Abstract
The authors tested the hypothesis that difficulty in identifying odors, a common finding in Parkinson's disease, is associated with more rapid progression of parkinsonian signs in 743 community-dwelling older people without dementia or Parkinson's disease at study onset. Odor identification ability was assessed at baseline with the 12-item Brief Smell Identification Test (mean = 9.0 correct, SD = 2.1), and parkinsonism was assessed annually for up to 5 years with a modified version of the Unified Parkinson's Disease Rating Scale. In an analysis adjusted for age, sex, and education, lower odor identification score was related to higher level of global parkinsonism at baseline (p < .001) and more rapid progression of global parkinsonism on follow-up (p = .002). This result mainly reflected an association of odor identification with worsening parkinsonian gait. The results suggest that impaired odor identification is associated with more rapid progression of parkinsonism in old age, particularly parkinsonian gait disturbance.
Parkinsonian signs are common in older persons without Parkinson's disease and have been associated with an increased risk of dementia (Richards, Stern, & Mayeux, 1993; Wilson, Schneider, Bienias, Evans, & Bennett, 2003; Louis, Tang, & Mayeux, 2004), disability (Louis, Tang, Schupf, & Mayeux, 2005), and mortality (Bennett et al., 1996; Wilson, Schneider, Beckett, Evans, & Bennett, 2002). In addition, these signs are often progressive (Wilson, Schneider et al., 2002; Wilson et al., 2003), with more rapid rates of progression associated with an increased risk of dementia (Wilson et al., 2003) and death (Wilson, Schneider et al., 2002). Little is known, however, about factors that predict the development and progression of parkinsonian signs in old age.
Impaired odor identification has been associated with a variety of age-related neurodegenerative conditions that impair cognitive and motor function, including Parkinson's disease (Doty, Stern, Pfeiffer, Gollomp, & Hurtig, 1992; Mesholam, Moberg, Mahr, & Doty, 1998), Alzheimer's disease (Doty et al., 1992; Serby, Larson, & Kalkstein, 1991), Huntington's disease (Nordin, Paulsen, & Murphy, 1995), and motor neuron disease (Hawkes, Shephard, Geddes, Body, & Martin, 1998). Because of these data, plus evidence of an association of olfactory dysfunction with cognitive decline (Graves et al., 1999; Swan & Carmelli, 2002; Wilson, Arnold, Tang, & Bennett, 2006), we hypothesized that difficulty identifying odors would be associated with progression of parkinsonian motor signs in old age. We tested this hypothesis with data from the Rush Memory and Aging Project, a longitudinal clinical-pathologic study of risk factors for common chronic conditions of old age. The ability to recognize familiar odors was assessed with a standard scale. At annual intervals for up to 5 years, a modified version of the motor portion of the Unified Parkinson's Disease Rating Scale was administered, and previously established composite measures of global parkinsonism and individual parkinsonian signs were derived. In analyses, we tested the hypothesis that lower odor identification is associated with more rapid progression of parkinsonian signs.
METHODS
Participants
People in this study are from the Rush Memory and Aging Project, a longitudinal clinical-pathologic investigation of risk factors for common chronic conditions of old age. They were recruited from diverse retirement facilities in and around Chicago. Following a group presentation about the project, persons rated their interest in participating. Study personnel subsequently met with those who expressed interest and explained the project in detail. Persons then signed an informed consent agreeing to annual clinical evaluations and an anatomical gift act agreeing to donate their brain, spinal cord, and selected nerves and muscles to Rush Investigators. The project was approved by the Institutional Review Board of Rush University Medical Center.
At baseline, participants had a uniform clinical evaluation that was repeated annually thereafter, with examiners blinded to previously collected data. The evaluation included a medical history, complete neurological examination with emphasis on parkinsonian signs, cognitive function testing, as previously described (Bennett et al., 2005; Wilson et al., 2005, 2006), and administration of the Brief Smell Identification Test (Doty, Marcus, & Lee, 1996). Of 989 who completed the clinical evaluation and had a valid score on the Brief Smell Identification Test, we excluded 9 people who had a history of Parkinson's disease and 80 people who on examination met the criteria for dementia of the joint working group of the National Institute of Neurologic and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (McKhann et al., 1984). These require a history of cognitive decline and evidence of impairment in two or more cognitive domains on examination, as described elsewhere (Wilson, Mendes de Leon et al., 2002).
Of the remaining 900 eligible people, 16 died within the following year and another 93 had not yet reached the date of their next annual evaluation at the time of these analyses. This left 791 people eligible for follow-up, and follow-up data were available on 743 of them (93.1%). Analyses are based on this group, which is described in Table 1. They completed two to six annual evaluations per individual (210 persons with two evaluations, 228 with three, 206 with four, 77 with five, and 22 with six).
Table 1.
Baseline characteristics of study participants*
| Characteristic | Value |
|---|---|
| Age, years | 80.9 (7.0) |
| Education, years | 14.5 (3.1) |
| Women, % | 74.6 |
| White non-Hispanic, % | 90.2 |
| MMSE score | 28.0 (2.0) |
| Clinical stroke, % | 10.8 |
| Cigarette smoking, %: | |
| Current | 3.7 |
| Former | 36.5 |
Values are mean and standard deviation unless otherwise indicated. MMSE is for Mini-Mental State Examination.
Assessment of Odor Identification
The Brief Smell Identification Test (Doty et al., 1996) was used to assess odor identification at the baseline clinical evaluation, as previously described (Wilson et al., 2006; Wilson, Arnold, Schneider, Tang, & Bennett, 2007). On this test, a microcapsule containing a familiar odor is scratched with a pencil and placed under the nose of the participant whose task is to match the smell with one of four specific choices. There are 12 items. The score is the number of odors correctly recognized; a score of 0.25 was assigned to missing responses to a maximum of two. If more than two responses were missing, the test was treated as missing. Performance on the Brief Smell Identification Test has been shown to correspond to the 40-item University of Pennsylvania Smell Identification Test (Doty, Shannon, & Dann, 1984), from which it was derived (Doty et al., 1996; Doty, Frye, & Aggarwal, 1989), and to differentiate persons with Parkinson's disease (Double et al., 2003) and mild cognitive impairment (Wang et al., 2002) from age-matched controls.
Assessment of Parkinsonian Signs
A modified form (Bennett, Shannon, Beckett, Goetz, & Wilson, 1997) of the motor section of the Unified Parkinson's Disease Rating Scale (Fahn & Elton, 1987) was administered at baseline and annually thereafter. The modifications were intended to make the scale applicable to persons without Parkinson's disease and to facilitate administration by nurse clinicians instead of physicians. There was a total of 17 items, from which previously established measure of gait disorder/postural reflex impairment (6 items), bradykinesia (4 items), rigidity (5 items), and tremor (2 items) were derived. The four sign scores were averaged to yield a measure of global parkinsonism. Scores on each measure could range from 0 to 100 and indicated the percent of the total possible item score obtained. The parkinsonian varibles have previously been shown to have excellent interrater reliability and short-term temporal stability (Bennett et al., 1997) and to be related to risk (Wilson et al., 2003) and progression (Wilson et al., 2000) of Alzheimer's disease.
Assessment of Cognitive Function
A battery of 20 cognitive tests was administered at baseline in an approximately 1-h session. The Mini-Mental State Examination was used to describe the cohort but not in analysis. The remaining 19 tests included 7 measures of episodic memory, 3 measures of semantic memory, 3 tests of working memory, 4 tests of perceptual speed, and 2 tests of visuospatial ability. To make use of all available data and minimize measurement error, a composite measure of global cognition based on all 19 tests was used in analyses. Raw scores on each test were converted to z scores, using the mean and standard deviation from all study participants, and averaged to yield the composite score. Psychometric information on the composite measure of global cognition and the 19 individual tests is contained in previous publications (Wilson et al., 2005, 2006).
Data Analysis
The associations of odor score to age and education were estimated with Pearson correlation coefficients and a t test was used to test for sex differences in odor score. The parkinsonian sign scores had negatively skewed distributions. The measures of global parkinsonism and gait disorder were subjected to a square root transformation, and the transformed scores were used in all analyses. Bradykinesia, rigidity, and tremor were relatively infrequent and so were treated as present or absent in analyses.
Generalized estimating equations (GEEs) (Liang & Zeger, 1986) were used to examine change in the parkinsonian sign scores over time and to test the relation of odor identification score to baseline parkinsonian score and, with this baseline effect controlled, to annual rate of change in parkinsonian score. The assumed distribution for the GEE models with transformed global parkinsonism and gait scores was a normal distrubution with an identity link function, and for the GEE models with a dichotomous outcome, we used a binomial distribution with a logit link function. The assumed working correlation structure for all outcomes was exchangeable.
The core model for each parkinsonian outcome measure included terms for time in years since baseline, odor identification score (centered at 8), and the interaction of time with odor identification score. The term for time indicates the average path of change in the parkinsonian measure for a person with an odor identification score of 8; the term for odor identification indicates the average difference in baseline parkinsonism associated with a 1-unit difference in the odor identification score; and the interaction represents the effect of a 1-unit difference in odor identification score on annual rate of change in parkinsonism. These and all subsequent models included terms to control for the potentially confounding effects of age, sex, and education on baseline parkinsonism and rate of change. In separate subsequent analyses of global parkinsonism and parkinsonian gait, terms were added for baseline level of global cognition and its interaction with time; those taking antipsychotic medications were excluded; terms were added for the contrast of current and of past smokers with those who never smoked and for the interaction of each indicator with time; and terms were added for clinically diagnosed stroke and its interaction with time.
Models were validated graphically and analytically. Programming was done in SAS (SAS Institute Inc., 2004), with the GEE analyses implemented in PROC GENMOD/SAS.
RESULTS
Odor Identification Performance
Scores on the Brief Smell Identification Test ranged from 0 to 12 (mean = 9.0, SD = 2.1), with higher scores indicating better ability to identify odors. Odor identification score was inversely related to age (r =−.21, p < .001) and unrelated to education (r = .03, p = .504). Women identified odors slightly better than men (t [degrees of freedom = 237] = 2.7, p = .007).
Odor Identification and Change in Global Parkinsonism
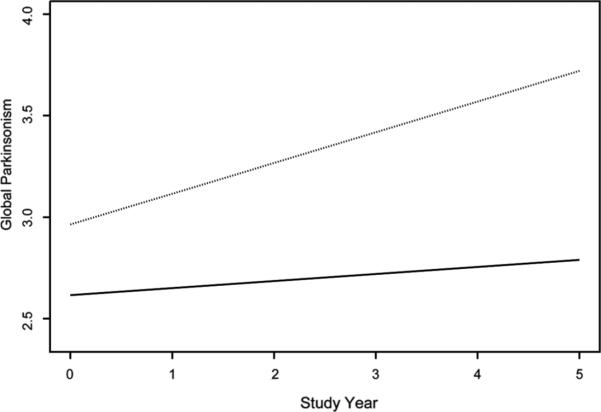
Table 2 shows the distributions of the raw parkinsonian sign scores. A square root transformation normalized the distributions of the composite and gait scores; the remaining three signs were treated as present versus absent in analyses. We constructed a GEE analysis to characterize change in the composite measure of parkinsonism and to test the hypothesis that difficulty in identifying odors at the time of the baseline evaluation would be associated with more rapid progression of global parkinsonism. The model had terms for time, odor score, and their interaction; in addition, terms for age, sex, education, and their interactions with time were included in this and all subsequent models. In this analysis, the global measure of parkinsonism increased a mean of 0.113 unit per year for a typical participant (i.e., an 80-year-old woman with 14 years of education), as shown by the term for time in Table 3. Odor identification was inversely related to baseline level of parkinsonism. With this baseline effect controlled, each additional point on the odor identification test was associated with a mean reduction of 0.023 unit, or about 20% (i.e., 0.023/0.113), in the predicted linear increase in global parkinsonism per year. As shown in Figure 1, which is based on this analysis, global parkinsonism progressively worsened in a typical person with a low odor identification performance (dotted line, score = 6, 10th percentile) but was virtually unchanged in a typical participant with a high odor identification score (solid line, score = 11, 90th percentile).
Table 2.
Distribution of parkinsonian sign scores at baseline
| Raw scores |
Scores used in analyses* |
||||
|---|---|---|---|---|---|
| Parkinsonian sign | Mean (SD) | Range | Mean (SD) | Range | Nonzero score% |
| Composite | 9.3 (7.0) | 0.0–47.9 | 2.8 (1.2) | 0.0–6.9 | |
| Gait | 19.2 (15.9) | 0.0–92.9 | 3.9 (1.9) | 0.0–9.6 | |
| Bradykinesia | 11.8 (11.9) | 0.0–63.3 | 73.4 | ||
| Rigidity | 3.6 (7.0) | 0.0–40.0 | 29.7 | ||
| Tremor | 2.6 (5.2) | 0.0–57.6 | 34.1 | ||
Based on square root transformation of raw scores or the presence of a nonzero score.
Table 3.
Relation of odor indentification score to global parkinsonism at baseline and change in global parkinsonism*
| Model term | Estimate | SE | P |
|---|---|---|---|
| Time | 0.113 | 0.021 | <.001 |
| Odor identification | −0.070 | 0.019 | <.001 |
| Time × Odor Identification | 0.023 | 0.007 | .002 |
From a generalized estimating equation model adjusted for age, sex, and education.
Figure 1.
Predicted rates of progression of the global measure of parkinsonism in persons with low (dotted line, score = 6, 10th percentile) and high (solid line, score = 11, 90th percentile) odor identification scores.
Level of cognitive function is related to both odor identification (Wilson et al., 2006) and parkinsonian signs (Wilson et al., 2003). Therefore, we repeated the analysis controlling for baseline level of cognition and its interaction with time using a previously established measure of global cognition based on 19 individual performance tests. In this analysis, the relation of odor identification to change in the composite parkinsonian measure was not substantially changed (estimate =−.023, SE = .008, p = .003). In subsequent analyses adjusted for other potentially confounding factors, the association of odor identification with change in global parkinsonism persisted after excluding persons on antipsychotic medication (13 people at baseline; estimate =−.021, SE = .007, p = .004); controlling for current and past cigarette smoking (estimate =−.023, SE = .007, p = .002); and controlling for clinical stroke (present in 10.8% at baseline; estimate =−.022, SE = .007, p = .003).
Odor Identification and Change in Parkinsonian Signs
We next examined the relation of odor identification to each of the four individual parkinsonian signs. We began with the measure of gait, which was treated as a continuous variable. In an initial analysis, gait disorder score increased by a mean of .237 unit per year (SE = .029, p < .001); higher odor identification score was associated with less severe parkinsonian gait disorder at baseline (estimate =−.112, SE = .033, p < .001) and less rapid worsening of the gait disorder (estimate =−.029, SE = .011, p = .006). Thus, gait deteriorated about twice as rapidly in a person with difficulty identifying odors (score = 6, 10th percentile) compared to a person with preserved odor identification ability (score = 11, 90th percentile). The association of low odor identification score with more rapid progression of gait disturbance persisted in subsequent analyses that controlled for baseline level of global cognition (estimate =−.027, SE = .011, p = .013), antipsychotic medication use (estimate =−.028, SE = .010, p = .007), cigarette smoking (estimate =−.029, SE = .010, p = .005), or stroke (estimate =−.029, SE = .010, p = .006).
We subsequently analyzed each of the other three parkinsonian signs, which were treated as present or absent in analyses. Odor identification was not related to baseline level of bradykinesia (estimate =−.022, SE = .042, p = .603) but had a nearly significant association with change in bradykinesia (estimate =−039, SE = .022, p = .077); it was inversely related to baseline levels of rigidity (estimate =−.081, SE = .033, p = .015) and tremor (estimate =−075, SE = .033, p = .023) but was not related to change in either sign (each p > .200).
DISCUSSION
In a group of more than 700 older persons examined annually for up to 5 years, we assessed the relation of odor identification to progression of parkinsonian signs. Lower ability to identify odors was associated with more impairment on a global measure of parkinsonism at baseline and more rapid progression of global parkinsonism during the study period. The results suggest that difficulty recognizing familiar odors is associated with increased severity and progression of parkinsonian motor impairment in old age.
We are not aware of any prior research relating olfactory function to change in parkinsonism. Difficulty recognizing familiar odors is a well-established feature of Parkinson's disease (Doty et al., 1992; Mesholam et al., 1998), but cross-sectional studies of people with Parkinson's disease (Doty, Deems, & Stellar, 1988; Doty, Ricklan, Deems, Reynolds, & Steller, 1989) or at risk for it (Marras et al., 2005) have not observed an association between odor identification and severity of parkinsonism. These studies were based on fewer than 100 participants, however, possibly limiting power to identify an association. Difficulty recognizing familiar odors has also been observed in other progressive neurodegenerative conditions which impair motor function including Alzheimer's disease (Mesholam et al., 1988; Serby et al., 1991), Huntington's disease (Nordin et al., 1995), and motor neuron disease (Hawkes et al., 1998), possibly due in part to impaired sniffing (Sobel et al., 2001).
Why might impaired odor identification be associated with severity and progression of parkinsonian motor signs, particularly gait disturbance? The neurobiological basis of parkinsonian gait disturbance in persons without Parkinson's disease is not well understood. Among older persons with and without Alzheimer's disease, parkinsonian gait has been associated with neurofibrillary pathology in the substantia nigra (Burns, Galvin, Roe, Morris, & McKeel, 2005; Liu et al., 1997; Schneider et al., 2006). Nigral dysfunction has been associated with olfactory impairment in Parkinson's disease (Siderowf et al., 2005), suggesting that such pathology might directly contribute to impaired olfaction, possibly because of nigral regulation of neurogensis in the olfactory bulb (Hoglinger et al., 2004). Alternatively, because neurofibrillary tangles are commonly seen in limbic regions involved in olfactory function (Kovacs, Cairns, & Lantos, 2001; Wilson et al., 2007), and the degree of tau pathology in the olfactory bulb is correlated with severity of tau pathology elsewhere in the brain (Tsuboi, Wszolek, Graff-Radford, Cookson, & Dickson, 2003), widespread Alzheimer's disease pathology affecting regions involved in gait and olfaction might contribute to the observed association between odor identification and change in parkinsonian signs. Another possibility is that Lewy bodies in olfactory systems might be contributing to the observed association given evidence linking olfactory dysfunction with the presence of Lewy bodies in dementia (McShane et al., 2001; Olichney et al., 2005) and that Lewy body pathology in the olfactory bulb and anterior olfactory nucleus appears to precede nigral involvement in Parkinson's disease (Braak et al., 2003). Although, cerebrovascular disease has been associated with olfactory impairment (Murphy et al., 2002) and parkinsonian signs (Winikates & Jankovic, 1999), olfaction is not affected in vascular parkinsonism (Katzenschlager, Zijlmans, Evans, Watts, & Lees, 2004), making cerebrovascular disease a less likely explanation for the observed association. Clinical-pathologic research on older persons with data on odor identification and parkinsonian signs proximate to death will be needed to investigate these and other possibilities.
This study has several strengths. On the basis of a structured medical history and clinical evaluation, persons with dementia or Parkinson's disease were excluded. There was a high rate of participation in follow-up among survivors, minimizing the likelihood that selective attrition biased results. We used previously established psychometrically sound measures of parkinsonian signs. A relatively large group of participants was studied so that there was adequate power to identify the associations of interest even after controlling for selected demographic and clinical variables.
The study also has notable limitations. Participants were selected; studies of these associations in defined populations of older persons are needed. The skewed distributions of three of the parkinsonian signs made it necessary to treat them as binary variables in analyses, limiting our ability to detect an association of odor identification with change in these signs. Because exclusion of Parkinson's disease was based on self-reported medical history, some undiagnosed persons may have been included in analyses. In addition, a longer period of follow-up observation would enhance our ability to characterize individual differences in rates of progression of parkinsonian signs and their relation to odor identification.
In summary, impaired odor identification was associated with more rapid progression of parkinsonian motor impairment, primarily affecting gait and postural stability. The findings add to evidence that age-related olfactory dysfunction may precede dysfunction in other behavioral systems and, therefore, that olfactory assessment may help identify people at risk for neurodegenerative conditions before critical neural systems underlying motor and cognitive function have been overly compromised.
Acknowledgments
This research was supported by National Institute on Aging grants R01 AG17917, R01 AG022018, and R01 AG024480, and the Illinois Department of Public Health. We thank the many Illinois residents for participating in the Rush Memory and Aging Project; Traci Colvin, MPH, and Tracy Hagman for coordinating the study; Zhaotai Cui, MS, for statistical programming; George Dombrowski, MS, and Greg Klein for data management; and Valerie J. Young for preparing the manuscript.
Footnotes
Publisher's Disclaimer: The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
REFERENCES
- Bennett DA, Beckett LA, Murray AM, Shannon KM, Goetz CG, Pilgram DM, Evans DA. Prevalence of parkinsonian signs and associated mortality in a community population of older people. New England Journal of Medicine. 1996;334:71–76. doi: 10.1056/NEJM199601113340202. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The rush memory and aging project: Study design and baseline characteristics of the study cohort. Neuropidemiology. 2005;25:169–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Shannon KM, Beckett LA, Goetz CG, Wilson RS. Metric properties of nurses' ratings of parkinsonian signs with a modified unified parkinson's disease rating scale. Neurology. 1997;49:1580–1587. doi: 10.1212/wnl.49.6.1580. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub V, de Vos RAI, Steur ENHJ, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiology of Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Burns JM, Galvin JE, Roe CM, Morris JC, McKeel DW. The pathology of the substantia nigra in Alzheimer's disease with extrapyramidal signs. Neurology. 2005;64:1397–1403. doi: 10.1212/01.WNL.0000158423.05224.7F. [DOI] [PubMed] [Google Scholar]
- Doty RL, Deems DA, Stellar S. Olfactory dysfunction in parkinsonism: A general deficit unrelated to neurologic signs, disease stage or disease duration. Neurology. 1988;38:1237–1244. doi: 10.1212/wnl.38.8.1237. [DOI] [PubMed] [Google Scholar]
- Doty RL, Frye RE, Agarwal U. Internal consistency reliability of the fractionated and whole University of Pennsylvania Smell Identification Test. Perception and Psychophysics. 1989;45:381–384. doi: 10.3758/bf03210709. [DOI] [PubMed] [Google Scholar]
- Doty RL, Marcus A, Lee WW. Development of the 12-item cross-cultural smell identification test (CC-SIT) Laryngoscope. 1996;106:353–356. doi: 10.1097/00005537-199603000-00021. [DOI] [PubMed] [Google Scholar]
- Doty RL, Riklan M, Deems DA, Reynolds C, Stellar S. The olfactory and cognitive dificits of Parkinson's disease: Evidence for independence. Annals of Neurology. 1989;25:166–171. doi: 10.1002/ana.410250210. [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: A standardized microencapsulated test of olfactory function. Physiology & Behavior. 1984;32:489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- Doty RL, Stern MB, Pfeiffer C, Gallomp SM, Hortig HI. Bilateral olfactory function in early stage treated and untreated Parkinson's disease. Journal of Neurology, Neurosurgery, and Psychiatry. 1992;55:138–142. doi: 10.1136/jnnp.55.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Double KL, Rowe DB, Hayes M, Chan DK, Blackie J, Corbett A, Joffe R, Fung VS, Morris J, Halliday GM. Identifying the pattern of olfactory deficits in Parkinson's disease using the brief smell identification test. Archives of Neurology. 2003;60:545–549. doi: 10.1001/archneur.60.4.545. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton R. Unified Parkinson's disease rating scale. In: Fahn S, Marsden C, Goldstein M, editors. Recent developments in Parkinson's disease. Vol. 12. MacMillan Healthcare Information; Florham Park, NJ: 1987. pp. 153–163. [Google Scholar]
- Graves AB, Bowen JD, Rajaram L, McCormick WC, McCurry SM, Schellenberg GD, Larson EB. Impaired olfaction as a marker for cognitive decline: Interaction with apolipoprotein E ε4 status. Neurology. 1999;53:1480–1487. doi: 10.1212/wnl.53.7.1480. [DOI] [PubMed] [Google Scholar]
- Hawkes CH, Shephard BC, Geddes JF, Body GD, Martin JE. Olfactory disorder in motor neuron disease. Experimental Neurology. 1998;150:248–253. doi: 10.1006/exnr.1997.6773. [DOI] [PubMed] [Google Scholar]
- Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nature Neuroscience. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- Katzenschlager R, Zijlmans J, Evans A, Watt H, Lees AJ. Olfactory function distinguishes vascular parkinsonism from Parkinson's disease. Journal of Neurology Neurosurgery and Psychiatry. 2004;75:1749–1752. doi: 10.1136/jnnp.2003.035287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs T, Cairns NJ, Lantos PL. Olfactory centres in Alzheimer's disease: Olfactory bulb is involved in early Braak's stages. Neuroreport. 2001;12:285–288. doi: 10.1097/00001756-200102120-00021. [DOI] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Liu Y, Stern Y, Chun M, Jacobs DM, Yau P, Goldman JE. Pathological correlates of extrapyramidal signs in Alzheimer's disease. Annals of Neurology. 1997;41:368–374. doi: 10.1002/ana.410410312. [DOI] [PubMed] [Google Scholar]
- Louis ED, Tang MX, Mayeux R. Parkinsonian signs in older people in a community-based study: Risk of incident dementia. Archives of Neurology. 2004;61:1273–1276. doi: 10.1001/archneur.61.8.1273. [DOI] [PubMed] [Google Scholar]
- Louis ED, Tang MX, Schupf N, Mayeux R. Functional correlates and prevalence of mild parkinsonian signs in a community population of older persons. Archives of Neurology. 2005;62:297–302. doi: 10.1001/archneur.62.2.297. [DOI] [PubMed] [Google Scholar]
- Marras C, Goldman S, Smith A, Barney P, Aston D, Comyns K, Kurell M, Langston JW, Ross GW, Tanner CM. Smell identification ability in twin pairs discordant for Parkinson's disease. Movement Disorders. 2005;20:687–693. doi: 10.1002/mds.20389. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlin E. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS/ADRDA work group under the auspices of department of health and human services task force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McShane RJ, Nagy Z, Esiri MM, King E, Joachim C, Sullivan N, Smith AD. Anosmia in dementia is associated with Lewy bodies rather than Alzheimer's pathology. Journal of Neurology Neurosurgery and Psychiatry. 2001;70:739–743. doi: 10.1136/jnnp.70.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesholam RI, Moberg PJ, Mahr RN, Doty RL. Olfaction in neurodegenerative disease: A meta-analysis of olfactory functioning in Alzheimer's and Parkinson's diseases. Archives of Neurology. 1998;55:84–90. doi: 10.1001/archneur.55.1.84. [DOI] [PubMed] [Google Scholar]
- Murphy C, Schubert CR, Cruisckshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. Journal of the American Medical Association. 2002;288:2307–2312. doi: 10.1001/jama.288.18.2307. [DOI] [PubMed] [Google Scholar]
- Nordin S, Paulsen JS, Murphy C. Sensory-and memory-mediated olfactory dysfunction in Huntington's disease. Journal of the International Neuro-psychological Society. 1995;1:281–290. doi: 10.1017/s1355617700000278. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Murphy C, Hofstetter CR, Foster K, Hansen LA, Thal LJ, Katzman R. Anosmia is very common in the Lewy body variant of Alzheimer's disease. Journal of Neurology Neurosurgery and Psychiatry. 2005;76:1342–1347. doi: 10.1136/jnnp.2003.032003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards M, Stern Y, Mayeux R. Subtle extrapyramidal signs can predict the development of dementia in elderly individuals. Neurology. 1993;43:2184–2188. doi: 10.1212/wnl.43.11.2184. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS OnlineDoc ®9.1.3. SAS Institute; Cary, NC: 2004. [Google Scholar]
- Serby M, Larson P, Kalkstein D. The nature and course of olfactory deficits in Alzheimer's disease. American Journal of Psychiatry. 1991;418:357–360. doi: 10.1176/ajp.148.3.357. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Li JL, Li Y, Wilson RS, Kordower JH, Bennett DA. Neurofibrillary tangles in the substantia nigra are related to gait impairment in older persons. Annals of Neurology. 2006;59:166–173. doi: 10.1002/ana.20723. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Wilson RS, Cochran EJ, Bienias JL, Arnold SE, Evans DA, Bennett DA. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology. 2003;60:1082–1089. doi: 10.1212/01.wnl.0000055863.87435.b2. [DOI] [PubMed] [Google Scholar]
- Siderowf A, Newberg A, Chou KL, Lloyd M, Colcher A, Hurtig HI, Stern MB, Doty RL, Mozley PD, Wintering N, Duda JE, Weintraub D, Moberg PJ. [99mTc]TRODAT-1 SPECT imaging correlates with odor identification in early Parkinson"s disease. Neurology. 2005;64:1716–1720. doi: 10.1212/01.WNL.0000161874.52302.5D. [DOI] [PubMed] [Google Scholar]
- Sobel N, Thomason ME, Stappen I, Tanner CM, Tetrud JW, Bower JM, Sullivan EV, Gabrieli JD. An impairment in sniffing contributes to the olfactory impairment in Parkinson's disease. Proceedings of the National Academy of Science of the United States of America. 2001;98:4154–4159. doi: 10.1073/pnas.071061598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, Carmelli D. Impaired olfaction predicts cognitive decline in nondemented older adults. Neuroepidemiology. 2002;21:58–67. doi: 10.1159/000048618. [DOI] [PubMed] [Google Scholar]
- Tsuboi Y, Wszolek ZK, Graff-Radford NR, Cookson N, Dickson DW. Tau pathology in the olfactory bulb correlates with Braak stage, Lewy body pathology and apolipoprotein ∊. Neuropathology and Applied Neurbiology. 2003;29:503–510. doi: 10.1046/j.1365-2990.2003.00453.x. [DOI] [PubMed] [Google Scholar]
- Wang QS, Tian L, Huang YL, Qin S, He LQ, Zhou JN. Olfactory identification and apolipoprotein E ε4 in mild cognitive impairment. Brain Research. 2002;951:77–81. doi: 10.1016/s0006-8993(02)03137-2. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Schneider JA, Tang Y, Bennett DA. The relation of cerebral Alzheimer's disease pathology to odor identification in old age. Journal of Neurology Neurosurgery and Psychiatry. 2007;78:30–35. doi: 10.1136/jnnp.2006.099721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Tang Y, Bennett DA. Odor identification and decline in different cognitive domains in old age. Neuroepidemiology. 2006;26:61–67. doi: 10.1159/000090250. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. Journal of the International Neuropsychology Society. 2005;11:400–407. [PubMed] [Google Scholar]
- Wilson RS, Bennett DA, Gilley DW, Beckett LA, Schneider JA, Evans DA. Progression of parkinsonism and loss of cognitive function in Alzheimer's disease. Archives of Neurology. 2000;57:855–860. doi: 10.1001/archneur.57.6.855. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Mendes de Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, Bennett DA. Participation in cognitively stimulating activities and risk of incident Alzheimer's disease. Journal of the American Medical Association. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Beckett LA, Evans DA, Bennett DA. Progression of gait disorder and rigidity and risk of death in older persons. Neurology. 2002;58:1815–1819. doi: 10.1212/wnl.58.12.1815. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Bienias JL, Evans DA, Bennett DA. Parkinsonianlike signs and risk of incident Alzheimer's disease in older persons. Archives of Neurology. 2003;60:539–544. doi: 10.1001/archneur.60.4.539. [DOI] [PubMed] [Google Scholar]
- Winikates J, Jankovic J. Clinical correlates of vascular parkinsonism. Archives of Neurology. 1999;56:98–102. doi: 10.1001/archneur.56.1.98. [DOI] [PubMed] [Google Scholar]