Abstract
Background
Leucocyte telomere length (LTL) chronicles the cumulative burden of oxidative stress and inflammation over a life course. Activation of the renin-angiotensin-aldosterone system (RAAS) is associated with increased oxidative stress and inflammation. Therefore, LTL may be related to circulating biomarkers of the RAAS.
Methods
We evaluated the cross-sectional relations of LTL (dependent variable) to circulating renin and aldosterone concentrations and the renin-aldosterone ratio (all logarithmically-transformed; independent variables) in 1203 Framingham Study participants (mean age 59 years, 51% women). We used multivariable linear regression and adjusted for age, blood pressure, hypertension treatment, smoking, diabetes, body mass index, hormone replacement therapy, serum creatinine and the urine sodium-creatinine ratio.
Results
Overall, multivariable-adjusted LTL was inversely related to renin (beta coefficient per unit increase [β]=-0.038; p= 0.036), directly related to aldosterone (β=0.099; p= 0.002), and inversely related to the renin-aldosterone ratio (β=-0.049; p= 0.003). Relations of LTL to biomarkers were stronger in those with hypertension, although a formal test of interaction was not statistically significant (p=0.20). Individuals with hypertension displayed significant associations of LTL with renin (β=-0.060; p= 0.005), aldosterone (β=0.134; p= 0.002) and renin-aldosterone ratio (β=-0.072; p<0.001). Participants with hypertension who were in the top tertile of the renin-aldosterone ratio had LTL that was 182 base pairs shorter relative to those in the lowest tertile.
Conclusions
In our community-based sample, LTL was shorter in individuals with a higher renin-aldosterone ratio, especially so in participants with hypertension. Additional investigations are warranted to confirm our observations.
Keywords: Telomere, Renin, Aldosterone, Hypertension, Epidemiology, Association, Salt, Oxidative stress
Introduction
Telomeres are tandem repeats of TTAGGG at the ends of chromosomes that preserve genomic integrity and progressively shorten with replication of cultured somatic cells.1 Telomeres demonstrate age-dependent shortening in proliferative somatic cells in vivo.2 Therefore, at any age, leukocyte telomere length (LTL) represents the balance between LTL at birth and the attrition that occurs thereafter. In clinical studies, shorter age-adjusted LTL has been associated with metabolic (oxidative stress, inflammation),3-6 structural (atherosclerosis and arterial stiffness)7-10 and environmental (cigarette smoking and obesity)11,12 indices of cardiovascular disease (CVD).
Telomere length is a record of the replicative history of somatic cells,13 because telomere repeats are lost with each cell division; such a loss is heightened with oxidative stress.14,15 These features might render LTL a valuable index of metabolic factors predisposing to atherosclerosis. Firstly, inflammation, which enhances the turnover rate of leukocytes, increases the pace of LTL shortening. Secondly, as oxidative stress increases the rate of telomere attrition per cell division, it also accelerates LTL shortening. Accordingly, LTL chronicles the cumulative burden of inflammation and oxidative stress over the lifetime of an individual. Both inflammation and oxidative stress are major determinants in atherosclerosis– a process that is contingent on the continued recruitment of leukocytes16 and increased oxidative stress at the interface of the endothelium with the blood.17 Such a process usually takes place over the course of many years, and it would be expressed by a higher pace of LTL attrition and a shorter LTL.
On a parallel note, substantial data implicate increased activation of components of the renin-angiotensin-aldosterone system (RAAS) in the atherosclerotic process. The two major components of the RAAS are its primarily vasoactive arm, the renin-angiotensin system (RAS), and its primarily sodium regulating arm, aldosterone, both of which evoke oxidative stress and inflammatory responses.18-21 We do not know, however, which of these two arms of the RAAS provokes comparatively more inflammation/oxidative stress in vivo, and, therefore, might increase atherosclerotic risk more relatively to the other. Given that LTL attrition chronicles the cumulative burden of inflammation/oxidative stress and is associated (inversely) with atherosclerosis,7,9,10 our objective was to relate LTL to circulating renin and aldosterone concentrations in a community-based sample to obtain insights into the issues noted above.
Methods
Study Sample
In 1971, 5124 offspring (and their spouses) of the original Framingham Heart Study participants were enrolled into the Framingham Offspring Study.22 The sixth examination of this cohort occurred from 1995 to 1998 and was attended by 3532 individuals. 1589 DNA samples from the sixth examination were available for LTL analysis. These subjects were selected to be biologically unrelated. Among the 1589 subjects, we excluded 386 individuals because of missing data on plasma renin or serum aldosterone (n=41), or inadequate quality or amount of DNA for LTL measurements (n=345). After these exclusions, 1203 individuals remained eligible for the present investigation. Participants included in the present investigation were not systematically different from the sample of eligible attendees who were not included (Appendix Table 1). The study protocol was approved by the Institutional Review Board at Boston University Medical Center, and all participants provided written informed consent.
Appendix Table 1. Comparison of mean levels of CVD risk factors in the study sample with the remainder of the attendees at examination cycle 6.
| Characteristic | Study Sample
(N=1203) |
Attendees at exam cycle 6 minus study sample
(N=2329) |
|---|---|---|
| Age, years | 59±9 | 59±10 |
| Women, % | 51.8 | 53.8 |
| Systolic BP, mmHg | 130±19 | 128±19 |
| Diastolic BP, mmHg | 76±9 | 75±10 |
| Total cholesterol, mg/dL | 205±38 | 206±42 |
| LDL cholesterol, mg/dL | 154±38 | 155±42 |
| HDL cholesterol, mg/dL | 51±16 | 51±16 |
| Triglycerides, mg/dL | 138±96 | 144±149 |
| Fasting blood glucose mg/dL | 105±29 | 103±27 |
| Current smokers, % | 13.8 | 16.0 |
| Body mass index, kg/m2 | 27.9±5.1 | 27.9±5.1 |
Values are means ± standard deviations or percentages.
Clinical Evaluation
At the sixth examination cycle (referred to as the ‘index’ examination), all attendees underwent standardized evaluations, including medical history and physical examination, anthropometry and laboratory assessment of cardiovascular risk factors. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of anti-hypertensive medications.23 Diabetes mellitus was defined as fasting glucose ≥126 mg/dl, or use of insulin or hypoglycemic medications.24 Participants were considered current cigarette smokers if they reported having smoked cigarettes regularly during the previous year.
Measurement of renin, aldosterone and urinary sodium
At the index examination, venous blood was drawn from fasting study participants— typically between 8 a.m. and 9 a.m.— with the participants in a recumbent position for about 5-10 minutes prior to the phlebotomy; participants were ambulatory prior to blood draw. Blood specimens were centrifuged immediately and serum/plasma stored at −80°C without repeat freeze-thaw cycles until their assay in 2002.
Serum aldosterone concentration was measured from extracted and fractionated serum using a highly sensitive and specific radioimmunoassay (Quest Diagnostics Inc., CA) with a sensitivity of <1 ng/dL.25 The intra-assay coefficient of variation (CV) ranges from 3.8% (for high concentrations) to 6% (low concentrations) with corresponding interassay CVs varying from 4.0 to 9.8%. Plasma renin concentration was measured with a highly sensitive and specific immunochemiluminometric assay (Nichols Advantage® Direct Renin assay).26 The intra-assay CV ranges from 3.7 percent (at high levels) to 7.2 percent (at low levels), with corresponding inter-assay CV ranging from 4.9 to 10 percent. This direct renin assay yields measurements that have a high degree of correlation with the plasma renin activity.27,28 Serum high-sensitivity C-reactive protein (CRP) was measured using a Dade Behring BN100 nephelometer, with an intra-assay CV of 2.2%.
Spot urine samples (3 ml) were collected at the index examination at the time of phlebotomy, temporarily stored at −4°C for up to 4 hours, and maintained at −20°C until analysis. Urine samples were thawed at room temperature, and urine sodium was measured using an automated ion-electrode method. Samples were analyzed in duplicate with an average intra-assay CV of 0.8%. Urinary creatinine concentration was determined using a modified Jaffe method with an average intra-assay CV of 1.7-3.8%. Urinary sodium excretion was expressed as mmol of sodium/gm of urinary creatinine (referred to as urine sodium index).29
LTL measurements
We performed Southern blot analyses and obtained the mean of the terminal restriction fragment lengths in DNA extracted from leukocytes. We refer to this mean as LTL. Details of the method to measure the LTL, including an illustrative figure (Appendix figure), are presented in the appendix. The CV for this approach (for samples measured in duplicate or triplicate on different gels and occasions and by two researchers) was 2.4%.
Appendix Figure.
Illustration of an autoradiogram showing the terminal restriction fragments (LTLs) (A), the molecular weight ladders (B) and the superimposition of A and B The position of each band of the MW ladder (y) a was determined by y= a0+a1*exp(-kb/a2).
The mean LTL length was calculated as follows: LTL =ΣODi/Σ(ODi/MWi), where ODi is optical density at a given position in the lane and MWi is molecular weight at that position. This formula accounts for the fact that longer telomeres bind more labeled probe and consequently appear darker on the X-ray film.
Statistical Analyses
We used multivariable linear regression models to relate serum aldosterone and plasma renin (independent variables modeled jointly) to LTL (dependent variable). Serum aldosterone and plasma renin concentrations were treated as continuous variables (natural-logarithmic-transformed values because of a positively skewed distribution). We also related LTL to the ratio of the two biomarkers, and present the results for the renin/aldosterone ratio (as opposed to the more conventionally-used aldosterone-renin ratio) for ease of interpretation (see results section below). The renin/aldosterone ratio was modeled as a continuous ratio and as tertiles. Because there was no evidence of effect modification of the relations of serum aldosterone and plasma renin and LTL by sex, all analyses were sex-pooled, with sex incorporated as a covariate.
Two sets of models were constructed: 1) adjusting for age and sex alone; 2). multivariable models additionally adjusting for covariates that are known to influence LTL,3,4,6,11,12 and plasma renin and aldosterone concentrations,30,31 age, sex, systolic and diastolic blood pressure, hypertension treatment, current smoking status, diabetes, serum creatinine, urine sodium index, body mass index, and hormone replacement therapy. We tested for effect modification by age and hypertension status by incorporating corresponding interaction terms in multivariable models.
Since any association of biomarkers of the RAAS with LTL may be mediated by their proinflammatory effects, we performed secondary analyses adjusting for CRP in the multivariable models, in addition to the covariates listed above. Antihypertensive agents differentially affect plasma renin and aldosterone concentration: diuretics raise aldosterone and renin levels; angiotensin converting enzyme inhibitors raise renin levels but lower aldosterone levels; beta blockers inhibit renin, and calcium channel blockers are generally neutral. Therefore, we conducted exploratory analyses to assess if the association of LTL with biomarkers varied among treated and untreated participants with hypertension by incorporating appropriate interaction terms for use of antihypertensive medications in a model that evaluated the subgroup of participants with hypertension.
Results
General Characteristics
The characteristics of our study sample (mean age 59 years; 51% women) are displayed in Table 1. Over 40% of men and women in our sample had hypertension; Appendix Table 2 details the use of antihypertensive medications in these individuals by drug class. The mean values and overall distribution of serum aldosterone were similar in men and women, whereas plasma renin levels were slightly higher in men, consistent with our prior reports on a larger sample.30,31
Table 1. Characteristics of Framingham Heart Study sample and non-hypertensive subgroup at examination 6.
| Entire sample
(n=1203) |
Non-hypertensive* sample | Hypertensive participants | ||||
|---|---|---|---|---|---|---|
| Men
(n=580) |
Women
(n=623) |
Men
(n=314) |
Women
(n=371) |
Men
(n=263) |
Women
(n=251) |
|
| Age, yrs | 60±10 | 59±9 | 57±9 | 56±9 | 64±9 | 62±8 |
| Body mass index, kg/m2 | 28.5±4.5 | 27.3±5.6 | 28.2±4.7 | 26.4±4.9 | 28.9±4.1 | 28.8±6.2 |
| Systolic blood pressure (mm Hg) | 131±18 | 129±20 | 121±10 | 118±12 | 143±18 | 145±19 |
| Diastolic blood pressure (mm Hg) | 77±9 | 74±9 | 75±7 | 71±7 | 80±11 | 78±10 |
|
| ||||||
| Hypertension at exam 6†, % | 46 | 40 | 0 | 0 | 100 | 100 |
|
|
||||||
| On any treatment, % | see data for hypertensives | not applicable | 70 | 65 | ||
| On diuretics, % | 11.0 | 25 | ||||
| On ACE inhibitors, % | 32 | 26 | ||||
| On beta blockers, % | 23 | 26 | ||||
| On calcium channel blockers, % | 26 | 19 | ||||
| On alpha-1 AR blockers, % | 2 | 0.4 | ||||
|
| ||||||
| Total cholesterol to HDL ratio | 4.82±1.5 | 3.93±1.4 | 4.86±1.6 | 3.80±1.5 | 4.77±1.4 | 4.12±1.3 |
| Diabetes‡, % | 16 | 10 | 12 | 5 | 20 | 18 |
| Current smoker, % | 13 | 15 | 16 | 16 | 10 | 13 |
| Prevalent cardiovascular disease, % | 14 | 7 | 9 | 3 | 21 | 14 |
| Premenopausal, % | 24 | 32 | 11 | |||
| Postmenopausal on HRT, % | not applicable | 26 | not applicable | 26 | not applicable | 27 |
| Postmenopausal no HRT, % | 50 | 42 | 61 | |||
|
| ||||||
| Serum creatinine (mg/dL) | 1.26±0.2 | 1.11±0.3 | 1.25±0.2 | 1.1±0.2 | 1.27±0.2 | 1.14±0.4 |
| Urine Na/Cr ratio mmol/g§ | 106 | 121 | 104 | 117 | 108 | 125 |
| Serum aldosterone ng/L (Q1, Q3) | 10 (7,14) | 10 (7, 15) | 10 (7, 14) | 10 (7, 14) | 10 (7, 14) | 10 (7, 15) |
| Plasma renin (μU/mL) (Q1, Q3) | 15 (8, 25) | 11 (6, 20) | 15 (9, 24) | 11 (7, 18) | 14 (7, 31) | 11 (5, 28) |
| Renin-aldosterone ratio (μU/mL/ ng/L) (Q1, Q3) | 1.5 (0.9, 2.6) | 1.1 (0.6, 2.0) | 1.55 (1.0, 2.3) | 1.2 (0.7, 1.9) | 1.4 (0.8, 3.2) | 1.0 (0.5, 2.4) |
Values are reported as mean (SD) for continuous traits and % for dichotomous traits. Because serum aldosterone, renin and ARR are skewed, median values [25%ile-75%ile] are shown. Na = sodium, Cr = creatinine, AR = adrenergic receptor.
Non-hypertensive participants at baseline exam 6 were defined by systolic BP <140mmHg, diastolic BP <90mmHg and absence of antihypertensive treatment.
Shown are the percentages of participants with treated and untreated hypertension.
Diabetes determined by use of hypoglycemic agents or fasting glucose ≥ 126mg/dL.
Note that Urine Na/Cr ratio was available for the subset of 1066 (511 men).
Appendix Table 2. Anti-HTN use at exam 6 by drug class.
| Individuals with Hypertension at exam 6 | Men
(n=263) |
Women
(n=251) |
|---|---|---|
| On diuretics alone | 5 | 22 |
| On diuretics with other agents | 24 | 39 |
|
| ||
| On ACE inhibitors alone | 50 | 30 |
| On ACE inhibitors with other agents | 35 | 34 |
|
| ||
| On beta blockers alone | 30 | 32 |
| On beta blockers with other agents | 30 | 32 |
|
| ||
| On calcium channel blockers alone | 34 | 19 |
| On calcium channel blockers with other agents | 35 | 28 |
Serum aldosterone and plasma renin were positively correlated (age- and sex-adjusted Spearman r =0.21, p =<0.001). The rate of LTL attrition with age for the entire cohort was 22±1.7 base pairs (bp) per year (r=-0.35, p<0.001). Age-adjusted LTL was longer in women than men (by 124 bp; men, 6.90 ±0.02 kb; women, 7.02±0.02 kb; p<0.001), and in ‘never smokers’ than current smokers (Never smokers, 7.0158±0.03 kb; Current smokers, 6.84± 0.04 kb; p =0.0012), though the relations were borderline statistically significant in the latter. Body mass index was related inversely to LTL (decrement of 11±3 bp per unit increase, p <0.001).
Associations between LTL with Aldosterone, Renin and Renin-Aldosterone Ratio
Table 2 displays the results of regression analyses relating aldosterone, renin and the renin-aldosterone ratio to LTL in the whole sample, and stratified by hypertension status. Modeled together, after adjustment for covariates LTL was inversely related to renin (p=0.036), and directly related to aldosterone (p=0.002). Results were similar when renin and aldosterone were modeled individually (Appendix Table 3). LTL was inversely related to the renin-aldosterone ratio (p=0.003).
Table 2. Regression of Leucocyte Telomere Length on log (aldosterone) and log (renin) conjointly, and on log (renin-aldosterone ratio).
| All Participants | Non-Hypertensive | Hypertensive | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model, adjustment | Variable | Beta | SE | P-value | Beta | SE | P-value | Beta | SE | P-value |
| Incorporating Aldosterone and renin conjointly | ||||||||||
| age, sex | log (aldosterone) | 0.082 | 0.03 | 0.004 | 0.063 | 0.04 | 0.15 | 0.092 | 0.04 | 0.02 |
| log (renin) | -0.039 | 0.02 | 0.012 | -0.009 | 0.03 | 0.77 | -0.053 | 0.02 | 0.003 | |
| Multivariable | log (aldosterone) | 0.099 | 0.03 | 0.002 | 0.058 | 0.05 | 0.22 | 0.134 | 0.04 | 0.002 |
| log (renin) | -0.038 | 0.02 | 0.036 | 0.001 | 0.03 | 0.99 | -0.060 | 0.02 | 0.005 | |
| Incorporating the Renin-aldosterone ratio | ||||||||||
| Age, sex | log (ratio) | -0.047 | 0.02 | 0.002 | -0.022 | 0.03 | 0.45 | -0.058 | 0.02 | <0.001 |
| Multivariable | log (ratio) | -0.049 | 0.02 | 0.003 | -0.014 | 0.03 | 0.67 | -0.072 | 0.02 | <0.001 |
multivariable models adjusted for age, sex, systolic BP, diastolic BP, HTN treatment (where appropriate), current smoking status, diabetes, serum creatinine, urine sodium/creatinine ratio, body mass index, hormone replacement therapy.
Beta is the regression coefficient per unit increase in log(predictor). SE = standard error of beta.
Appendix Table 3. Regression of Leucocyte telomere length on log (aldosterone), log (renin) individually.
| All Participants | Non-Hypertensive | Hypertensive | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model, adjustment | Variable | Beta | (SE) | P-value | Beta | (SE) | P-value | Beta | (SE) | P-value |
| Incorporating Aldosterone alone | ||||||||||
| Age, sex | log (aldosterone) | 0.067 | 0.03 | 0.0176 | 0.058 | 0.04 | 0.16 | 0.079 | 0.04 | 0.047 |
| Multivariable | log (aldosterone) | 0.085 | 0.03 | 0.0056 | 0.058 | 0.04 | 0.185 | 0.122 | 0.04 | 0.006 |
| Incorporating Renin alone | ||||||||||
| Age, sex | log (renin) | -0.030 | 0.02 | 0.0485 | 0.007 | 0.03 | 0.81 | -0.048 | 0.02 | 0.007 |
| Multivariable | log (renin) | -0.026 | 0.02 | 0.142 | 0.016 | 0.03 | 0.61 | -0.053 | 0.02 | 0.013 |
multivariable models adjusted for age, sex, systolic BP, diastolic BP, HTN treatment (where appropriate), current smoking status, diabetes, serum creatinine, urine sodium/creatinine ratio, body mass index, hormone replacement therapy and CRP.
Beta is the regression coefficient per unit increase in log(predictor). SE = standard error of beta.
We did not observe effect modification by hypertension status (p =0.20 for interaction). Nevertheless, we stratified all analyses by hypertension status (Table 2), given the limited power to detect significant interactions and the fundamental role of the RAS in hypertension. In these analyses, we observed in participants with hypertension statistically significant relations of LTL with renin (inverse; p=0.005), aldosterone (positive; p=0.002) and renin-aldosterone ratio (inverse; p<0.001). Relations of LTL to RAAS biomarkers were not statistically significant in non-hypertensive individuals. It is noteworthy that we had adequate statistical power to detect associations of a similar magnitude in the subsets without and with hypertension; we had 80% power to detect partial correlations of the biomarkers with LTL of 0.107 (R2=1.1%) and 0.124 (R2=1.54%), respectively in the two groups.
Figure 1 displays the adjusted least square mean LTL according to tertiles of the renin-aldosterone ratio in the overall sample, and in nonhypertensive and hypertensive participants. In the overall sample, individuals in the top tertile of the ratio had a LTL that was 108 bases shorter relative to those in the lowest tertile. Corresponding differences among nonhypertensive and hypertensive individuals (top compared to lowest tertile) were 59 and 182 bases, respectively (Figure 1).
Figure 1.
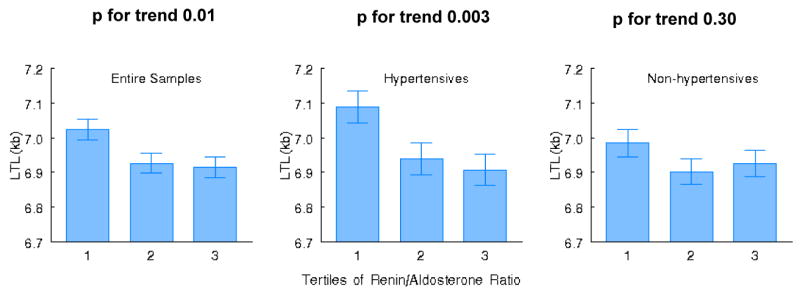
Least square means for adjusted leukocyte telomere length (LTL) according to tertile of the renin-aldosterone ratio in the entire sample (left panel), hypertensive individuals (middle panel) and nonhypertensive participants (right panel). P values indicate trend across tertiles of the ratio.
Table 3 displays the results of analyses with additional adjustment for CRP. The associations seen in Table 2 remain robust after additionally adjusting for CRP.
Table 3. Regression of Leucocyte Telomere Length on log (aldosterone) and log (renin) conjointly, and on log (renin-aldosterone ratio): Additional adjustment for CRP.
| All Participants | Non-Hypertensive | Hypertensive | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model, adjustment | Variable | Beta | SE | P-value | Beta | (SE) | P-value | Beta | (SE) | P-value |
| Incorporating Aldosterone and renin conjointly | ||||||||||
| age, sex | log (aldosterone) | 0.082 | 0.03 | 0.004 | 0.063 | 0.04 | 0.15 | 0.092 | 0.04 | 0.02 |
| log (renin) | -0.039 | 0.02 | 0.012 | -0.009 | 0.03 | 0.77 | -0.053 | 0.02 | 0.003 | |
| Multivariable | log (aldosterone) | 0.099 | 0.03 | 0.002 | 0.051 | 0.05 | 0.29 | 0.146 | 0.04 | 0.001 |
| log (renin) | -0.034 | 0.02 | 0.06 | -0.002 | 0.03 | 0.95 | -0.055 | 0.02 | 0.01 | |
| Incorporating the Renin-aldosterone ratio | ||||||||||
| Age, sex | log (ratio) | -0.047 | 0.02 | 0.002 | -0.022 | 0.03 | 0.45 | -0.058 | 0.02 | <0.001 |
| Multivariable | log (ratio) | -0.046 | 0.02 | 0.007 | -0.010 | 0.03 | 0.75 | -0.070 | 0.02 | <0.001 |
multivariable models adjusted for age, sex, systolic BP, diastolic BP, HTN treatment (where appropriate), current smoking status, diabetes, serum creatinine, urine sodium/creatinine ratio, body mass index, hormone replacement therapy and CRP.
Beta is the regression coefficient per unit increase in log(predictor). SE = standard error of beta.
We performed secondary analysis focusing on the association of biomarkers with LTL in untreated (n=166) versus treated (n=347) participants with hypertension. There was no effect modification by antihypertensive medication use (all p values for interaction terms were ≥0.50).
Discussion
Principal Findings and Related Considerations
We observed that across a wide range of sodium intake (expressed in urinary sodium output), and after adjusting for sex, smoking, and blood pressure status, age-adjusted LTL correlated positively with plasma aldosterone and negatively with plasma renin. Thus, shortened age-adjusted LTL was more frequently expressed in individuals with the phenotype of a high renin-aldosterone ratio, i.e., a more active RAS. In large measure, this association was driven by the individuals with hypertension. Both angiotensin II18,21 and aldosterone20,21 provoke inflammation/oxidative stress. One potential explanation for our finding may be that a preferential reliance on the RAS over aldosterone to maintain blood pressure could generate more inflammation/oxidative stress and pose greater atherosclerotic risk. This speculation is based on findings that shortened LTL ostensibly reflects an increased cumulative burden of inflammation/oxidative stress and is strongly associated with atherosclerosis.7,9,10
Individuals with higher plasma renin levels have been reported to have increased mortality risk, thereby implicating increased activity of the RAS in disease states.32-35 The stronger associations we noted in hypertensive individuals is consistent with greater activation of the RAS in those with elevated blood pressure. Angiotensin II is a powerful activator of the p53/p21 pathways, which play a key role in cellular senescence.36 Indeed, shortened LTL has been observed in a host of aging-related diseases, particularly atherosclerotic CVD,6,8-10,37 the common denominator for which is increased burden of oxidative stress and inflammation.
The positive association of LTL with aldosterone concentrations in our investigation conflicts with a recent report by Benetos et al.,38 who observed that LTL was inversely correlated with plasma aldosterone in 75 normotensive and mildly hypertensive men. That report was based on a small sample (n=75) and the investigators adjusted only for age (raising the issue of residual confounding). No measurements of plasma renin were available in that study.
We note that LTL is heritable11,39-42 and that shortened LTL is associated with a host of risk factors to CVD and diminished lifespan, including, male gender,6,11,43 insulin resistance,4,44 low socio-economic status,45 cigarette smoking,11,12 and generally unhealthy lifestyle.43 The shorter LTL in smokers than non-smokers is in line with the concept that the smoking-induced increase burden of oxidative stress and inflammation46-48 accelerates not only cardiovascular aging but systemic aging, as well.49
Postulated Mechanisms Underlying the Principal Findings
There is considerable interaction between angiotensin II and aldosterone and the targets of both agents are diverse and not mutually exclusive.18-21 For instance, angiotensin II is a determinant in aldosterone release from the adrenal gland and has a major impact on renal sodium reabsorption in the proximal tubules, while the targets of aldosterone include the heart and vasculature. That said, we can still broadly divide the RAAS into its renin-angiotensin (RAS) arm and the aldosterone arm. For a given sodium intake, the relative dependency on aldosterone versus angiotensin II for maintaining blood pressure homeostasis might be an important determinant of the correlates of LTL. Our findings raise the possibility that a preponderance of angiotensin II activity (as reflected by renin levels) relative to aldosterone for the modulation of blood pressure (as evidenced by a high renin-aldosterone ratio) may promote an increased burden of inflammation and oxidative stress, expressed in a shorter LTL. This proposed paradigm may serve as a theoretical framework that integrates blood pressure regulation, sodium homeostasis, vascular tone, RAAS and vascular aging, and it merits further study.
Strengths and Limitations
The strengths of this work include the community-based sample, the blinded assessments of LTL and biomarkers of the RAAS, and adjustment for multiple confounders in multivariable analyses. However, several limitations of our study should be noted. First, circulating biomarkers may not adequately reflect the degree of activation of the RAAS in tissues. Second, whereas LTL might be a record of the cumulative burden of oxidative stress and inflammation during the lifetime of the individual, the single measures of renin and aldosterone reflect the current status of the RAAS in ambulatory individuals. Additionally, we did not evaluate biomarkers of oxidative stress at the index examination. However, we have previously reported4 an inverse association of LTL and urinary excretion of isoprostanes (an index of systemic oxidative stress) measured at an examination approximately fours years after the examination at which LTL measurements were obtained. Last, our sample comprised predominantly middle-aged to elderly whites of European descent. The generalizability of our findings to younger individuals or other ethnicities is unknown.
Conclusions
Our observations in a moderate-sized community based sample indicate that individuals, primarily those with hypertension, with a higher renin-aldosterone ratio display shorter LTL. We hypothesize, based on these observations, that a greater dependency on angiotensin II versus aldosterone to regulate blood pressure entails a relative increase in the cumulative burden of oxidative stress and inflammation and heightens the atherosclerotic risk. In this regard, a recent study showed that shortened LTL predicts coronary artery disease events10. Both cross-sectional and longitudinal studies are warranted to confirm the link between LTL and the RAAS.
Acknowledgments
This project was supported by a NIA grant AG021593 and AG028321; NHLBI NO1-HC 25195; 2K24 HL 4334 and R01HL67288.
Appendix
LTL Length Analysis
A major problem with the LTL length analysis is that the DNA may migrate at slightly different rates in different regions of the gel. This is due to a host of technical reasons. For this reason, we developed techniques that resolved each digested DNA sample and the molecular weight ladder on the same lane, thereby eliminating the effect of variation in DNA migration in different lanes. Samples were digested overnight with restriction enzymes digest set, HinfI (5.2 U)/Rsa I (5.2 U) (Roche). DNA samples (3 μg each) and DNA ladders (1 kb DNA ladder plus 23.1kb fragment of λ DNA/Hind III fragments (Invitrogen, Carlsbad, CA)) were resolved on a 0.5% agarose gel (20 cm × 20 cm) at 50 V (GNA-200 Pharmacia Biotech). After 16 hr, the DNA was depurinated for 15 min in 0.25 N HCl, denatured 30 min in 0.5 mol/L NaOH/1.5 mol/L NaCl and neutralized for 30 min in 0.5 mol/L Tris, pH 8/1.5 mol/L NaCl. The DNA was transferred for 1 hr to a positively charged nylon membrane (Roche) using a vacuum blotter (Boeckel Scientific, Feasterville, PA). The membranes were spotted at 4 sites with diluted telomeric probe [digoxigenin 3′-end labeled 5′-(CCTAAA)3] (arrows, Figure1 A,B,C) and then hybridized at 65 °C with the probe overnight in 5 × SSC, 0.1% Sarkosyl, 0.02% SDS and 1% blocking reagent (Roche). The membranes were washed 3 times at room temperature in 2 × SSC, 0.1% SDS each for 15 min and once in 2 × SSC for 15 min. The digoxigenin-labeled probe was detected by the digoxigenin luminescent detection procedure (Roche) and exposed on X-ray film. After scanning the LTL signal by densitometry (Figure 1A), the membrane was stripped and re-probed with a molecular weight marker probe (Figure 1B). The superimposition of A and B, using the 4 spotted sites of telomeric probe (arrows), yields the image shown in Figure 1C. In this way, variation in DNA migration in different lanes did not affect the analysis.
Footnotes
Ramachandran S. Vasan:
Research Grant: NIH grant, Amount: >= $10,000
Serkalem Demissie: No disclosures
Masayuki Kimura: No disclosures
L. Adrienne Cupples: No disclosures
Nader Rifai: No disclosures
Charles White: No disclosures
Thomas J Wang: No disclosures
Jeffrey P Gardner: No disclosures
Xiaogian Cao: No disclosures
Emelia J Benjamin:
Research Grant: NIH grant, Amount: >= $10,000
Daniel Levy: No disclosures
Abraham Aviv:
Research Grant: NIH grant, Amount: >= $10,000
Publisher's Disclaimer: Disclaimer: The manuscript and its contents are confidential, intended for journal review purposes only, and not to be further disclosed.
Reference List
- 1.Wong JM, Collins K. Telomere maintenance and disease. The Lancet. 2003;362:983–988. doi: 10.1016/S0140-6736(03)14369-3. [DOI] [PubMed] [Google Scholar]
- 2.Blackburn EH. Telomere states and cell fates. Nature. 2000;408:53–56. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- 3.Aviv A, Valdes A, Gardner JP, Swaminathan R, Kimura M, Spector TD. Menopause Modifies the Association of Leukocyte Telomere Length with Insulin Resistance and Inflammation. J Clin Endocrinol Metab. 2006;91:635–640. doi: 10.1210/jc.2005-1814. [DOI] [PubMed] [Google Scholar]
- 4.Demissie S, Levy D, Benjamin EJ, Cupples LA, Gardner JP, Herbert A, Kimura M, Larson MG, Meigs JB, Keaney JF, Aviv A. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5:325–330. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 5.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. From the Cover: Accelerated telomere shortening in response to life stress. PNAS. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Walston J, Kimura M, Aviv A. Leukocyte Telomere Length and Cardiovascular Disease in the Cardiovascular Health Study. Am J Epidemiol. 2007;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 7.Benetos A, Okuda K, Lajemi M, Kimura M, Thomas F, Skurnick J, Labat C, Bean K, Aviv A. Telomere Length as an Indicator of Biological Aging : The Gender Effect and Relation With Pulse Pressure and Pulse Wave Velocity. Hypertension. 2001;37:381–385. doi: 10.1161/01.hyp.37.2.381. [DOI] [PubMed] [Google Scholar]
- 8.Benetos A, Gardner JP, Zureik M, Labat C, Xiaobin L, Adamopoulos C, Temmar M, Bean KE, Thomas F, Aviv A. Short Telomeres Are Associated With Increased Carotid Atherosclerosis in Hypertensive Subjects. Hypertension. 2004;43:182–185. doi: 10.1161/01.HYP.0000113081.42868.f4. [DOI] [PubMed] [Google Scholar]
- 9.Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. White Cell Telomere Length and Risk of Premature Myocardial Infarction. Arterioscler Thromb Vasc Biol. 2003;23:842–846. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- 10.Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, Packard CJ, Samani NJ. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. The Lancet. 369:107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 11.Nawrot TS, Staessen JA, Gardner JP, Aviv PA. Telomere length and possible link to X chromosome. The Lancet. 2004;363:507–510. doi: 10.1016/S0140-6736(04)15535-9. [DOI] [PubMed] [Google Scholar]
- 12.Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. Obesity, cigarette smoking, and telomere length in women. The Lancet. 366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 13.Stewart SA, Weinberg RA. Telomeres: Cancer to Human Aging. Annual Review of Cell and Developmental Biology. 2006;22:531–557. doi: 10.1146/annurev.cellbio.22.010305.104518. [DOI] [PubMed] [Google Scholar]
- 14.Hall DB, Holmlin RE, Barton JK. Oxidative DNA damage through long-range electron transfer. Nature. 1996;382:731–735. doi: 10.1038/382731a0. [DOI] [PubMed] [Google Scholar]
- 15.von Zglinicki T. Role of Oxidative Stress in Telomere Length Regulation and Replicative Senescence. Ann NY Acad Sci. 2000;908:99–110. doi: 10.1111/j.1749-6632.2000.tb06639.x. [DOI] [PubMed] [Google Scholar]
- 16.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 17.Gutierrez J, Ballinger SW, rley-Usmar VM, Landar A. Free Radicals, Mitochondria, and Oxidized Lipids: The Emerging Role in Signal Transduction in Vascular Cells. Circ Res. 2006;99:924–932. doi: 10.1161/01.RES.0000248212.86638.e9. [DOI] [PubMed] [Google Scholar]
- 18.Brasier AR, Recinos A, III, Eledrisi MS. Vascular Inflammation and the Renin-Angiotensin System. Arterioscler Thromb Vasc Biol. 2002;22:1257–1266. doi: 10.1161/01.atv.0000021412.56621.a2. [DOI] [PubMed] [Google Scholar]
- 19.Fiebeler A, Luft FC. The mineralocorticoid receptor and oxidative stress. Heart Fail Rev. 2005;10:47–52. doi: 10.1007/s10741-005-2348-y. [DOI] [PubMed] [Google Scholar]
- 20.Funder JW. Aldosterone, mineralocorticoid receptors and vascular inflammation. Molecular and Cellular Endocrinology. 2004;217:263–269. doi: 10.1016/j.mce.2003.10.054. [DOI] [PubMed] [Google Scholar]
- 21.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 22.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 23.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 Report. JAMA. 2003;289 doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 24.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 25.Ito T, Woo J, Haning R, Horton R. A radioimmunoassay for aldosterone in human peripheral plasma including a comparison of alternate techniques. J Clin Endocrinol Metab. 1972;34:106–112. doi: 10.1210/jcem-34-1-106. [DOI] [PubMed] [Google Scholar]
- 26.de Bruin RA, Bouhuizen A, Diederich S, Perschel FH, Boomsma F, Deinum J. Validation of a New Automated Renin Assay. Clin Chem. 2004;50:2111–2116. doi: 10.1373/clinchem.2004.032052. [DOI] [PubMed] [Google Scholar]
- 27.Ferrari P, Shaw SG, Nicod J, Saner E, Nussberger J. Active renin versus plasma renin activity to define aldosterone-to-renin ratio for primary aldosteronism. J Hypertens. 2004;22:377–381. doi: 10.1097/00004872-200402000-00023. [DOI] [PubMed] [Google Scholar]
- 28.Perschel FH, Schemer R, Seiler L, Reincke M, Deinum J, Maser-Gluth C, Mechelhoff D, Tauber R, Diederich S. Rapid screening test for primary hyperaldosteronism: ratio of plasma aldosterone to renin concentration determined by fully automated chemiluminescence immunoassays. Clin Chem. 2004;50:1650–1655. doi: 10.1373/clinchem.2004.033159. [DOI] [PubMed] [Google Scholar]
- 29.Flack JM, Grimm RH, Jr, Staffileno BA, Dnsc, Elmer P, Yunis C, Hedquist L, Dudley A. New salt-sensitivity metrics: variability-adjusted blood pressure change and the urinary sodium-to-creatinine ratio. Ethn Dis. 2002;12:10–19. [PubMed] [Google Scholar]
- 30.Kathiresan S, Larson MG, Benjamin EJ, Corey D, Murabito JM, Fox CS, Wilson PW, Rifai N, Meigs JB, Ricken G, Lifton RP, Levy D, Vasan RS. Clinical and genetic correlates of serum aldosterone in the community: the Framingham Heart Study. Am J Hypertension. 2005;18:657–665. doi: 10.1016/j.amjhyper.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 31.Newton-Cheh C, Guo CY, Gona P, Larson MG, Benjamin EJ, Wang TJ, Kathiresan S, O'Donnell CJ, Musone SL, Camargo AL, Drake JA, Levy D, Hirschhorn JN, Vasan RS. Clinical and genetic correlates of aldosterone-to-renin ratio and relations to blood pressure in a community sample. Hypertension. 2007;49:846–856. doi: 10.1161/01.HYP.0000258554.87444.91. [DOI] [PubMed] [Google Scholar]
- 32.Alderman MH, Madhavan S, Ooi WL, Cohen H, Sealey JE, Laragh JH. Association of the renin-sodium profile with the risk of myocardial infarction in patients with hypertension. N Engl J Med. 1991;324:1098–1104. doi: 10.1056/NEJM199104183241605. [DOI] [PubMed] [Google Scholar]
- 33.Alderman MH, Ooi WL, Cohen H, Madhavan S, Sealey JE, Laragh JH. Plasma renin activity: a risk factor for myocardial infarction in hypertensive patients. Am J Hypertens. 1997;10:1–8. doi: 10.1016/s0895-7061(96)00301-9. [DOI] [PubMed] [Google Scholar]
- 34.Brunner HR, Laragh JH, Baer L, Newton MA, Goodwin FT, Krakoff LR, Bard RH, Buhler FR. Essential hypertension: renin and aldosterone, heart attack and stroke. N Engl J Med. 1972;286:441–449. doi: 10.1056/NEJM197203022860901. [DOI] [PubMed] [Google Scholar]
- 35.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, Jacques PF, Rifai N, Selhub J, Robins SJ, Benjamin EJ, D'Agostino RB, Vasan RS. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 36.Minamino T, Komuro I. Vascular Cell Senescence: Contribution to Atherosclerosis. Circ Res. 2007;100:15–26. doi: 10.1161/01.RES.0000256837.40544.4a. [DOI] [PubMed] [Google Scholar]
- 37.Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 38.Benetos A, Gardner JP, Kimura M, Labat C, Nzietchueng R, Dousset B, Zannad F, Lacolley P, Aviv A. Aldosterone and Telomere Length in White Blood Cells. J Gerontol A Biol Sci Med Sci. 2005;60:1593–1596. doi: 10.1093/gerona/60.12.1593. [DOI] [PubMed] [Google Scholar]
- 39.Andrew T, Aviv A, Falchi M, Surdulescu GL, Gardner JP, Lu X, Kimura M, Kato BS, Valdes AM, Spector TD. Mapping genetic loci that determine leukocyte telomere length in a large sample of unselected female sibling pairs. Am J Hum Genet. 2006;78:480–486. doi: 10.1086/500052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Njajou OT, Cawthon RM, Damcott CM, Wu SH, Ott S, Garant MJ, Blackburn EH, Mitchell BD, Shuldiner AR, Hsueh WC. Telomere length is paternally inherited and is associated with parental lifespan. PNAS. 2007;104:12135–12139. doi: 10.1073/pnas.0702703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slagboom PE, Droog S, Boomsma DI. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994;55:876–882. [PMC free article] [PubMed] [Google Scholar]
- 42.Vasa-Nicotera M, Brouilette S, Mangino M, Thompson JR, Braund P, Clemitson JR, Mason A, Bodycote CL, Raleigh SM, Louis E, Samani NJ. Mapping of a major locus that determines telomere length in humans. Am J Hum Genet. 2005;76:147–151. doi: 10.1086/426734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bekaert S, De Meyer T, Rietzschel ER, De Buyzere ML, De Bacquer D, Langlois M, Segers P, Cooman L, Van Damme P, Cassiman P, Van Criekinge W, Verdonck P, De Backer GG, Gillebert TC, Van Oostveldt P. Telomere length and cardiovascular risk factors in a middle-aged population free of overt cardiovascular disease. Aging Cell. 2007;6:639–647. doi: 10.1111/j.1474-9726.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 44.Gardner JP, Li S, Srinivasan SR, Chen W, Kimura M, Lu X, Berenson GS, Aviv A. Rise in Insulin Resistance Is Associated With Escalated Telomere Attrition. Circulation. 2005;111:2171–2177. doi: 10.1161/01.CIR.0000163550.70487.0B. [DOI] [PubMed] [Google Scholar]
- 45.Cherkas LF, Aviv A, Valdes AM, Hunkin JL, Gardner JP, Surdulescu GL, Kimura M, Spector TD. The effects of social status on biological aging as measured by white-blood-cell telomere length. Aging Cell. 2006;5:361–365. doi: 10.1111/j.1474-9726.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- 46.Churg A. Interactions of exogenous or evoked agents and particles: the role of reactive oxygen species. Free Radical Biology and Medicine. 2003;34:1230–1235. doi: 10.1016/s0891-5849(03)00175-8. [DOI] [PubMed] [Google Scholar]
- 47.Repine J, Bast A, Lankhorst I, The Oxidative Stress Study Group Oxidative Stress in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 1997;156:341–357. doi: 10.1164/ajrccm.156.2.9611013. [DOI] [PubMed] [Google Scholar]
- 48.van der Vaart H, Postma DS, Timens W, Ten Hacken NHT. Acute effects of cigarette smoke on inflammation and oxidative stress: a review. Thorax. 2004;59:713–721. doi: 10.1136/thx.2003.012468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bernhard D, Moser C, Backovic A, Wick G. Cigarette smoke--an aging accelerator? Exp Gerontol. 2007;42:160–165. doi: 10.1016/j.exger.2006.09.016. [DOI] [PubMed] [Google Scholar]


