Abstract
The discovery of new uses for older, clinically approved drugs is one way to expedite drug development for cancer. Thiocolchicoside, a semisynthetic colchicoside from the plant Gloriosa superba, is a muscle relaxant and used to treat rheumatologic and orthopedic disorders because of its analgesic and anti-inflammatory mechanisms. Given that activation of the transcription factor NF-κB plays a major role in inflammation and tumorigenesis, we postulated that thiocolchicoside would inhibit NF-κB and exhibit anticancer effects through the modulation of NF-κB–regulated proteins. We show that thiocolchicoside inhibited proliferation of leukemia, myeloma, squamous cell carcinoma, breast, colon, and kidney cancer cells. Formation of tumor colonies was also suppressed by thiocolchicoside. The colchicoside induced apoptosis, as indicated by caspase-3 and poly(ADP-ribose) polymerase cleavage, and suppressed the expression of cell survival [e.g., Bcl-2, X-linked inhibitor of apoptosis (XIAP), MCL-1, bcl-xL, cIAP-1, cIAP-2, and cFLIP] proteins. Cell proliferation biomarkers such as c-MYC and phosphorylation of phosphoinositide 3-kinase and glycogen synthase kinase 3β were also blocked by thiocolchicoside. Because most cell survival and proliferation gene products are regulated by NF-κB, we studied the effect of thiocolchicoside on this transcription factor and found that thiocolchicoside inhibited NF-κB activation, degradation of inhibitory κBα (IκBα), IκBα ubiquitination, and phosphorylation, abolished the activation of IκBα kinase, and suppressed p65 nuclear translocation. This effect of thiocolchicoside on the NF-κB pathway led to inhibition of NF-κB reporter activity and cyclooxygenase-2 promoter activity. Our results indicate that thiocolchicoside exhibits anticancer activity through inhibition of NF-κB and NF-κB–regulated gene products, which provides novel insight into a half-century old drug.
Introduction
Thiocolchicoside is a semisynthetic drug derived from colchicoside, a natural glucoside present in the plant Gloriosa superba. Thiocolchicoside has been used clinically for >35 years as a muscle relaxant as well as an anti-inflammatory and analgesic drug (1). Given these properties, thiocolchicoside has long been used to treat a number of orthopedic, traumatic, and rheumatologic conditions. Furthermore, clinical trials showed that thiocolchicoside is an efficient and safe treatment for patients with acute low back pain accompanied by muscle spasm (2) and is at least as effective as tizanidine, the standard drug used to treat low back pain (3, 4). Biochemical studies indicated that thiocolchicoside can interact with γ-aminobutyric acid receptors, given that it was able to inhibit the binding of both [3H]γ-aminobutyric acid and [3H]strychnine to rat cerebrocortical and spinal cord membranes, respectively, in vitro and in vivo (5–7). Whereas these findings suggest that thiocolchicoside, a γ-aminobutyric acid agonist, induces depression of the central nervous system and, in turn, myorelaxation (8), the mechanism of its anti-inflammatory effect is still unknown.
One possible mechanism by which thiocolchicoside may exert its anti-inflammatory effect is by modulating the NF-κB pathway, which is commonly involved in inflammation and tumorigenesis. NF-κB is a transcription factor that resides in the cytoplasm in its resting stage and then, when activated, translocates to the nucleus and mediates the transcription of >400 different genes (9). Various inflammatory agents can activate NF-κB, including cytokines [e.g., tumor necrosis factor (TNF)], carcinogens, tumor promoters, cigarette smoke, environmental pollutants, ionizing radiation, and stress (9). NF-κB activation has been shown to control the expression of genes linked to inflammation, apoptosis, survival, proliferation, invasion, angiogenesis, metastasis, chemoresistance, tumor cell transformation, and radioresistance (9).
Because of the critical role of NF-κB in inflammation and tumorigenesis, we postulated that thiocolchicoside mediates its anti-inflammatory effect through modulation of the NF-κB pathway. Indeed, our results show that thiocolchicoside inhibits NF-κB activated by inflammatory cytokines (TNF), okadaic acid (OA), tumor promoter [phorbol 12-myristate 13-acetate (PMA)], and lipopolysaccharide (LPS) through inhibition of phosphorylation, ubiquitination, and degradation of inhibitory κBα (IκBα), the inhibitor of NF-κB. Thiocolchicoside also inhibited the phosphorylation and nuclear translocation of p65, the major isoform of NF-κB. Thiocolchicoside inhibition of NF-κB leads to suppression of NF-κB–regulated proteins, which are responsible for the anticancer effect of thiocolchicoside on various cancer cell lines, characterized by induction of apoptosis and inhibition of cell proliferation as well as colony formation. Together, our results provide a new role for thiocolchicoside as an anticancer agent.
Materials and Methods
Reagents
A 100 mmol/L solution of thiocolchicoside, kindly provided by Sarv Bio Labs, was prepared in water, stored at +4°C, and then diluted as needed in cell culture medium. Bacteria-derived recombinant human TNF-α was kindly provided by Genentech.
Penicillin, streptomycin, Iscove’s modified Dulbecco’s medium, DMEM, RPMI 1640, and fetal bovine serum (FBS) were purchased from Invitrogen. The proteasome inhibitor N-acetyl-leucylleucyl-norleucinal (ALLN) was purchased from Calbiochem.
PMA, OA, LPS, and anti-β-actin antibody were purchased from Sigma-Aldrich. Antibodies against p65, poly (ADP-ribose) polymerase (PARP), inhibitor of apoptosis protein 1, BCL-2, BCL-xL, c-MYC, and caspase-3 were purchased from Santa Cruz Biotechnology. The antibody against the inhibitory subunit of NF-κB (IκBα) was purchased from Imgenex. Anti-XIAP antibodies were purchased from BD Biosciences. Anti-phosphorylated IκBα (Ser32/36) and anti-phosphorylated p65 (Ser536) were purchased from Cell Signaling Technology. Anti-IκBα kinase α (IKKα), anti-IKKβ, and anticellular caspase-8–like inhibitory protein (c-FLIP) antibodies were kindly provided by Imgenex.
Cell lines
All cell lines used in this study were purchased from American Type Culture Collection. KBM5 cells were cultured in Iscove’s modified Dulbecco’s medium supplemented with 15% FBS. U266, RPMI-8226, Jurkat, MM.1S, Caco-2, andHT-29 cells were cultured in RPMI 1640, and A293, HCT-116, MCF-7, and MCF-10A cells were cultured in DMEM supplemented with 10% FBS. SCC4 cells were cultured in DMEM containing 10% FBS, nonessential amino acids, pyruvate, glutamine, and vitamins. All media were also supplemented with 100 units/mL penicillin and 100 μg/mL streptomycin.
Cytotoxicity assay
Cell proliferation and cell viability experiments were assayed by the modified tetrazolium salt MTT assay as described previously (10). For the cell proliferation assay, 2,000 cells were incubated with various concentrations of thiocolchicoside, in triplicate, for 1, 3, and 5 days in 96-well plates at 37°C. For the cell viability assay, 5,000 cells were incubated with various concentrations of thiocolchicoside, in triplicate, for 24 hours in 96-well plates at 37°C. Thereafter, an MTT solution was added to each well. After 2 hours of incubation at 37°C, lysis buffer (20% SDS and 50% dimethylformamide) was added, the cells were incubated overnight at 37°C, and then absorbance was measured at 570 nm using a 96-well multi-scanner (MRX Revelation, Dynex Technologies).
Clonogenic assay for HCT-116
The clonogenic assay or colony formation assay is an in vitro cell survival assay based on the ability of a single cell to grow into a colony (11). HCT-116 cells have been used for the clonogenic assay, because these are adherent cells and give a good response for this assay. To test the ability of thiocolchicoside to inhibit single cells to grow into colonies, 500 cells were seeded in six-well plates and incubated overnight to allow attachment. The following day, the cells were treated with various concentrations of thiocolchicoside, in triplicate, for 24 hours. The next day, the medium was changed, and the cells were incubated for 9 days to form colonies. Medium was replaced after 4 days. At the end of the ninth day, medium was removed, and 0.3 mL of clonogenic acid reagent was added. Cells were incubated for 30 minutes and washed twice, and blue colonies were counted (12).
Electrophoretic mobility shift assay
To assess NF-κB activation, we did electrophoretic mobility shift assay (EMSA) as described previously (13).
In brief, nuclear extracts prepared from TNF-treated cells (1.5 × 106/mL) were incubated with 32P end-labeled 45-mer double-stranded NF-κB oligonucleotide (15 μg of protein with 16 fmol of DNA) from the HIV long terminal repeat, 5′-TTGTTACAAGGGACTTTCCGCTGGGGAC-TTTCCAGGGAGGCGTGG-3′ (boldface indicates NF-κB–binding sites) for 30 minutes at 37°C, and the DNA-protein complex formed was separated from free oligonucleotide on 6.6%native polyacrylamide gels. The dried gels were visualized with a Storm 820 PhosphorImager, and radioactive bands were quantitated using ImageQuant software (GE Healthcare).
Western blot analysis
To determine the levels of protein expression in whole-cell extracts or in the cytoplasm or nucleus of treated cells (1.5 × 106 cells in 1 mL of medium), we prepared extracts, and 30 μg of proteins were fractionated by SDS-PAGE. After electrophoresis, the proteins were electrotransferred to nitrocellulose membranes, blotted with the relevant antibody, and detected with an electrogenerated chemiluminescence reagent (GE Healthcare).
IKK assay
To determine the effect of thiocolchicoside on TNF-induced IKK activation, IKK assay was done by a method we described previously (14). In brief, the IKK complex from whole-cell extracts was precipitated with antibody against IKKβ and then treated with protein A/G-agarose beads (Pierce). After 2 hours, the beads were washed with lysis buffer and then resuspended in a kinase assay mixture containing 50 mmol/L HEPES (pH 7.4), 20 mmol/L MgCl2, 2 mmol/L DTT, 20 μCi of [γ-32P]ATP, 10 μmol/L unlabeled ATP, and 2 μg of substrate glutathione transferase-IκBα (amino acids 1–54). After incubation at 30°C for 30 minutes, the reaction was terminated by boiling with SDS sample buffer for 7 minutes. Finally, the protein was resolved on 10% SDS-PAGE, the gel was dried, and the radioactive bands were visualized with a Storm820. To determine the total amounts of IKKα and IKKβ in each sample, 30 μg of whole-cell protein were resolved on 7.5% $SDS-PAGE, electrotransferred to a nitrocellulose membrane, and then blotted with either anti-IKKα or anti-IKKβ antibody.
NF-κB–dependent reporter gene expression assay
The effect of thiocolchicoside on NF-κB–dependent reporter gene transcription induced by TNF and various genes was analyzed by secretory alkaline phosphatase (SEAP) assay, with the following modification. In brief, A293 cells (5 × 105 per well) were plated in six-well plates and transiently transfected by the calcium phosphate method with pNF-κB-SEAP (0.5 μg). To examine TNF-induced reporter gene expression, we transfected the cells with 0.5 μg of the SEAP expression plasmid and 2 μg of the control plasmid pCMV-FLAG1 DNA for 24 hours.
We then treated the cells for 24 hours with thiocolchicoside and then stimulated them with 1 nmol/L TNF. The cell culture medium was harvested after 24 hours of TNF treatment. To examine reporter gene expression induced by various genes, we transfected A293 cells with 0.5 μg of pNF-κBSEAP plasmid with 1 μg of an expressing plasmid and 0.5 μg of the control plasmid pCMV-FLAG1 for 24 hours, treated them with 100 μmol/L thiocolchicoside, and then harvested them from culture medium after an additional 24 hours of incubation. Culture medium was analyzed for SEAP activity according to the protocol essentially as described by the manufacturer (Clontech) using a Victor 3 microplate reader (Perkin-Elmer Life and Analytical Sciences).
Immunocytochemical analysis of NF-κB p65 localization
The effect of thiocolchicoside on the nuclear translocation of p65 was examined by immunocytochemistry as described previously (10). In brief, treated cells were plated on a poly(L-lysine)–coated glass slide by centrifugation (Shandon Cytospin 4, ThermoFisher Scientific), air-dried, and fixed with 4% paraformaldehyde after permeabilization with 0.2% Triton X-100. After washing in PBS, the slides were blocked with 5% normal goat serum for 1 hour and then incubated with rabbit polyclonal anti-human p65 antibody at a 1:200 dilution. After overnight incubation at 4°C, the slides were washed, incubated with goat anti-rabbit IgG-Alexa Fluor 594 (Invitrogen) at a 1:200 dilution for 1 hour, and counterstained for nuclei with Hoechst 33342 (50 ng/mL) for 5 minutes. Stained slides were mounted with mounting medium purchased from Sigma-Aldrich and analyzed under a fluorescence microscope (Labophot-2). Pictures were captured using a Photometrics Coolsnap CF color camera (Nikon) and Meta-Morph version 4.6.5 software (Molecular Devices).
Luciferase assay
The effect of thiocolchicoside on cyclooxygenase-2 (COX-2) promoter activity induced by TNF was analyzed using a luciferase assay. A293 cells (2.5 × 105 per well) were seeded in six-well plates. After overnight culture, the cells in each well were transfected by the calcium phosphate method with 0.5 μg of DNA consisting of COX-2 promoter luciferase reporter. The COX-2 promoter (−375 ± 59) was provided by Dr. Xiao-Chun Xu (University of Texas M.D. Anderson Cancer Center). After 24 hours of transfection, the cells were incubated with thiocolchicoside for 24 hours, then exposed to 1 nmol/L TNF for 20 hours, and harvested. Luciferase activity was measured using the luciferase assay system (Promega) and detected using the Victor3 microplate reader (Perkin-Elmer Life and Analytical Sciences).
Statistical analysis
Results from at least three independent experiments were analyzed for statistical significant differences using the Student’s t test. They are expressed as the mean ± SD. P values below 0.05 were considered as statistically significant.
Results
The present studies were designed to investigate the effect of thiocolchicoside on the NF-κB cell signaling pathway and on NF-κB–mediated cellular responses. Human myeloid KBM5 cells were used for most studies, because TNF induces robust activation of NF-κB in these cells, and also these cells have extensively been used in our laboratory to study the mechanism of inhibition of NF-κB by various phytochemicals. In addition to this, other cell types were also used to determine the specificity of this effect.
Thiocolchicoside inhibits cell proliferation of various cancer cell lines
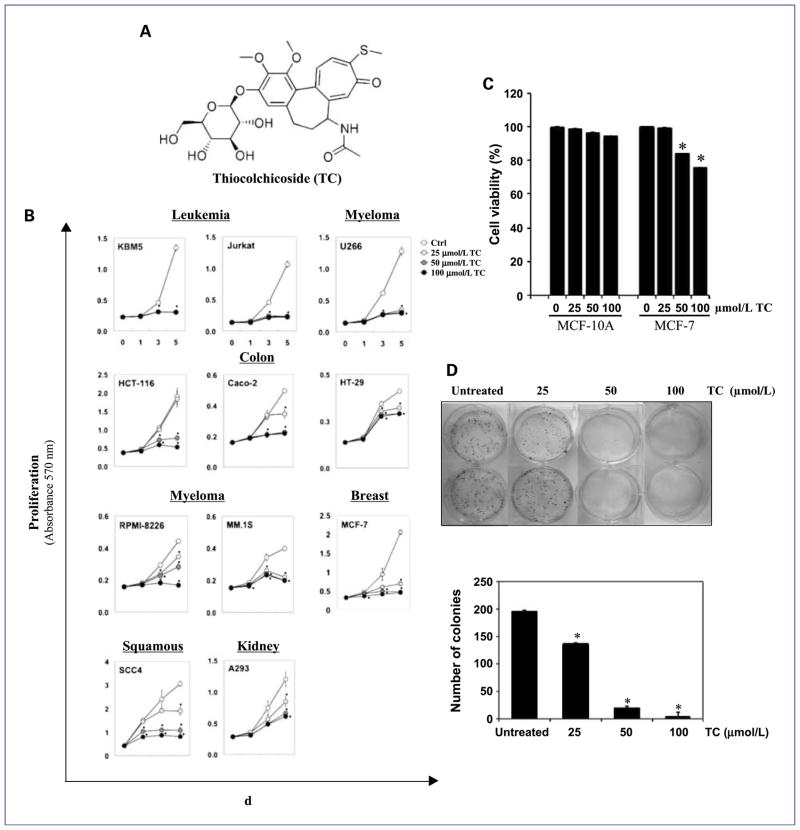
We investigated the effect of thiocolchicoside (Fig. 1A) on the proliferation of various cancer cells using the MTT method, which detects the mitochondrial activity of the cells. For this, we treated different cell lines (KBM5, Jurkat, U266, HCT-116, Caco-2, HT-29, RPMI-8226, MM.1S, MCF-7, SCC4, and A293) with 25, 50, and 100 μmol/L thiocolchicoside for 5 days (Fig. 1B). Results showed that thiocolchicoside inhibited the proliferation of all cancer cell lines investigated. Proliferation of KBM5, Jurkat, and U266 was completely inhibited after 3 and 5 days at all three concentrations tested. HCT-116 cells showed no effect at 25 μmol/L, but proliferation was strongly inhibited at 50 and 100 μmol/L. Caco-2 cells showed no effect at 25 μmol/L after 3 days, but proliferation was strongly inhibited at 5 days, as it was with 50 and 100 μmol/L of thiocolchicoside. RPMI-8226 and SCC4 cells showed concentration-dependent inhibition with 100 μmol/L having a complete inhibitory effect. HT-29 and A293 cells showed a less strong response to 100 μmol/L thiocolchicoside than the other cells. MM.1S and MCF-7 cells also showed an inhibitory effect at all concentrations tested.
Fig. 1.
Thiocolchicoside suppresses cell proliferation and colony formation of various cancer cell lines. A, the structure of thiocolchicoside. B, thiocolchicoside inhibits cell proliferation of various cancer cell lines. Leukemia (KBM5, Jurkat), colon (HCT-116, Caco-2, HT-29), myeloma (U266, RPMI-8266, MM.1S), breast (MCF-7), squamous (SCC4), and kidney (A293) cells were treated with 25, 50, and 100 μmol/L thiocolchicoside for 1, 3, and 5 d, and cell proliferation was assessed by the MTT method. C, thiocolchicoside has no effect on normal cells. MCF-10A (nontransformed breast epithelial cells) cells and MCF-7 (transformed breast epithelial cells) cells were treated with 25, 50, and 100 μmol/L thiocolchicoside for 24 h, and cell viability was assessed by the MTT method. D, thiocolchicoside inhibits colony formation of HCT-116 cells. HCT-116 cells were plated in six-well plates and treated with 25, 50, and 100 μmol/L of thiocolchicoside for 24 h. After 1 d, medium was changed, and cells were incubated for 9 d for colony formation (top). After 9 d, cells were stained with crystal violet, and number of colonies was counted (bottom). Data are presented as mean (±SD), and * indicates P < 0.05, when compared with control.
To investigate whether thiocolchicoside is selectively active against cancer cells and not against normal cells, we compared its effect on cell viability of MCF-10A (non-transformed breast epithelial cells) and MCF-7 (transformed breast epithelial cells) using the MTT method (Fig. 1C). Cells were treated with 25, 50, and 100 μmol/L thiocolchicoside for 24 hours, and cell viability was measured. Results show that thiocolchicoside reduced cell viability of MCF-7 cells in a dose-dependent manner. But, thiocolchicoside had no significant effect on cell viability of nontransformed MCF-10A cells. This result indicates that thiocolchicoside is only active against cancer cells and not against normal cells.
We also examined whether thiocolchicoside inhibited colony formation of HCT-116 cells in a long-term assay (Fig. 1D). We found that thiocolchicoside had minimal effect on colony formation of HCT-116 cells at 25 μmol/L but 50 and 100 μmol/L treatment completely suppressed the colony-forming ability of these tumor cells.
Thiocolchicoside inhibits antiapoptotic proteins, induces PARP and caspase-3 cleavage, and inhibits expression of proteins involved in cell proliferation
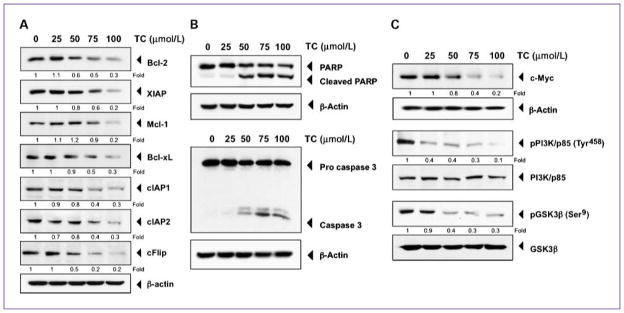
To determine the mechanism responsible for thiocolchicoside-induced inhibition of cancer cell proliferation, we analyzed the expression of various proteins linked with cell proliferation and apoptosis by Western blot. Thiocolchicoside dose-dependently inhibited the antiapoptotic proteins Bcl-2, XIAP, Mcl-1, Bcl-xL, cIAP-1, cIAP-2, and cFlip (Fig. 2A). By contrast, thiocolchicoside induced PARP and caspase-3 cleavage, confirming the proapoptotic effect of thiocolchicoside (Fig. 2B).
Fig. 2.
Thiocolchicoside induces apoptosis and inhibits proteins involved in cell proliferation. A, thiocolchicoside downregulates the expression of antiapoptotic proteins. KBM5 cells were treated with 25, 50, 75, and 100 μmol/L of thiocolchicoside for 24 h; whole-cell extracts were prepared and analyzed by Western blot using antibodies against Bcl-2, XIAP, Mcl-1, Bcl-xL, cIAP1, cIAP2, and cFlip. B, thiocolchicoside induces apoptosis. KBM5 cells were treated with 25, 50, 75, and 100 μmol/L of thiocolchicoside for 24 h. Whole-cell extracts were prepared and analyzed by Western blot using antibodies against PARP and caspase-3. C, thiocolchicoside downregulates the expression of proteins involved in cell proliferation. KBM5 cells were treated with 25, 50, 75, and 100 μmol/L of thiocolchicoside for 24 h. Whole-cell extracts were prepared and analyzed by Western blot using antibodies against c-Myc, the phosphorylated PI3K/p85, and phosphorylated GSK3β proteins. Unphosphorylated proteins of PI3K/p85 and GSK3β as well as β-actin were used as a loading control. Numbers below each panel indicate fold differences after normalization to β-actin.
Furthermore, thiocolchicoside inhibited c-myc as well as phosphorylation of the p85 subunit of phosphoinositide 3-kinase (PI3K) and GSK3β without having any effect on the nonphosphorylated form of these two proteins, which are involved in cell proliferation (Fig. 2C). Together, these results show that thiocolchicoside inhibits cancer cell growth by inducing apoptosis and inhibiting cell proliferation.
Thiocolchicoside inhibits TNF-induced NF-κB activation
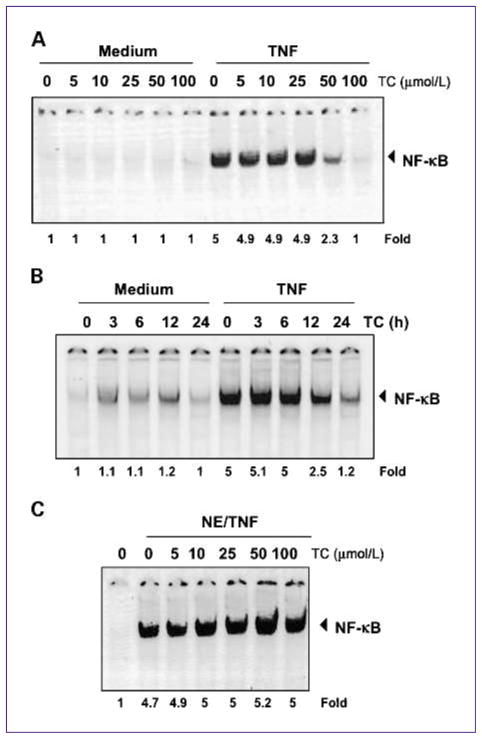
Because the transcription factor NF-κB is involved in cell proliferation and apoptosis, we investigated the effect of thiocolchicoside on NF-κB activation. TNF is the main NF-κB activator, so we used this cytokine to stimulate NF-κB, which is retained in the cytoplasm when inactive. EMSA showed that thiocolchicoside suppressed TNF-induced NF-κB activation in a dose-dependent (Fig. 3A) and time-dependent (Fig. 3B) manner. Thiocolchicoside alone did not activate NF-κB.
Fig. 3.
Thiocolchicoside inhibits TNF-dependent NF-κB activation. A, thiocolchicoside inhibits TNF-dependent NF-κB activation in a dose-dependent manner. KBM5 cells were preincubated with indicated concentrations of thiocolchicoside for 24 h, treated with 0.1 nmol/L TNF for 30 min, and then subjected to EMSA to test for NF-κB activation. B, thiocolchicoside inhibits TNF-dependent NF-κB activation in a time-dependent manner. KBM5 cells were preincubated with 100 μmol/L thiocolchicoside for the indicated times, treated with 0.1 nmol/L TNF for 30 min, and then subjected to EMSA to test for NF-κB activation. C, the direct effect of thiocolchicoside on NF-κB complex was investigated. Nuclear extracts were prepared from untreated cells or cells treated with 0.1 nmol/L TNF and incubated for 30 min with the indicated concentrations of thiocolchicoside. They were then assayed for NF-κB activation by EMSA. Numbers below panels indicate fold differences normalized to the control.
We next sought to determine whether thiocolchicoside directly modified the binding of NF-κB complex to the DNA. EMSA showed that thiocolchicoside did not modify the DNA-binding ability of the NF-κB complex (Fig. 3C). Therefore, we concluded that thiocolchicoside inhibits NF-κB activation indirectly rather than directly.
Suppression of NF-κB activation by thiocolchicoside was not unique to TNF
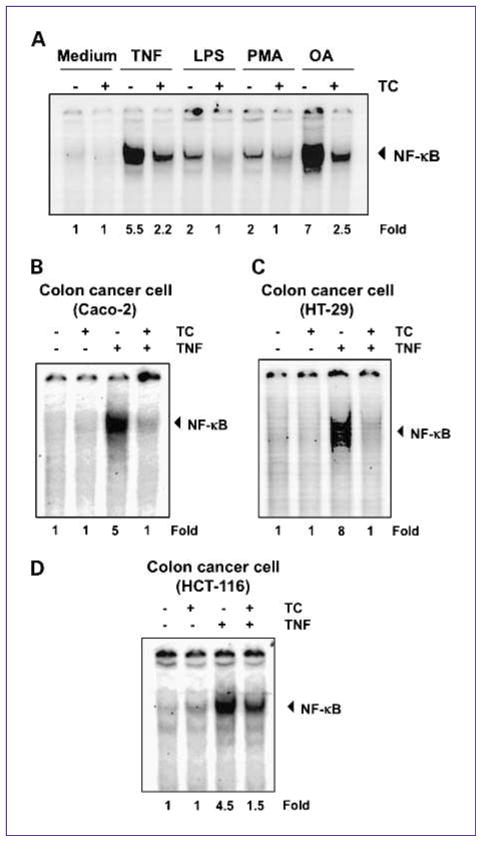
A wide variety of carcinogens, tumor promoters, and inflammatory agents has been shown to activate NF-κB, including LPS, PMA, and OA, through mechanisms that may differ. We investigated whether thiocolchicoside abrogates NF-κB activation by all these agents. EMSA showed that all of these agents activated NF-κB and that thiocolchicoside suppressed activation (Fig. 4A).
Fig. 4.
Thiocolchicoside-induced NF-κB inhibition is neither inducer nor cell type specific. A, thiocolchicoside suppresses NF-κB activation by different stimuli. KBM5 cells were preincubated with 100 μmol/L thiocolchicoside for 24 h and then treated with 0.1 nmol/L TNF or 10 μg/mL LPS for 30 min, 500 nmol/L OA for 4 h or 25 μg/mL PMA for 2 h. The cells were then analyzed for NF-κB activation by EMSA. B–D, thiocolchicoside induced NF-κB inhibition is not cell type specific. Caco-2, HT-29, and HCT-116 cells were incubated with 100 μmol/L thiocolchicoside for 24 h and then incubated with 0.1 nmol/L TNF for 30 min. Nuclear extracts were then prepared and assayed for NF-κB activation by EMSA. Numbers below panels indicate fold differences normalized to the control.
Inhibition of NF-κB activation by thiocolchicoside was not cell type specific
Because distinct signal transduction pathways can mediate NF-κB induction in different cell types (15), we examined the effect of thiocolchicoside on TNF-induced NF-κB activation in three different human colon cancer cells: Caco-2, HT-29, and HCT-116 cells. EMSA showed that thiocolchicoside inhibited TNF-activated NF-κB in all three cell types (Fig. 4B–D).
Thiocolchicoside inhibited TNF-dependent IκBα phosphorylation, ubiquitination, and degradation
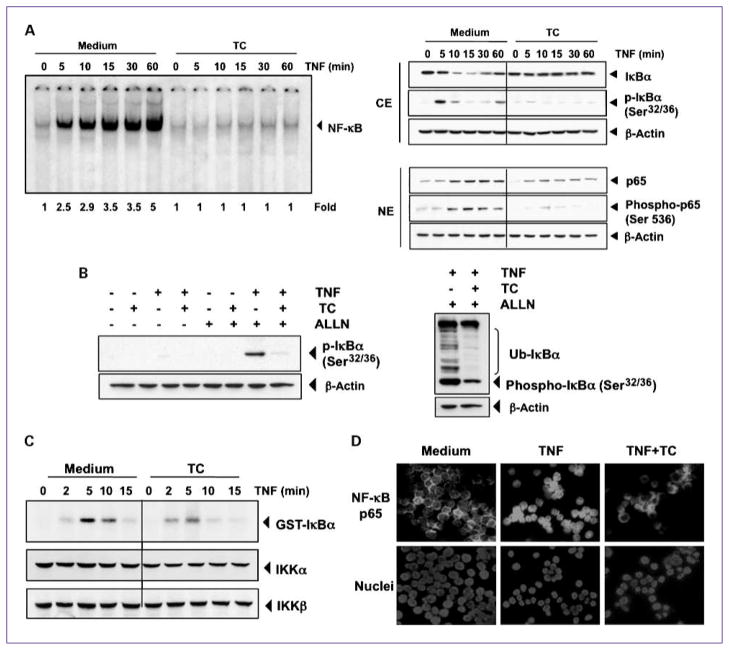
The translocation of NF-κB to the nucleus is preceded by the proteolytic degradation of IκBα (16), so we next sought to determine whether thiocolchicoside-induced NF-κB inhibitory activity was due to inhibition of IκBα degradation. EMSA showed that NF-κB was activated with increasing TNF incubation times and that thiocolchicoside pretreatment strongly decreased this activation (Fig. 5A, left). Western blot analysis showed that TNF induced IκBα degradation in control cells after 10 minutes, and thiocolchicoside inhibited this degradation (Fig. 5A, right).
Fig. 5.
Thiocolchicoside inhibits TNF-dependent IκBα phosphorylation, IκBα degradation, p65 phosphorylation, and p65 nuclear translocation. A, thiocolchicoside inhibits TNF-induced activation of NF-κB. KBM-5 cells were incubated with 100 μmol/L thiocolchicoside for 24 h, treated with 0.1 nmol/L TNF for the indicated times, and then analyzed for NF-κB activation by EMSA (left). A, right, effect of thiocolchicoside on TNF-induced IκBα degradation, p65 phosphorylation, and p65 nuclear translocation. Cells were incubated with 100 μmol/L thiocolchicoside for 24 h and treated with 0.1 nmol/L TNF for the indicated times. Cytoplasmic extracts (CE) and nuclear extracts (NE) were prepared, fractionated on SDS-PAGE, and electrotransferred to nitrocellulose membrane. Western blot analysis was done using the indicated antibody. An anti-β-actin antibody was the loading control. B, effect of thiocolchicoside on the phosphorylation of IκBα by TNF. Cells were preincubated with 100 μmol/L thiocolchicoside for 24 h, incubated with 50 μg/mL ALLN for 30 min, and then treated with 0.1 nmol/L TNF for 10 min. Cytoplasmic extracts were fractionated and then subjected to Western blot analysis using a phospho-specific anti-IκBα antibody. An anti-β-actin antibody was used as loading control (left). B, right, thiocolchicoside inhibits ubiquitination of IκBα. Cells were preincubated with 100 μmol/L thiocolchicoside for 24 h, incubated with 50 μg/mL ALLN for 30 min, and then treated with 0.1 nmol/L TNF for 10 min. Cytoplasmic extracts were fractionated and then subjected to Western blot analysis using a phospho-specific anti-IκBα antibody. An anti-β-actin antibody used as the loading control. C, direct effect of thiocolchicoside on IKK activation induced by TNF. Whole-cell extracts were immunoprecipitated with antibody against IKKβ and analyzed by an immune complex kinase assay. To examine the effect of thiocolchicoside on the level of expression of IKK proteins, whole-cell extracts were fractionated on SDS-PAGE and examined by Western blot analysis using anti-IKKα and anti-IKKβ antibodies. D, immunocytochemical analysis of p65 localization. Cells were incubated with 100 μmol/L thiocolchicoside for 24 h and then treated with 1 nm TNF for 15 min. Cells were subjected to immunocytochemical analysis.
Thiocolchicoside also inhibited IκBα phosphorylation, which occurred in control cells after 5 minutes and disappeared simultaneously with IκBα degradation (Fig. 5A, right). These results indicate that thiocolchicoside inhibited both TNF-induced NF-κB activation and IκBα degradation, as well as phosphorylation.
To further confirm that inhibition of TNF-induced IκBα degradation was due to inhibition of IκBα phosphorylation and ubiquitination, we used the proteasome inhibitor ALLN to block the degradation of IκBα (17). Western blot analysis using an antibody that recognizes the serine-phosphorylated form of IκBα showed that TNF induced IκBα phosphorylation and that thiocolchicoside suppressed both this phosphorylation (Fig. 5B, left) and IκBα ubiquitination (Fig. 5B, right).
Thiocolchicoside inhibited TNF-induced activation of IKK
Because IKK is required for TNF-induced phosphorylation of IκBα and because thiocolchicoside inhibited the phosphorylation of IκBα, we determined the effect of thiocolchicoside on TNF-induced IKK activation. Results of the immune complex kinase assay showed that TNF induced the activation of IKK in a time-dependent manner and that thiocolchicoside down-modulated the TNF activation of IKK (Fig. 5C). Neither TNF nor thiocolchicoside affected the expression of IKKα or IKKβ proteins (Fig. 5C).
Thiocolchicoside inhibited nuclear translocation of p65
p65 is a subunit of NF-κB that has nuclear localization signals, and p65 is retained in the cytoplasm by IκBα. We next examined whether the degradation of IκBα leads to nuclear translocation of p65. We found that TNF induced the nuclear translocation of p65 in as little as 10 minutes after incubation (Fig. 5A, right) and that thiocolchicoside suppressed p65 translocation. Phosphorylation of p65 at Ser536 was also suppressed by thiocolchicoside (Fig. 5A, right). Using an immunocytochemical assay, we further confirmed that thiocolchicoside suppressed the translocation of p65 from the cytoplasm to the nucleus (Fig. 5D).
Thiocolchicoside repressed TNF-induced NF-κB– dependent reporter gene expression
Although EMSA showed that thiocolchicoside blocked NF-κB activation, DNA binding alone does not always correlate with NF-κB–dependent gene transcription, suggesting that there are additional regulatory steps. Therefore, we investigated by a NF-κB–dependent reporter gene expression assay whether thiocolchicoside could suppress the TNF-induced NF-κB reporter activity. TNF induced NF-κB–regulated SEAP reporter gene expression, and thiocolchicoside suppressed the expression in a dose-dependent manner (Fig. 6A).
Fig. 6.
Thiocolchicoside represses NF-κB–dependent reporter gene expression induced by TNF and by overexpression of various signaling intermediates. A, thiocolchicoside inhibits the NF-κB–dependent reporter gene expression induced by TNF. A293 cells were transiently transfected with a NF-κB–containing plasmid for 24 h. After transfection, the cells were incubated with the indicated concentrations of thiocolchicoside for 24 h and then treated with 1 nmol/L TNF for an additional 24 h. The supernatants of the culture media were assayed for SEAP activity. Data are presented as mean (±SD). B, thiocolchicoside inhibits the NF-κB–dependent reporter gene expression induced by TNF, TNFR1, TRADD, TRAF2, NIK, IKK, and p65. Cells were transiently transfected with a NF-κB–containing plasmid alone or with the indicated plasmids. After transfection, cells were incubated with 100 μmol/L thiocolchicoside for 24 h and then incubated with the relevant plasmid for an additional 24 h. TNF-treated cells were incubated with 100 μmol/L thiocolchicoside for 24 h and then treated with 1 nmol/L TNF for an additional 24 h. The supernatants of the culture media were assayed for SEAP activity. Data are presented as mean (±SD). C, thiocolchicoside inhibits the COX-2 promoter activity induced by TNF. Cells were transiently transfected with a COX-2 promoter linked to the luciferase reporter gene plasmid for 24 h and treated with the indicated concentrations of thiocolchicoside for 24 h. Cells were then treated with 1 nmol/L TNF for an additional 24 h, lysed, and subjected to a luciferase assay. Data are presented as mean (±SD), and * indicates P < 0.05 when compared with their respective control.
Thiocolchicoside repressed NF-κB–dependent reporter gene expression induced by TNF receptor-1, TNFR1-associated death domain, TNFR-associated factor 2, NF-κB–inducing kinase, IKKβ, and p65
TNF has been shown to activate NF-κB activation through sequential interaction with the TNF receptor 1 (TNFR1), TNFR1-associated death domain (TRADD), TNFR-associated factor 2 (TRAF2), NF-κB–inducing kinase (NIK), and IKKβ, resulting in phosphorylation of IκBα (18, 19). To determine the effect of thiocolchicoside on NF-κB–dependent reporter gene expression, cells were transiently transfected with TNFR1-, TRADD-, TRAF2-, NIK-, IKKβ-, and p65-expressing plasmids and then monitored for NF-κB–dependent SEAP expression. We found that cells transfected with any of these plasmids expressed the NF-κB–regulated reporter gene and that the reporter expression induced by each of them was suppressed by thiocolchicoside (Fig. 6B).
Thiocolchicoside inhibited TNF-induced COX-2 promoter activity
TNF induces COX-2, which has NF-κB binding sites in its promoter (20). Because we found that downregulation of NF-κB by thiocolchicoside suppressed the expression of NF-κB–regulated gene products, we also examined the effect of thiocolchicoside on TNF-induced COX-2 promoter activity using a COX-2 promoter-luciferase reporter plasmid. We found that TNF induced COX-2 promoter activity and that thiocolchicoside suppressed this activity in a dose-dependent manner (Fig. 6C). This result suggests that thiocolchicoside inhibits NF-κB–regulated gene expression by suppressing NF-κB binding to the COX-2 promoter.
Discussion
In our investigation of the effect of thiocolchicoside, a commonly used muscle relaxant drug, on cell proliferation of various cancer cells and on the NF-κB signaling pathway, we found that thiocolchicoside inhibited cell proliferation of various cancer cell lines and not of normal cells, inhibited the colony-forming ability of tumor cells, and induced apoptosis by inhibiting antiapoptotic proteins and by inducing cleavage of caspase-3 and PARP. In addition, thiocolchicoside suppressed NF-κB activation stimulated by carcinogens and inflammatory stimuli in a dose- and time-dependent manner. Thiocolchicoside-induced NF-κB inhibition was not cell type specific, and the mechanism involved was found to be through inhibition of IKK activation, IκBα phosphorylation, ubiquitination, and degradation, as well as through p65 phosphorylation and translocation. Finally, we found that thiocolchicoside inhibited NF-κB–dependent reporter assay and suppressed NF-κB binding to the COX-2 promoter.
To our knowledge, this is the first report on the effect of thiocolchicoside on cancer cell proliferation as well as on NF-κB activation. Our results show that thiocolchicoside inhibits cell proliferation of a wide variety of cancer cells, but not of normal cells, including leukemia, colon, myeloma, breast, squamous, and kidney cancer cells, indicating that the inhibitory effect of thiocolchicoside on cancer cells is not cell type specific. Moreover, we found that thiocolchicoside inhibits the colony-forming ability of colon cancer cells.
We also found that thiocolchicoside inhibits NF-κB activation. NF-κB is a sequence-specific transcription factor that is involved in the inflammatory and innate immune responses and controls >400 genes (21). The molecular identification of its p50 subunit as a member of the reticuloendotheliosis (REL) family provided the first evidence that NF-κB is linked with cancer, because v-REL is an oncoprotein of the REL retrovirus (REV-T; ref. 22). Since then, numerous points of evidence have confirmed the involvement of activated NF-κB in multiple types of cancer (21). Moreover, NF-κB is one of the major figures in the inflammatory network, and this inflammation is now regarded as a “secret killer” in diseases such as atherosclerosis, rheumatoid arthritis, multiple sclerosis, asthma, Alzheimer disease, depression, fatigue, neuropathic pain, lack of appetite, and cancer (23).
Because thiocolchicoside has been shown to have anti-inflammatory properties, we postulated that thiocolchicoside inhibits the activation of the NF-κB transcription factor. Our results show that, indeed, thiocolchicoside suppressed NF-κB activation in different cancer cells, indicating that the suppression is not cell type specific. Moreover, thiocolchicoside inhibited NF-κB activation induced by a variety of stimuli, suggesting that thiocolchicoside must act at a step common to all these activators. In addition, our results showed that thiocolchicoside blocked NF-κB activation without directly interfering with the DNA binding of NF-κB. NF-κB activation in response to different stimuli requires IKK activation, which phosphorylates IκBα at Ser32 and Ser36, leading to degradation of IκBα (16). We found that this inhibition was mediated through the inhibition of IKK by thiocolchicoside, which led to the suppression of phosphorylation and ubiquitination of IκBα and then to its degradation. Thiocolchicoside also inhibited p65 phosphorylation and translocation.
To investigate whether thiocolchicoside also modulates NF-κB–regulated genes, we studied the effect of thiocolchicoside on several proteins regulated by NF-κB, including Bcl-2, XIAP, Mcl-1, Bcl-xL, cIAP1, cIAP2, and cFlip. All of these proteins are involved in apoptosis, and their downregulation by thiocolchicoside may explain the thiocolchicoside-induced apoptosis as observed by PARP and caspase-3 cleavage. We also found that the expression of gene products involved in proliferation (c-Myc, phosphorylated PI3K/p85, and phosphorylated GSK3β) were strongly downregulated by thiocolchicoside, which may explain the inhibited proliferation of various cancer cells by this drug.
Finally, we investigated the effect of thiocolchicoside on an NF-κB–dependent reporter assay. Thiocolchicoside inhibited the NF-κB–dependent reporter assay induced by TNF in a dose-dependent manner. Furthermore, overexpression of proteins in the NF-κB signaling pathway (TNFR1, TRADD, TRAF2, NIK, IKKβ, p65, and IκBα) stimulated NF-κB promoter activity, whereas the inhibitor of NF-κB, IκBα, had no effect. The canonical NF-κB signaling pathway is triggered in response to microbial and viral infections as well as by proinflammatory cytokines, such as TNF (16). TRADD is an adaptor protein that interacts with TNFR1 and with another adaptor protein, TRAF2, to activate NF-κB signaling (18, 24). The IKK complex, which is composed of the two catalytic subunits (IKKα and IKKβ) and the scaffolding protein IKKγ/NEMO (25–27), is recruited to the TNFR1 adaptor proteins, where it is activated and subsequently phosphorylates the specific inhibitors of NF-κB, the IκB proteins. Moreover, thiocolchicoside dose-dependently inhibits the binding of NF-κB proteins to the COX-2 promoter, which contains NF-κB binding sites in its sequence (20).
Until now, thiocolchicoside has been described as a muscle relaxant with anti-inflammatory properties (1), but our results show that thiocolchicoside is also effective against cancer cells and thus may have potential as an anticancer agent, which gives a new use to an existing drug. Thiocolchicoside (Muscoril) has been used for >35 years as a muscle relaxant, anti-inflammatory, and analgesic drug (1), and clinical trials (2–4, 28) proved its efficacy and safety. Peak plasma concentration of thiocolchicoside in healthy humans varied from 0.18 to 0.64 μmol/L, depending on the mode and route of administration (29, 30). These concentrations are much lower than the concentration used to study NF-κB inhibitory effects of thiocolchicoside in vitro. The mechanism of thiocolchicoside on NF-κB inhibition in vivo remains to be worked out. Constitutive activation of NF-κB has been associated with a wide variety of tumors and has also been linked with chemotherapy- and radiotherapy-induced resistance in many cancers (31). Therefore, inhibition of NF-κB has been a widely sought target for cancer prevention. In in vitro and in vivo animal models, inhibition of constitutive and inducible NF-κB has been shown to enhance therapeutic potentials of many drugs (21).
The results of our study show that thiocolchicoside is a potent inhibitor of NF-κB activation, which may explain its antiproliferative, proapoptotic, and anti-inflammatory effects. Further studies are needed to explore its in vivo potential against cancer and other diseases.
Acknowledgments
We thank Virginia M. Mohlere for carefully editing this article.
Grant Support
NIH M.D. Anderson’s Cancer Center support grant NIH CA-16 672, NIH program project grant NIH CA-124787-01A2, and Center for Targeted Therapy at University of Texas M.D. Anderson Cancer Center, where Dr. Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research. Simone Reuter was supported by Fonds National de la Recherche Luxembourg grant PDR-08-017.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Janbroers JM. Review of the toxicology, pharmacodynamics and pharmacokinetics of thiocolchicoside, a GABA-agonist muscle relaxant with anti-inflammatory and analgesic actions. Acta Therapeutica. 1987;13:221–35. [Google Scholar]
- 2.Tuzun F, Unalan H, Oner N, et al. Multicenter, randomized, double-blinded, placebo-controlled trial of thiocolchicoside in acute low back pain. Joint Bone Spine. 2003;70:356–61. doi: 10.1016/s1297-319x(03)00075-7. [DOI] [PubMed] [Google Scholar]
- 3.Ketenci A, Ozcan E, Karamursel S. Assessment of efficacy and psychomotor performances of thiocolchicoside and tizanidine in patients with acute low back pain. Int J Clin Pract. 2005;59:764–70. doi: 10.1111/j.1742-1241.2004.00454.x. [DOI] [PubMed] [Google Scholar]
- 4.Soonawalla DF, Joshi N. Efficacy of thiocolchicoside in Indian patients suffering from low back pain associated with muscle spasm. J Indian Med Assoc. 2008;106:331–5. [PubMed] [Google Scholar]
- 5.Balduini W, Cimino M, Depoortere H, Cattabeni F. Characterization of [3H]thiocolchicoside binding sites in rat spinal cord and cerebral cortex. Eur J Pharmacol. 1999;376:149–57. doi: 10.1016/s0014-2999(99)00371-4. [DOI] [PubMed] [Google Scholar]
- 6.Balduini W, De Angelis V, Mazzoni E, Depoortere H, Cattabeni F, Cimino M. Autoradiographic localization of [3H]thiocolchicoside binding sites in the rat brain and spinal cord. Neuropharmacology. 2001;40:1044–9. doi: 10.1016/s0028-3908(01)00023-5. [DOI] [PubMed] [Google Scholar]
- 7.Cimino M, Marini P, Cattabeni F. Interaction of thiocolchicoside with [3H]strychnine binding sites in rat spinal cord and brainstem. Eur J Pharmacol. 1996;318:201–4. doi: 10.1016/s0014-2999(96)00884-9. [DOI] [PubMed] [Google Scholar]
- 8.Biziere K, Huguet F, Narcisse G, Breteau M. Affinity of thiocolchicoside and thiocolchicoside analogues for the postsynaptic GABA receptor site. Eur J Pharmacol. 1981;75:167–8. doi: 10.1016/0014-2999(81)90080-7. [DOI] [PubMed] [Google Scholar]
- 9.Ahn KS, Aggarwal BB. Transcription factor NF-κB: a sensor for smoke and stress signals. Ann N Y Acad Sci. 2005;1056:218–33. doi: 10.1196/annals.1352.026. [DOI] [PubMed] [Google Scholar]
- 10.Pandey MK, Sandur SK, Sung B, Sethi G, Kunnumakkara AB, Aggarwal BB. Butein, a tetrahydroxychalcone, inhibits nuclear factor (NF)-κB and NF-κB-regulated gene expression through direct inhibition of IκBα kinase β on cysteine 179 residue. J Biol Chem. 2007;282:17340–50. doi: 10.1074/jbc.M700890200. [DOI] [PubMed] [Google Scholar]
- 11.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–9. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 12.Takada Y, Sethi G, Sung B, Aggarwal BB. Flavopiridol suppresses tumor necrosis factor-induced activation of activator protein-1, c-Jun N-terminal kinase, p38 mitogen-activated protein kinase (MAPK), p44/p42 MAPK, Akt, inhibits expression of antiapoptotic gene products, and enhances apoptosis through cytochrome c release and caspase activation in human myeloid cells. Mol Pharmacol. 2008;73:1549–57. doi: 10.1124/mol.107.041350. [DOI] [PubMed] [Google Scholar]
- 13.Chaturvedi MM, Mukhopadhyay A, Aggarwal BB. Assay for redox-sensitive transcription factor. Methods Enzymol. 2000;319:585–602. doi: 10.1016/s0076-6879(00)19055-x. [DOI] [PubMed] [Google Scholar]
- 14.Sung B, Pandey MK, Aggarwal BB. Fisetin, an inhibitor of cyclin-dependent kinase 6, down-regulates nuclear factor-κB-regulated cell proliferation, antiapoptotic and metastatic gene products through the suppression of TAK-1 and receptor-interacting protein-regulated IκBα kinase activation. Mol Pharmacol. 2007;71:1703–14. doi: 10.1124/mol.107.034512. [DOI] [PubMed] [Google Scholar]
- 15.Bonizzi G, Piette J, Merville MP, Bours V. Distinct signal transduction pathways mediate nuclear factor-κB induction by IL-1β in epithelial and lymphoid cells. J Immunol. 1997;159:5264–72. [PubMed] [Google Scholar]
- 16.Ghosh S, Karin M. Missing pieces in the NF-κB puzzle. Cell. 2002;109 (Suppl):S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 17.Vinitsky A, Michaud C, Powers JC, Orlowski M. Inhibition of the chymotrypsin-like activity of the pituitary multicatalytic proteinase complex. Biochemistry. 1992;31:9421–8. doi: 10.1021/bi00154a014. [DOI] [PubMed] [Google Scholar]
- 18.Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 19.Simeonidis S, Stauber D, Chen G, Hendrickson WA, Thanos D. Mechanisms by which IκB proteins control NF-κB activity. Proc Natl Acad Sci U S A. 1999;96:49–54. doi: 10.1073/pnas.96.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamamoto K, Arakawa T, Ueda N, Yamamoto S. Transcriptional roles of nuclear factor κ B and nuclear factor-interleukin-6 in the tumor necrosis factor α-dependent induction of cyclooxygenase-2 in MC3T3-1 cells. J Biol Chem. 1995;270:31315–20. doi: 10.1074/jbc.270.52.31315. [DOI] [PubMed] [Google Scholar]
- 21.Prasad S, Ravindran J, Aggarwal BB. NF-κB and cancer: how intimate is this relationship? Mol Cell Biochem. 2010;336:25–37. doi: 10.1007/s11010-009-0267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilmore TD, Starczynowski DT, Kalaitzidis D. RELevant gene amplification in B-cell lymphomas? Blood. 2004;103:3243–4. doi: 10.1182/blood-2003-11-4019. author reply 4–5. [DOI] [PubMed] [Google Scholar]
- 23.Heidland A, Klassen A, Rutkowski P, Bahner U. The contribution of Rudolf Virchow to the concept of inflammation: what is still of importance? J Nephrol. 2006;19 (Suppl 10):S102–9. [PubMed] [Google Scholar]
- 24.Hsu H, Xiong J, Goeddel DV. The TNF receptor 1-associated protein TRADD signals cell death and NF-κ B activation. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 25.Roff M, Thompson J, Rodriguez MS, et al. Role of IκBα ubiquitination in signal-induced activation of NFκB in vivo. J Biol Chem. 1996;271:7844–50. doi: 10.1074/jbc.271.13.7844. [DOI] [PubMed] [Google Scholar]
- 26.Sakurai H, Suzuki S, Kawasaki N, et al. Tumor necrosis factor-α-induced IKK phosphorylation of NF-κB p65 on serine 536 is mediated through the TRAF2, TRAF5, and TAK1 signaling pathway. J Biol Chem. 2003;278:36916–23. doi: 10.1074/jbc.M301598200. [DOI] [PubMed] [Google Scholar]
- 27.Yamaoka S, Courtois G, Bessia C, et al. Complementation cloning of NEMO, a component of the IκB kinase complex essential for NF-κB activation. Cell. 1998;93:1231–40. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]
- 28.Ketenci A, Basat H, Esmaeilzadeh S. The efficacy of topical thiocolchicoside (Muscoril) in the treatment of acute cervical myofascial pain syndrome: a single-blind, randomized, prospective, phase IV clinical study. Agri. 2009;21:95–103. [PubMed] [Google Scholar]
- 29.Sandouk P, Bouvier d’Yvoire M, Chretien P, Tillement JP, Scherrmann JM. Single-dose bioavailability of oral and intramuscular thiocolchicoside in healthy volunteers. Biopharm Drug Dispos. 1994;15:87–92. doi: 10.1002/bdd.2510150108. [DOI] [PubMed] [Google Scholar]
- 30.Trellu M, Filali-Ansary A, Francon D, et al. New metabolic and pharmacokinetic characteristics of thiocolchicoside and its active metabolite in healthy humans. Fundam Clin Pharmacol. 2004;18:493–501. doi: 10.1111/j.1472-8206.2004.00277.x. [DOI] [PubMed] [Google Scholar]
- 31.Aggarwal BB, Gehlot P. Inflammation and cancer: how friendly is the relationship for cancer patients? Curr Opin Pharmacol. 2009;9:351–69. doi: 10.1016/j.coph.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]