [PubMed] [Google Scholar]


