Abstract
Bone regeneration is a complex event that requires the interaction of numerous growth factors. Fibroblast growth factor (Fgf)-ligands have been previously described for their importance in osteogenesis during development. In the current study, we investigated the role of Fgf-18 during bone regeneration. By utilizing a unicortical tibial defect model, we revealed that mice haploinsufficient for Fgf-18 have a markedly reduced healing capacity as compared with wild-type mice. Reduced levels of Runx2 and Osteocalcin but not Vegfa accompanied the impaired bone regeneration. Interestingly, our data indicated that upon injury angiogenesis was not impaired in Fgf-18+/− mice. Moreover, other Fgf-ligands and Bmp-2 could not compensate for the loss of Fgf-18. Finally, application of FGF-18 protein was able to rescue the impaired healing in Fgf-18+/− mice. Thus, we identified Fgf-18 as an important mediator of bone regeneration, which is required during later stages of bone regeneration. This study provides hints on how to engineering efficiently programmed bony tissue for long bone repair.
Introduction
Fracture healing requires the interplay of numerous growth factors to conduct different events during repair such as inflammation and angiogenesis, callus formation, and remodeling of the bone. Interestingly, fracture healing is thought to recapitulate aspects of skeletal development,1,2 which opens up prospects to study fracture healing with a developmental lens. The fibroblast growth factor (Fgf) family and their receptors have been implicated in the regulation of numerous processes during skeletal development. For instance, depletion of Fgf-2 resulted in decreased bone mass and bone formation in femurs postnatally,3 depletion of Fgf-9 in delayed vascularization and chondrocyte hypertrophy4 and depletion of Fgf-18 in smaller cranial vaults, deformed thoracic cavities, and shortened long bones.5,6 Besides effects on osteogenesis in vivo, Fgf-18 was also found to block chondrocyte proliferation5,6 through activation of Fgfr3.7 Of note, Fgf-18 homozygotic mice Fgf-18 die perinatally. Moreover, various skeletal syndromes are associated with activating mutations in the fibroblast growth factor receptors (FGFR) 1–3, such as craniosynostosis8–10 or chondrodysplasias due to mutations in FGFR3,11–13 which highlights the importance of Fgfs in skeletal pathology. In a recent characterization of Fgfs expression levels during repair of nonstable tibial fractures, a different temporal activation of a cluster of Fgf genes was identified.14 Moreover, we have previously demonstrated that bone regeneration is impaired in Fgf-9+/− mice through decreased osteogenesis and angiogenesis.15 Among the 23 members of Fgf family, Fgf-18 is unique in consideration of the fact that is expressed by perichondrial cells and regulated by Runx2, a master gene of skeletogenesis.16 First described in 1998,17,18 Fgf-18 is considered to inhibit chondrocyte proliferation and hypertrophy and promote differentiation of osteoblasts.5,6 Further, it has been proposed that Fgf-18 is a combined target of the canonical Wnt-pathway and Runx2, conducting the pro-osteogenic effects of these transcription factors.19
Given this compelling evidence for the importance of Fgf-18 during skeletal development, in the current study we investigated the role of Fgf-18 in bone regeneration.
Materials and Methods
Skeletal injuries
All experiments using animals were performed in accordance with Stanford University Animal Care and Use Committee Guidelines. Ten- to 12-week-old age- and sex-matched Fgf-18+/− and C57/Bl6 wild-type (WT) littermates were used for all studies. Fgf-18+/− mice were previously described6 and kindly provided by Dr. David Ornitz (Washington University, St. Louis, MO). Genotyping was performed by polymerase chain reaction (PCR) analysis on genomic DNA. Tibia injures were performed as previously described.15 Briefly, after deeply anesthezing the mice, with an intraperitoneal injection of 100 mg/kg ketamine, 20 mg/kg xylazine, and 3 mg/kg acetopromazine the right legs were shaved and the skin was disinfected. Then, an incision was performed over the proximal medial diaphysis, followed by a division of the anterior tibial muscle. The medial surface of the tibia was exposed and the periosteum preserved. A unicortical defect was created with a 1 mm drill bit under constant irrigation. The anterior tibial muscle was reapproximated, the skin was closed, and mice were allowed to recover. Postoperative pain control was achieved by administration of 0.1 mg/kg buprenorphine. Mice were sacrificed after 3, 5, and 7 days, which represent the time points of inflammation/angiogenesis, hard callus formation, and remodeling. For rescue or gain-of-function experiments, defects were either treated with a 1-mm-diameter collagen sponge (Helistat; Integra Lifesciences Corporation, Plainsboro, NJ) soaked with phosphate-buffered saline (PBS) as control or with a collagen sponge soaked with 2 μg of FGF-18 (Santa Cruz Biotechnologies, Santa Cruz, CA) or vascular endothelial growth factor A (VEGFA) (R&D Systems, Minneapolis, MN) Sponges were inserted in the defects and filled out the generated bone marrow space.
Histology and immunohistochemistry
After 3, 5, and 7 days postoperatively, tibias were harvested and fixed in 4% paraformaldehyde overnight, decalcified, and paraffin embedded. Between five and eight animals were chosen for each time point and tibias were longitudinally sectioned at 9 μm. The 1 mm defect area was represented in about 60 sections. To evaluate new bone formation, every sixth slide was stained with Aniline blue, which detects the osteoid matrix or with hematoxylin and eosin according to standard procedures. Sections were photographed with a Leica digital imaging system at 5× and evaluated with Photoshop (Adobe, San Jose, CA). All images were cropped with a rectangular that covered the entire defect area (1×106 pixels). The selection of aniline blue-positive pixels was partially automated with the magic wand tool (tolerance: 60, no-contiguous). Cortical surfaces or bone chips from the drill injuries were manually deselected. The number of positive pixels was recorded and an average for each tissue sample was generated. Thereafter, averages for each group were calculated.
For immunohistochemistry, antigen retrieval was performed by incubating slides with Proteinase K (Sigma Aldrich, St. Louis, MO) at 37°C for 10 min. Primary antibodies against VEGFA (Lifespan Biosciences, Seattle, WA) were used at dilution of 1:100 and platelet endothelial cell adhesion molecule (PECAM) (Pharmingen, San Diego, CA) at dilution of 1:400. Antibodies against Runx2 and Osteocalcin (Santa Cruz Biotechnology) were used at dilution of 1:50. A rabbit or rat biotinylated secondary antibody followed by the AB reagent and NovaRed (Vector Laboratories, Burlingame, CA) were used for detection. Rabbit and rat IgG (Calbiochem, La Jolla, CA), used as negative controls, did not produce any staining (data not shown). Immunohistochemistry against anti-proliferative cell nuclear antigen (PCNA), was performed using a kit according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). Results were obtained from at least three animals per time point, and immunohistochemistry was carried out in duplicates. PCNA-positive cells or vessels, indicated by round or oval PECAM-positive structures containing a lumen, were counted within the defect areas by two blinded independent examiners at 20×magnification. Results are presented as mean±standard deviation. Tartrate-resistant acid phosphatase (TRAP) staining was performed using a leukocyte acid phosphatase kit (Sigma, St. Louis, MO).
Microcomputed tomography imaging
Microcomputed tomography (μCT) was performed, using a high-resolution MicroCAT II™ (ImTek Inc., Knoxville, TN) small animal imaging system, with the following settings: X-ray voltage of 80 kVp, anode current of 500 μA, and an exposure time of 500 ms for each of the 360 rotational steps. The two-dimensional projection images were used to reconstruct tomograms with a Feldkamp algorithm, using a commercial software package (Cobra EXXIM; EXXIM Computing Corp., Livermore, CA), resulting into a resolution of 80 μm. The duration of one scan was 9.5 min. Three-dimensional reconstructions were generated by MicroView software (GE Healthcare, London, Canada). Each mouse was scanned with a CT-phantom (GE Healthcare), containing air bubble, water, and hydroxyapatite rod, which served for calibration of each scan. For determining bone mineral density (BMD), standardized regions in the diaphysis of the tibias were chosen and analyzed with the BMD tool in MicroView. The threshold range was set between 900 and 3500. The software automatically performed data analysis and calculations. Measurements were performed on four mice of each strain.
μCT-angiographies
To assess neovascularization of bone defects in Fgf-18+/− and WT mice, animals underwent systemic perfusion with a radio-opaque contrast agent as previously described.15,20 Briefly, 7 days after the creation of unicortical tibial defects, mice were deeply anesthetized, the thoracic cavity was opened, and the heart was exposed. The left ventricle was punctured, the right atrium incised, and the entire vascular system was flushed with normal saline using a 22G catheter. Then, Microfil MV-120 (Flow Tech, Inc Carver, MA) was injected until the entire vascular system was reliably perfused. After polymerization for 2 h at room temperature, tibias were harvested, fixed overnight in 10% neutral buffered formalin, and decalcified in 19% ethylenediaminetetraacetic acid. Samples were processed for imaging by Numirabio company (www.numirabio.com) and underwent μCT scanning (μCT40; ScanCo Medical, Zurich, CH) with the following parameters: 10 μm isotropic voxel resolution at 200 ms exposure time, 2000 views, and 5 frames per view. The μCT-generated DICOM files were converted into a file format compatible with the segmentation software Seg3D (Scientific Computing and Imaging Institute, University of Utah, Salt Lake City, UT). A consistent threshold was used across the samples to extract the vasculature from the data sets using Seg3D. After the samples were imaged, measurements were made on the actual samples to determine the area of damage. For quantification of the neovascularization, the voxel count associated with the region of interest was obtained after the segmentation process; that is, the number of voxels associated with the vasculature were counted using Seg3D. The voxel count was then multiplied by the voxel resolution cubed to obtain volume measurements.
RNA isolation, RT-PCR, and quantitative RT-PCR
RNA isolation was performed as previously described.15 For RNA isolation, tibial defects of eight mice for each group were harvested, tibias were skeletonized, and defects were excised and homogenized in Trizol (Invitrogen). RNA was purified according to the manufacturer's protocol. Purified RNA was treated with DNAse I (Ambion, Austin, TX) to clear genomic DNA and reverse transcribed using the SuperScript First Strand Synthesis System (Invitrogen). Real-time (RT)-PCR and quantitative RT-PCR (QRT-PCR) were performed as previously described.15,21
Statistical analysis
Student's t-test was used for statistical analyses. A p-value<0.05 was considered statistically significant.
Results
Fgf-18+/− mice tibia did not display an overt phenotype
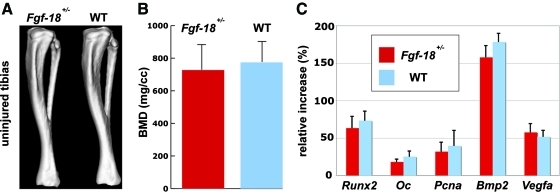
As first we evaluated, whether tibias of Fgf-18+/− mice exhibited any gross phenotypic differences. CT-scans of Fgf-18+/− and WT mice did not reveal any noticeable differences (Fig. 1A). BMDs were in a similar range and were not statistically different (Fgf-18+/−: 730±155 mg/cc; WT: 774±128 mg/cc) (Fig. 1B). QRT-PCR analysis of diaphysis of Fgf-18+/− and WT mice tibia did not reveal significant differences in the gene expression levels of osteogenic markers such as Runx2 and Osteocalcin as well as proliferative and pro-osteogenic genes, like Pcna and Bmp-2 and the angiogenic gene Vegfa (Fig. 1C).
FIG. 1.
No apparent differences in Fgf-18+/− and WT tibia. (A) Computed tomography scans of uninjured Fgf-18+/− and WT tibia revealed no obvious differences in shape. (B) Measurement of bone mineral density revealed no significant difference between Fgf-18+/− and WT tibia. (C) Quantitative real-time PCR analysis did not show differences in the gene expression profile for osteogenic, proliferative, and vascular markers. BMD, bone mineral density; Oc, osteocalcin; PCR, polymerase chain reaction; WT, wild type. Color images available online at www.liebertonline.com/tea
Bone regeneration is impaired in Fgf-18+/− mice
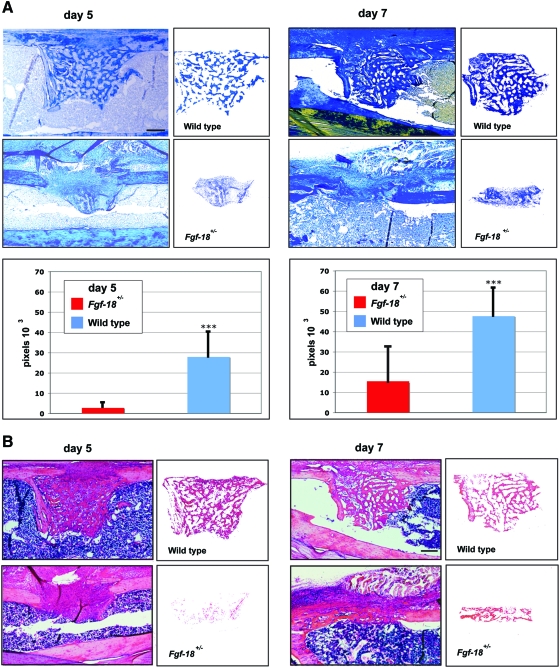
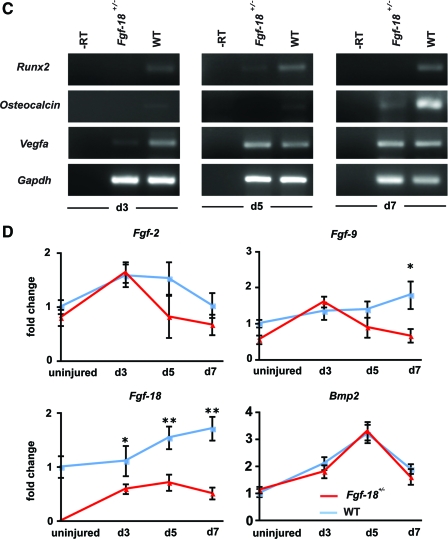
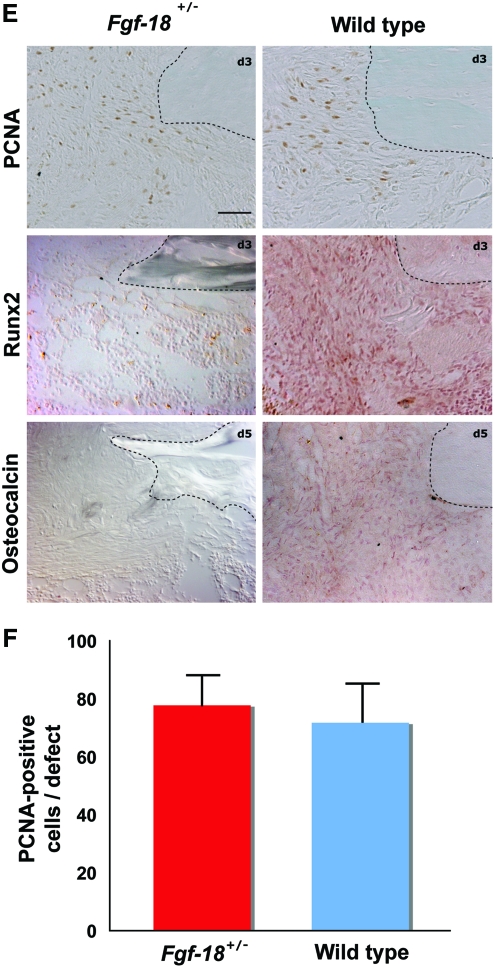
To evaluate the bone regeneration capacity of Fgf-18 haploinsufficient mice, Fgf-18+/− and WT mice underwent a tibial unicortical defect procedure, which represents an intramembranous healing model. Bone regeneration rates were determined during the hard callus (5 days postoperatively) and remodeling phase (7 days postoperatively). Aniline Blue staining revealed that new bone formation in Fgf-18+/− mice was significantly impaired as compared with WT mice (Fig. 2A). These findings were also confirmed by hematoxylin and eosin staining on adjacent slides (Fig. 2B). Histomorphometry indicated that in Fgf-18+/− mice bone formation rate was 90% reduced at postoperative day 5 and 67% reduced at postoperative day 7 as compared with WT mice (*p<0.0005). The severe impairment of bone regeneration in Fgf-18+/− mice prompted us to investigate, whether the expression of osteogenic genes were affected during the phases of healing. Indeed, expression of Runx2, an early marker of osteogenic differentiation, as well as, Osteocalcin, a late osteogenic marker, was lower at postoperative days 3, 5, and 7 in Fgf-18+/− compared with WT mice defects (Fig. 2C). In contrast, expression levels of Vegfa were lower in Fgf-18+/− mice only at postoperative day 3, but similar to WT defects at postoperative days 5 and 7 (Fig. 2C). QRT-PCR analysis of three pro-osteogenic Fgf ligands (Fgf-2, 9, and -18) revealed low levels of Fgf-2 and Fgf-9 in uninjured Fgf-18+/− tibia, whereas levels of Fgf-18 were not detectable in uninjured Fgf-18+/− tibia (Fig. 2D). Interestingly, in Fgf-18+/− defects, Fgf-2 and Fgf-9 genes were upregulated to levels similar to WT defects at postoperative day 3 and not significantly different from WT defects with the exception of lower levels of Fgf-9 in Fgf-18+/− defects at day 7. In sharp contrast and as expected, levels of Fgf-18 expression were significantly lower in haploinsufficient Fgf-18 defects than WT, at all time points analyzed. Interestingly, expression levels of Bmp-2, the prototypical osteogenic Bmp, were not impaired in tibial injuries of Fgf-18+/− mice (Fig. 2D). Moreover, immunohistochemistry for PCNA revealed similar staining in Fgf-18+/− and WT defects at postoperative day 3, thus suggesting that cell proliferation was not a limiting factor responsible for the impaired healing in Fgf-18+/− mice (Fig. 2E, F). However, immunoreactivity of Runx2 at postoperative day 3 and Osteocalcin at postoperative day 5 were lower in Fgf-18+/− defects than in WT defects, thereby mirroring the gene expression data.
FIG. 2.
Bone regeneration is impaired in Fgf-18+/− tibia. (A) Aniline blue staining of unicortical tibial defects revealed markedly impaired bone regeneration in Fgf-18+/− as compared with WT tibia at days 5 and 7 (upper panels). Histomorphometry performed on aniline blue-stained slides revealed a marked impairment of bone regeneration in Fgf-18+/− mice at both time points. ***p<0.0005. (B) Hematoxylin and eosin staining of adjacent defects of Fgf-18+/− and WT tibia at days 5 and 7 paralleled these findings. (C) real-time-PCR (RT-PCR) analysis time course of Runx2, Osteocalcin, and Vegfa harvested from defects of Fgf-18+/− and WT tibia. (D) Quantitative RT-PCR analysis of Fgf-2, -9, and -18 and Bmp2. *p<0.05 and **p<0.005. (E) Immunohistochemistry for PCNA, Runx2, and Osteocalcin in Fgf-18+/− and WT mice. No differences were observed in staining for PCNA; however, reduced immunoreactivity for Runx2 and Osteocalcin were observed in defects of Fgf-18+/− tibia. (F) Quantification of PCNA-positive cells revealed no statistical differences in proliferation between Fgf-18+/− and WT defects. Scale bars: (A, B) 200 μm, (E) 50 μm. PCNA, proliferative cell nuclear antigen. Color images available online at www.liebertonline.com/tea
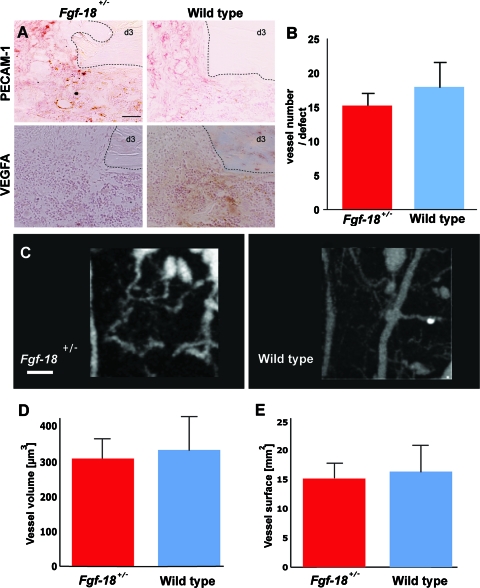
Angiogenesis is not impaired in Fgf-18+/− tibial defects
To elucidate how Fgf-18 might affect bone regeneration, we investigated the impact of Fgf-18 haploinsufficency on angiogenesis, a crucial event during the inflammation phase of fracture healing. Gene expression analysis indicated that expression of Vegfa was only mildly impaired during the inflammation stage (Fig. 2B). As a next step to investigate angiogenesis in Fgf-18+/− and WT defects, we performed immunohistochemistry for PECAM-1 (CD31), an endothelial marker. As shown in Figure 3A, an intense staining and vessel formation were observed in both Fgf-18+/− and WT defects at postoperative day 3. Moreover, immunohistochemistry for VEGFA paralleled this finding, revealing similar VEGF staining in both Fgf-18+/− and WT defects (Fig. 3A). Quantification of the vessel numbers within the defects as indicated by positive PECAM-1 stain and their round or oval structure revealed no statistical differences between Fgf-18+/− and WT defects (Fig. 3B). To further validate that angiogenesis was not impaired in Fgf-18+/− mice, we performed μCT angiography of the defects at postoperative day 7 (Fig. 3C). Quantification of both vessel volume (309 μm3 in Fgf-18+/− and 332 μm3 in WT mice) (Fig. 3D) and vessel surface area (15.4 mm2 in Fgf-18+/− and 16.1 mm2 in WT mice) (Fig. 3E) revealed similar levels in both defects. Taken together, the above results strongly suggest that angiogenesis is not impaired in Fgf-18+/− tibial defects.
FIG. 3.
Angiogenesis is not impaired in Fgf-18+/− tibia. (A) At day 3 immunohistochemistry for PECAM-1 and vascular endothelial growth factor A (VEGFA) did not reveal differences between Fgf-18+/− and WT tibia. (B) Quantification of vessel numbers as indicated by PECAM staining revealed no statistical differences between Fgf-18+/− and WT defects. (C) Microcomputed tomography-angiography of Fgf-18+/− and WT tibial defects at 7 day postoperatively. (D) Quantification of the vessel volume and (E) vessel surface area in the defects of Fgf-18+/− and WT mice did not reveal significant differences between the two groups. Scale bars: (A) 50 μm, (C) 200 μm. Dotted lines indicate the bony edges of the defects. PECAM, platelet endothelial cell adhesion molecule. Color images available online at www.liebertonline.com/tea
Bone remodeling is impaired in Fgf-18+/− tibial defects
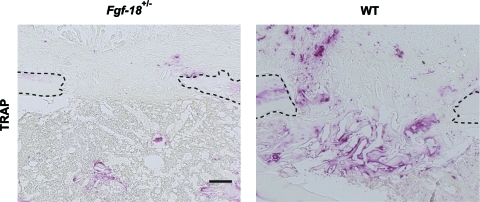
Having demonstrated an impairment of osteogenesis, but not angiogenesis in Fgf-18+/− mice, we sought to examine the effect of Fgf-18 on osteoclastogenesis. Staining for TRAP at day 7 revealed little staining in Fgf-18+/− tibial defects, whereas in WT defects, staining for TRAP was strong and found in the regenerating part of the bone (Fig. 4). This observation indicated that remodeling in Fgf-18+/− tibial defects was impaired; however, it must be noted that this effect could also be due to the decreased amount of bone regeneration occurring in Fgf-18+/− tibial defects.
FIG. 4.
Osteoclastogenesis is impaired in Fgf-18+/− tibia. Tartrate resistant acid phosphatase (TRAP) staining of Fgf-18+/− and WT tibial defects at 7 day postoperatively revealed a marked decrease in staining in Fgf-18+/− tibial defects. Scale bar: 200 μm. Dotted lines indicate the bony edges of the defects. Color images available online at www.liebertonline.com/tea
Bone regeneration in Fgf-18+/− tibial defects can be rescued with FGF-18
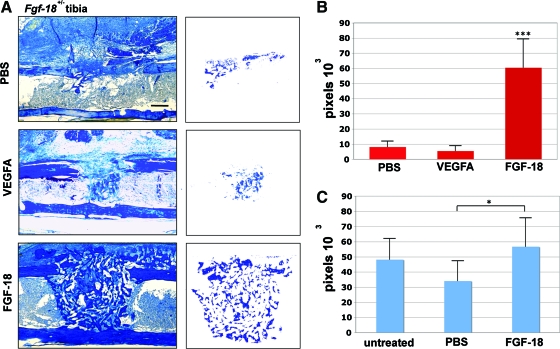
Finally, we investigated whether application of VEGFA or FGF-18 could rescue the impaired healing of tibial defects in Fgf-18+/− mice. VEGFA, well known for the capability to induce angiogenesis, has been previously demonstrated to accelerate osteogenesis.15,22 After 7 days, no improvement of bone regeneration could be achieved by application of VEGF (2 μg)-soaked collagen sponges. Likewise, application of PBS-soaked collagen sponges as control did not accelerate bone regeneration in Fgf-18+/− tibial defects (Fig. 5A). In contrast, application of FGF-18 protein rescued defects in Fgf-18+/− mice (Fig. 5A). Quantification of bone regeneration revealed that application of FGF-18 to Fgf-18+/− tibial defects increased bone regeneration by 744% as compared with PBS alone and 1105% as compared with treatment with VEGFA (p<0.0005 for both) (Fig. 5B). The rescue of bone regeneration in Fgf-18+/− tibial defects encouraged us to test the effects of FGF-18 in WT defects. Application on WT defects of collagen sponges soaked with FGF-18 slightly increased bone healing as compared with untreated WT defects, although this trend was not statistically significant (Fig. 5C). However, bone regeneration with FGF-18 in WT defects was significantly increased as compared with PBS-soaked collagen sponges (p<0.05).
FIG. 5.
Rescue of Fgf-18+/− tibial defects by application of FGF-18 but not VEGF. (A) Aniline blue staining of Fgf-18+/− tibial defects treated with phosphate-buffered saline (PBS), 2 μg VEGFA, or 2 μg FGF-18 7 days postoperatively. The injury site is segregated to the right column. Scale bar: 200 μm. (B) Histomorphometry revealed rescue of Fgf-18+/− tibial defects with FGF-18 protein but not with PBS or VEGFA, ***p<0.0005. (C) Histomorphometry of untreated WT defects or WT defects treated with PBS or 2 μg FGF-18 revealed a significant increase of healing with FGF-18 treatment as compared with PBS control at 7 day postoperatively. Moreover, there was a trend to increased healing with FGF-18 as compared with untreated WT defects; however, this was not significant, *p<0.05. Color images available online at www.liebertonline.com/tea
Discussion
The coming of regenerative medicine age has empowered tissue engineering as major discipline aimed to define and optimize techniques for regenerating tissues and organs. In this work, we investigated the role of Fgf-18 in bone regeneration, predicated on the severe impairment of the skeletal system of Fgf-18 knock out mice. Bone regeneration, but not proliferation or angiogenesis, was severely impaired in Fgf-18+/− mice, accompanied by downregulation of Runx2 and Osteocalcin. Other pro-osteogenic Fgf- ligands or Bmp-2 could not compensate for the haploinsufficency of Fgf-18; however, it was possible to rescue the tibial defects with FGF-18 protein.
The role of Fgf-18 in osteogenesis is not fully elucidated; however, there is substantial evidence for its relevance in promoting maturation and proliferation of osteoblasts from both developmental and in vitro studies. For instance, it has been previously reported that the osteogenic markers Osteopontin and Osteocalcin are decreased in embryonic long bones of Fgf-18−/− mice.5,6 Moreover, the expression of Runx2 was reduced in the trabecular bone, but not in the perichondrium/periosteum and endosteum of Fgf-18−/− mice,6 suggesting that Fgf-18 is upstream of Runx2 and that it may be involved in osteoblast maturation. On the contrary, in vitro data indicated that forced expression of Runx2 augmented the expression of Fgf-18 through canonical Wnt-signaling.19 Our data suggest that Fgf-18 is an important mediator of osteogenesis since we demonstrated decreased expression of Runx2 during bone regeneration in Fgf-18+/− mice compared with WT. This observation suggests that perhaps a reciprocal loop between Runx2 and Fgf-18 may exist in concurring and promoting osteoblast differentiation. Moreover, results obtained by TRAP staining suggest that Fgf-18 may also play a functional role during late stage of osteogenesis and its turnover.
Despite questions about genes interacting with Fgf-18, it remains unclear through which receptors FGF-18 ligand triggers osteogenic differentiation. For instance, in Stat1−/− mice, which develop increased bone mass, protein levels of FGF-18 were increased in osteoblasts lining trabecular femoral bone, whereas levels of FGFR-3 were decreased,23 suggesting that FGF-18 may act independently of FGFR-3. Further, it has been proposed that osteogenic differentiation of murine mesenchymal cells in the presence of dexamethasone is dependent on FGF-18 triggering signal through FGFR-1 and R-2, but not R-3.24 Indeed, it will be of interest to investigate in our model system through which receptors Fgf-18 is signaling.
It has been reported by Shimoaka et al. that in vitro FGF-18 induced osteoblast proliferation in a dose-dependent manner, similarly to FGF-2.25 Moreover, FGF-18 stimulated osteoclast function.25 In contrast to our in vivo data, the authors found that in vitro FGF-18 had an inhibitory effect on osteogenic differentiation25; however, these data are not consistent with the in vivo role described for Fgf-18.5,6 It must be pointed out that similar discrepancies between the in vivo and in vitro osteogenic effect has also been described for other Fgf ligands.21,26–29
An interesting observation emerging from our study was the different effect of Fgf-18 haploinsufficiency on the phenotype of long bone repair compared with what was previously observed in Fgf-9 haploinsufficiency.15 Unlike defects in Fgf-9+/− mice, angiogenesis was not impaired in Fgf-18+/− tibial defects. Moreover, cell proliferation was impaired in Fgf-9+/− but not Fgf-18+/− mice. However, it was possible to rescue the defects in both Fgf-haploinsufficient mice by treatment with the corresponding protein. It is noteworthy that application of VEGFA to defects created in Fgf-18+/− mice did not accelerate healing, whereas this effect was promptly observed in Fgf-9+/− mice. These distinct results strongly indicate that angiogenesis was neither a target nor a limiting step in Fgf-18+/− tibial defects. The observation that Fgf-18+/− mice did not exhibit marked impairment either of angiogenesis or Vegfa expression upon bone injury is in agreement with data obtained from one of the original developmental studies by Liu and colleagues showing no differences in the expression level of Vega and Mmp9 genes between E15.5 Fgf-18−/− and WT embryos.6 Therefore, our observation strengths the concept that tissue regeneration recapitulates developmental programs.
Interestingly, we found that temporal endogenous expression of other Fgf ligands, such as Fgf-2 and Fgf-9, could not compensate for the lack of Fgf-18. Initial endogenous expression levels of Fgf-2 and Fgf-9 were lower in Fgf-18+/− mice than WT, but expression was similar to WT at postoperative days 3 and 5. The coincidence of angiogenesis and expression of Fgf-9 in Fgf-18+/− mice at postoperative day 3 further supports the role of Fgf-9 as an important initial trigger for angiogenesis.15 Although Fgf-9 and Fgf-18 share some functional redundancy during limb development,4 the effects we observed upon bone regeneration were distinct, indicating that both individual growth factors are required to allow sufficient bone regeneration.
In conclusion, we revealed that Fgf-18 is required for sufficient bone regeneration. Reduced levels of Fgf-18 resulted in downregulation of Runx2 and Osteocalcin gene expression upon bone healing, whereas neither cell proliferation nor angiogenesis was affected. The above results provide new aspects of the complex biology of bone repair in the context of FGF-mediated signaling. Collectively, the data gathered from this study and a previous study15 highlight a distinct but yet converging role of two different Fgf ligands, such as FGF-9 and−18 in promoting bone repair. Moreover, the study provides valuable hints on how to achieve efficiently programmed bony tissue regeneration in injured long bone.
Acknowledgments
The authors would like to thank Dr. David Ornitz (Washington University, St. Louis, MO) for providing Fgf-18+/− mice. This work was supported by the Oak Foundation, NIH R21DE019274 and NIH R01DE019434 to M.T.L., and the German Research Foundation (DFG BE 4169-1) to B.B.
Disclosure Statement
No competing financial interests exist.
References
- 1.Schindeler A. McDonald M.M. Bokko P. Little D.G. Bone remodeling during fracture repair: the cellular picture. Semin Cell Dev Biol. 2008;19:459. doi: 10.1016/j.semcdb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Gerstenfeld L.C. Cullinane D.M. Barnes G.L. Graves D.T. Einhorn T.A. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 3.Montero A. Okada Y. Tomita M. Ito M. Tsurukami H. Nakamura T., et al. Disruption of the fibroblast growth factor-2 gene results in decreased bone mass and bone formation. J Clin Invest. 2000;105:1085. doi: 10.1172/JCI8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hung I.H. Yu K. Lavine K.J. Ornitz D.M. FGF9 regulates early hypertrophic chondrocyte differentiation and skeletal vascularization in the developing stylopod. Dev Biol. 2007;307:300. doi: 10.1016/j.ydbio.2007.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohbayashi N. Shibayama M. Kurotaki Y. Imanishi M. Fujimori T. Itoh N., et al. FGF18 is required for normal cell proliferation and differentiation during osteogenesis and chondrogenesis. Genes Dev. 2002;16:870. doi: 10.1101/gad.965702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Z. Xu J. Colvin J.S. Ornitz D.M. Coordination of chondrogenesis and osteogenesis by fibroblast growth factor 18. Genes Dev. 2002;16:859. doi: 10.1101/gad.965602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson D. Blanc A. Filion D. Wang H. Plut P. Pfeffer G., et al. Fibroblast growth factor (FGF) 18 signals through FGF receptor 3 to promote chondrogenesis. J Biol Chem. 2005;280:20509. doi: 10.1074/jbc.M410148200. [DOI] [PubMed] [Google Scholar]
- 8.Jabs E.W. Li X. Scott A.F. Meyers G. Chen W. Eccles M., et al. Jackson-Weiss and Crouzon syndromes are allelic with mutations in fibroblast growth factor receptor 2. Nat Genet. 1994;8:275. doi: 10.1038/ng1194-275. [DOI] [PubMed] [Google Scholar]
- 9.Bellus G.A. Gaudenz K. Zackai E.H. Clarke L.A. Szabo J. Francomano C.A., et al. Identical mutations in three different fibroblast growth factor receptor genes in autosomal dominant craniosynostosis syndromes. Nat Genet. 1996;14:174. doi: 10.1038/ng1096-174. [DOI] [PubMed] [Google Scholar]
- 10.Wilkie A.O. Craniosynostosis: genes and mechanisms. Hum Mol Genet. 1997;6:1647. doi: 10.1093/hmg/6.10.1647. [DOI] [PubMed] [Google Scholar]
- 11.Shiang R. Thompson L.M. Zhu Y.Z. Church D.M. Fielder T.J. Bocian M., et al. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell. 1994;78:335. doi: 10.1016/0092-8674(94)90302-6. [DOI] [PubMed] [Google Scholar]
- 12.Rousseau F. Mutations in the gene encoding fibroblast growth factor receptor-3 in achondroplasia. Nature. 1994;371:252. doi: 10.1038/371252a0. [DOI] [PubMed] [Google Scholar]
- 13.Bellus G.A. McIntosh I. Smith E.A. Aylsworth A.S. Kaitila I. Horton W.A., et al. A recurrent mutation in the tyrosine kinase domain of fibroblast growth factor receptor 3 causes hypochondroplasia. Nat Genet. 1995;10:357. doi: 10.1038/ng0795-357. [DOI] [PubMed] [Google Scholar]
- 14.Schmid G.J. Kobayashi C. Sandell L.J. Ornitz D.M. Fibroblast growth factor expression during skeletal fracture healing in mice. Dev Dyn. 2009;238:766. doi: 10.1002/dvdy.21882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Behr B. Leucht P. Longaker M.T. Quarto N. Fgf-9 is required for angiogenesis and osteogenesis in long bone repair. Proc Natl Acad Sci U S A. 2010;107:11853. doi: 10.1073/pnas.1003317107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinoi E. Bialek P. Chen Y.T. Rached M.T. Groner Y. Behringer R.R., et al. Runx2 inhibits chondrocyte proliferation and hypertrophy through its expression in the perichondrium. Genes Dev. 2006;20:2937. doi: 10.1101/gad.1482906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu M.C. Qiu W.R. Wang Y.P. Hill D. Ring B.D. Scully S., et al. FGF-18, a novel member of the fibroblast growth factor family, stimulates hepatic and intestinal proliferation. Mol Cell Biol. 1998;18:6063. doi: 10.1128/mcb.18.10.6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohbayashi N. Hoshikawa M. Kimura S. Yamasaki M. Fukui S. Itoh N. Structure and expression of the mRNA encoding a novel fibroblast growth factor, FGF-18. J Biol Chem. 1998;273:18161. doi: 10.1074/jbc.273.29.18161. [DOI] [PubMed] [Google Scholar]
- 19.Reinhold M.I. Naski M.C. Direct interactions of Runx2 and canonical Wnt signaling induce FGF18. J Biol Chem. 2007;282:3653. doi: 10.1074/jbc.M608995200. [DOI] [PubMed] [Google Scholar]
- 20.Bolland B.J. Kanczler J.M. Dunlop D.G. Oreffo R.O. Development of in vivo muCT evaluation of neovascularisation in tissue engineered bone constructs. Bone. 2008;43:195. doi: 10.1016/j.bone.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Quarto N. Longaker M.T. FGF-2 inhibits osteogenesis in mouse adipose tissue-derived stromal cells and sustains their proliferative and osteogenic potential state. Tissue Eng. 2006;12:1405. doi: 10.1089/ten.2006.12.1405. [DOI] [PubMed] [Google Scholar]
- 22.Street J. Bao M. deGuzman L. Bunting S. Peale F.V., Jr. Ferrara N., et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002;99:9656. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao L. Naganawa T. Obugunde E. Gronowicz G. Ornitz D.M. Coffin J.D., et al. Stat1 controls postnatal bone formation by regulating fibroblast growth factor signaling in osteoblasts. J Biol Chem. 2004;279:27743. doi: 10.1074/jbc.M314323200. [DOI] [PubMed] [Google Scholar]
- 24.Hamidouche Z. Fromigue O. Nuber U. Vaudin P. Pages J.C. Ebert R., et al. Autocrine fibroblast growth factor 18 mediates dexamethasone-induced osteogenic differentiation of murine mesenchymal stem cells. J Cell Physiol. 2010;224:509. doi: 10.1002/jcp.22152. [DOI] [PubMed] [Google Scholar]
- 25.Shimoaka T. Ogasawara T. Yonamine A. Chikazu D. Kawano H. Nakamura K., et al. Regulation of osteoblast, chondrocyte, and osteoclast functions by fibroblast growth factor (FGF)-18 in comparison with FGF-2 and FGF-10. J Biol Chem. 2002;277:7493. doi: 10.1074/jbc.M108653200. [DOI] [PubMed] [Google Scholar]
- 26.Canalis E. Centrella M. McCarthy T. Effects of basic fibroblast growth factor on bone formation in vitro. J Clin Invest. 1988;81:1572. doi: 10.1172/JCI113490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mansukhani A. Bellosta P. Sahni M. Basilico C. Signaling by fibroblast growth factors (FGF) and fibroblast growth factor receptor 2 (FGFR2)-activating mutations blocks mineralization and induces apoptosis in osteoblasts. J Cell Biol. 2000;149:1297. doi: 10.1083/jcb.149.6.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagai H. Tsukuda R. Mayahara H. Effects of basic fibroblast growth factor (bFGF) on bone formation in growing rats. Bone. 1995;16:367. doi: 10.1016/8756-3282(94)00049-2. [DOI] [PubMed] [Google Scholar]
- 29.Behr B. Panetta N.J. Longaker M.T. Quarto N. Different endogenous threshold levels of Fibroblast Growth Factor-ligands determine the healing potential of frontal and parietal bones. Bone. 2010;47:281. doi: 10.1016/j.bone.2010.05.008. [DOI] [PubMed] [Google Scholar]