Abstract
Zinc deficiency impairs cellular immunity. Up-regulation of mRNA levels of IFN-γ, IL-12Rβ2, and T-bet are essential for Th1 differentiation. We hypothesized that zinc increases Th1 differentiation via up-regulation of IFN-γ and T-bet expression. To test this hypothesis, we used zinc-deficient and zinc-sufficient HUT-78 cells (a Th0 cell line) under different condition of stimulation in this study. We also used TPEN, a zinc-specific chelator, to decrease the bioavailability of zinc in the cells. We measured intracellular free zinc, cytokines, and the mRNAs of T-bet, IFN-γ, and IL-12Rβ2. In this study, we show that in zinc-sufficient HUT-78 cells (a Th0 cell line), mRNA levels of IFN-γ, IL-12Rβ2, and T-bet in PMA/PHA-stimulated cells were increased in comparison to zinc-deficient cells. Although intracellular free zinc was increased slightly in PMA/PHA-stimulated cells, Con-A-stimulated cells in 5 μM zinc medium showed a greater sustained increase in intracellular free zinc in comparison to cells incubated in 1 μM zinc. The cells pre-incubated with TPEN showed decreased mRNA levels of IFN-γ and T-bet mRNAs in comparison to cells without TPEN incubation. We conclude that stimulation of cells by Con-A via TCR, release intracellular free zinc which functions as a signal molecule for generation of IFN-γ and T-bet, and IL-12Rbβ2 mRNAs required for Th1 cell differentiation. These results suggest that zinc increase Th1 cell differentiation by up-regulation of IFN-γ and T-bet, and IL-12Rbβ2 mRNAs.
Keywords: zinc, T-bet, IFN-γ, IL-12Rβ2
1. INTRODUCTION
Zinc is an essential trace element and plays an important role in immune functions in both humans and experimental animals [1, 2]. Abnormalities of cellular immunity such as decreased thymulin activity, reduced ratio of CD4+/CD8+ T cells, and decreased production of IL-2 [1, 2], and decreased natural killer cell (NK) activity have been observed in zinc deficient humans and these abnormalities are corrected by zinc supplementation [1]. Our studies have shown that serum thymulin activity is decreased when a mild but specific deficiency of zinc is induced in human volunteers [1, 2]. Thus, it is evident that zinc is essential for T cell function in humans and that impaired cell-mediated immunity is an important consequence of mild zinc deficiency [3]. We previously reported that zinc increased IL-2 and IFN-γ mRNAs and its cytokine production in HUT-78 (Th0 human malignant lymphoblastic cell line) [4], and zinc supplementation increased the ex vivo generation of IL-2 cytokines in PMNC isolated from elderly subjects [5]. However, the mechanism of zinc action on Th1 differentiation is not clear. In this study, we investigated the effect of zinc on gene expression of IFN-γ, IL-12Rβ2, and T-bet, a transcription factor required for Th1 differentiation. We also examined the intracellular free zinc concentration under zinc-deficient and zinc-sufficient conditions in unstimulated and stimulated HUT-78 cells.
2. MATERIALS AND METHODS
2.1. Cell culture and media
Zinc-deficient and zinc-sufficient media was prepared as previously described [4, 6]. Characterization of low and high zinc media and maintenance of viable cells in these media have been previously described [4]. For cell stimulation, we used three different techniques, to determine which mechanism of stimulation resulted in release of free zinc (a signal molecule). PHA (10 μg/mL) activates PKC-α through TCR/CD2 receptor. PMA (5 ng/mL) activates PKC-α directly and Con-A (25 μg/mL) activates PKC-θ via TCR. Ionomycin (1 μg/mL) activates PKC-α.
2.2. ELISA assay of cytokines and real time RT-PCR
HUT-78 cells were incubated with either zinc-deficient or zinc-sufficient medium for 4d, and then stimulated for 6h with PMA/PHA or PMA/ionomycin. Media were collected for cytokine assay by ELISA kits (R&D Systems). Cells were harvested for isolation of total RNAs. Real time RT-PCR was conducted using CybrGreen reagent (AB Systems) [6].
2.3. TPEN study
To examine the specificity of zinc on T-bet and IFN-γ gene expression, we incubated HUT-78 cells with N,N′,N′,N′-tetrakis-(2-pyridylmethyl)-ethylenediamine (TPEN), a zinc specific chelator, or pyrithione, a zinc ionophore for 30min, and then the cells were stimulated with Con-A for 24h. The cells were then harvested for real time RT-PCR assay.
2.4. Intracellular free zinc assay
Determination of intracellular free zinc was performed using a modified method previously described using the fluorescent zinc probe, Zinpyr-1 (Neurobiotex, Galveston TX) [7]. Free zinc was measured using the Zinc Tool (Neurobiotek). Free zinc was determined using the Tsien equation using cells treated with 10 μM TPEN to determine fmin and in the presence of additional 75 μM zinc in the presence of pyrithione (20 μM) to determine (fmax).
2.5. Statistical analysis
Data were expressed as the mean and standard deviation from three separate experiments. The differences between zinc-deficient and zinc-sufficient groups were determined using the Student’s t-test.
3. RESULTS
The results show that PMA/ionomycin stimulation induced HUT-78 cells to produce more IL-2 and IFN-γ cytokines than PMA/PHA stimulation (Table 1). Under both stimulated conditions, IL-2 and IFN-γ cytokines were increased in zinc-sufficient HUT-78 cells relative to that in zinc-deficient cells (Table 1), which is consistent with our previous report [4]. The effect of different concentrations of zinc, iron, and copper on IL-2 and IFN-γ cytokines in HUT-78 cells after PMA/ionomycin stimulation is shown in Table 2. The results indicate that only zinc deficiency inhibited the generation of IL-2 and IFN-γ cytokines after stimulation, compared to the cells incubated in 15 μM or 50 μM zinc. Different concentration of Fe and Cu in the media did not affect the production of IL-2 and IFN-γ cytokines in HUT-78 cells after stimulation (Table 2).
Table 1.
Effect of zinc on cytokine production in HUT-78 Cells1
| Cytokine | No stimulation | PMA/PHA | PMA/Ionomycin | |||
|---|---|---|---|---|---|---|
| Zn− | Zn+ | Zn− | Zn+ | Zn− | Zn+ | |
| IL-2 | 14.3±4.52 | 12.0±5.2 | 145.1±18.5 | 338.9±18.63 | 446.5±147.7 | 1199.5±35.33 |
| IFN-γ | 7.7±4.2 | 10.1±0.8 | 5.3±4.2 | 20.3±1.63 | 7.7±0.8 | 44.0±8.33 |
Zinc-treated HUT-78 cells were stimulated with PHA (10 μg/mL)/PMA (5 ng/mL) or PHA (5 ng/mL)/ionomycin (1 μg/mL) for 6h.
Mean± S.D. pg/ml cytokine generated;
p= ≤0.05 difference between cells maintained in 1 μM Zn vs 15 μM Zn media (n=3).
Table 2.
Effect of different concentration of Cu, Fe, Zn on IL-2 and IFN-γ production in HUT-78 cells (n=3)1
| IL-2 (pg/mL)
|
p value2 | IFN-γ (pg/mL)
|
p value2 | |||||
|---|---|---|---|---|---|---|---|---|
| 1 μM | 15 μM | 50 μM | 1 μM | 15 μM | 50 uM | |||
| Cu | 1518.3±280.2 | 1515.0±311.2 | 1422.8±415.8 | ns | 78.5±2.4 | 86.9±6.1 | 85.0±6.4 | ns |
| Fe | 1603.8±368.7 | 1609.3±328.9 | 1603.4±301.6 | ns | 100.3±11.7 | 88.1±4.4 | 84.1±9.5 | ns |
| Zn | 627.2±130.7a | 1544.6±340.4 | 1530.2±283.9 | <0.05 | 57.2±1.0 a | 99.3±6.1 | 100.0±2.9 | <0.05 |
:HUT-78 cells were incubated for 4 days in medium to which had been added varying concentrations of 2 other trace metals, Cu and Fe. Media to which Cu and Fe were added, contained 15 μM Zn, thus, 1 μM Cu and 1 μM Fe represent medium deficient in Cu and Fe respectively. Low zinc media (1 μM Zn) represents medium with normal Cu and Fe but contains only 1 μM Zn. Cells were then stimulated with PMA/ionomycin for 6 hr and the supernatants harvested for cytokines generated as assessed by ELISA.
:p value by ANOVA analysis, ns indicates non-significant: a): p<0.05; n=3, Dunnett post hoc Multiple comparison for 1 μM vs 15 μM or 50 μM conditions for both IL-2 and IFN-γ production.
We examined the effect of TPEN on T-bet and IFN-γ mRNAs in HUT-78 cells after Con-A stimulation. The results indicate that TPEN-treated cells exhibited a significant decrease in the relative mRNA levels of T-bet and IFN-γ after stimulation, compared to the stimulated cells without TPEN treatment or the cells treated with zinc plus pyrithione (p<0.05; Table 3). Without Con-A stimulation, neither zinc nor pyrithione alone, led to an increase in the mRNA levels of T-bet and IFN-γ (Table 3), suggesting that zinc specifically up-regulated T-bet and IFN-γ mRNAs in Con-A-stimulated HUT-78 cells.
Table 3.
Effect of TPEN on T-bet and IFN-γ mRNAs in HUT-78 cells after Con-A Stimulation1
| T-bet/GAPDH mRNA | IFN-γ/GAPDH mRNA | |
|---|---|---|
| A: Con-A, 24h | 0.088±0.021* | 0.0087±0.0032 |
| B: TPEN 30 min; Con-A, 24h | 0.015±0.016 | 0.0027±0.0031 |
| C: Zinc plus pyrithione, 30 min; Con-A, 24h | 0.095±0.016* | 0.0120±0.0066* |
| D: Zinc (extracellular 20 uM zinc), 30 min | 0.001±0.001 | 0.0003±0.0004 |
| E: Pyrithione, 30 min | 0.017±0.010 | 0.0034±0.0037 |
:HUT-78 cells were incubated with 5 μM TPEN, a zinc-specific chelator, or 20 μM zinc plus 10 μM pyrithione, a zinc ionophore for 30 min, and then following change of media, were continously incubated with Con-A for 24h. The cells were harvested for determination of T-bet and IFN-γ mRNAs by real time RT-PCR.
:p<0.05 (n=3) by ANOVA analysis (Dunnett post hoc Multiple comparison for Gr B vs Gr A, C, D, or E).
We also examined the effect of zinc on the gene expression of Th1 and Th2 type cytokines, T-bet, a major transcription factor for Th1 differentiation, and GATA3, a major transcription factor for Th2 differentiation, in HUT-78 cells stimulated with PMA/PHA and IL-12 (a macrophage-producing cytokine which initiates Th1 differentiation, Table 4). After 6h of PMA/PHA stimulation, mRNA for IFN-γ, IL-12Rβ2 and T-bet were significantly increased in zinc-sufficient cells in comparison to the zinc-deficient cells. The difference in IL-2 mRNA between zinc-deficient and zinc-sufficient cells only approached significance. This may have been due to a small n and a large SD. In contrast, after IL-12 stimulation, IL-2 mRNA level was non-significantly increased while IL-12Rβ2 mRNA was significantly increased in zinc-sufficient cells compared to that found in zinc-deficient cells. Compared to PMA/PHA, 15 μM zinc decreased IL-4 mRNA in IL-12-stimulated cells (Table 4), suggesting that high zinc may have decreased the Th2 cytokine generation. PMA/PHA- or IL-12-stimulated cells produced only trace amount of GATA-3/18S RNA (×10−9). There were no significant differences in GATA3 mRNA level between zinc-deficient and zinc-sufficient cells (data not shown). The data described above provide evidence that zinc increases the gene expression of T-bet, IFN-γ, IL-12Rβ2, which are the primary players in the differentiation of Th0 or naïve CD4 T cells into the Th1 cells.
Table 4.
Effect of zinc on relative mRNA levels for Th1 and Th2 type cytokines, T-bet in HUT-78 cells1
| Relative Cytokine mRNA | PMA/PHA stimulation | p value | IL-12 stimulation | p value | ||
|---|---|---|---|---|---|---|
| Zn− | Zn+ | Zn− | Zn+ | |||
| IL-2 (×10−3) | 1.2±1.2 | 5.8±9.6 | 0.06 | 0.85±0.79 | 2.5±4.9 | 0.162 |
| IFN-γ (×10−6) | 0.45±0.18 | 8.7±8.3 | 0.011 | 4.1±0.97 | 3.3±0.31 | 0.13 |
| IL-4 (×10−7) | 2.0±0.42 | 1.7±0.37 | 0.20 | 2.6±0.4 | 1.2±0.30 | 0.004 |
| IL-12Rβ2 (×10−5) | 0.1±0.03 | 0.59±0.14 | 0.019 | 0.65±0.08 | 3.58±0.67 | 0.004 |
| T-bet (×10−5) | 0.23±0.09 | 0.84±0.34 | 0.007 | 1.2±0.15 | 1.4±0.79 | 0.33 |
; zinc-treated HUT-78 cells were stimulated with PMA (5 ng/mL)/PHA (10 μg/mL) or IL-12 (100 pg/mL). Relative mRNA levels to 18S RNA were determined by real time RT-PCR (n=3).
To investigate whether or not an increase in intracellular free zinc accompanies Con-A-induced TCR-initiated Th1 stimulation, we measured the intracellular free zinc concentration in different concentration of zinc-treated Con-A-stimulated HUT-78 cells. As shown in Figure 1A, stimulation of HUT-78 cells in general resulted in an increased intracellular free zinc but only via TCR using Con-A, free zinc was significantly increased and sustained from 30 to 60min (p<0.001). These results suggest that the use of PMA may lead to release of Ca++ through the activation of PKC-α, but not the sustained release of free zinc, whereas activation via TCR may induce the sustained release of free zinc through the activation of PKC-θ. To determine whether the increase in cellular free zinc was the result of an influx of extracellular zinc or the release of intracellular free zinc, HUT-78 cells were maintained and then stimulated with Con-A in normal media (5 μM Zn) or stimulated in zinc-deficient media (1 μM Zn) and then free zinc was assayed. The data indicated that in Con-A-stimulated cells, the increase in free zinc occurred within 30 min and continues up to 150 min then it declined (Figure 1B). This increase in free zinc is blunted when cells are stimulated in the relative absence of extracellular free zinc (1 μM Zn medium). Therefore, the increase in cellular free zinc following TCR stimulation in HUT-78 cells appears to be from two sources, extracellular as well as from intracellular sources.
Figure 1. Activation of TCR increases intracellular free zinc.
A. Cells were incubated for 24hr in normal media to which had been added 15 μM zinc (to load cells with zinc) and then stimulated in normal media (5 μM zinc) in the presence of PHA, PMA or Con-A for 30 and 60 minutes to determine which pathway was involved in differentiation or activation of Th0 or naïve cells and resulted in an increase in cellular free zinc. B. To determine the time course for an increase in cellular free zinc, HUT-78 cells were stimulated in normal or in zinc-deficient medium. Stimulation in normal versus zinc-deficient media allowed us to determine if the increase in cellular free zinc was due to an influx of extracellular free zinc or only a release of free zinc within the cellular compartment under Con-A stimulation. It appears that the increase in cellular free zinc is a result of both extracellular zinc influx and the release of free zinc from intracellular sources following Con-A stimulation (n=3).
4. DISCUSSION
Interactions of peptide antigen with the TCR initiates the response for differentiation of naïve CD4+ T cells into Th1 or Th2 cells, but the direction of the differentiation pathway to different cell function type appears to be predominately driven by the cytokines such as IFN-γ for Th1 and IL-4 for Th2 cell differentiation in the microenvironment [8]. Thus, zinc regulation of cytokine, cytokine signaling, availability of co-stimulatory molecules, and induction of key transcription factors appear to play major roles in determining Th type differentiation.
Two important cytokines necessary for in vitro differentiation of Th1 effector cells from naïve precursors are IFN-γ and IL-12 [8]. IL-12 from activated antigen presenting cells (APC) and the expression of IL-12Rβ2 are necessary for sustaining T-bet transcription and differentiation of naïve CD4+ cells to Th1 type T cells [9]. Maintenance of the expression of IL-12Rβ2 is required for normal Th1 differentiation [10]. In this study, we demonstrate that zinc increased IFN-γ cytokine and the gene expression of IFN-γ and IL-12Rβ2 in stimulated HUT-78 cells. We have also observed that zinc increased the production of IL-12 in HL-60 (human pre-myelocytic leukemia cell line) after PMA stimulation (unpublished data). These data suggest that zinc is involved in the differentiation of Th1 effector cells from naïve precursors.
T-bet is a T-box protein with zinc finger structures expressed in Th1 cells. The expression of T-bet activates IFN-γ expression in cell lines or primary activated T cells and suppresses IL-4 and IL-5 production in differentiating and differentiated Th2 cells [8]. In this study, we demonstrate for the first time that free zinc increased and the gene expression of T-bet and IFN-γ were up-regulated in Con-A-stimulated stimulated HUT-78 cells, suggesting that zinc was involved in differentiation of Th0 to Th1 cells. However, the role of intracellular free zinc in gene expression of IFN-γ and T-bet, and IL-12Rbβ2 in PMA/PHA-stimulated cells is not clear at present.
A regulatory function of zinc requires a strict regulation of the cellular zinc content and its distribution. Approximately 30–40% of the cellular zinc is localized in the nucleus, 50% in the cytosol and cytosolic organelles and the remainder is associated with the membrane [11]. Free zinc is involved in extracellular signal recognition, second messenger metabolism, protein phosphorylation and dephosphorylation and activity of transcription factors [11, 12]. One study demonstrated that the cellular level of free zinc in monocytes can control cytokines secretion by interacting with several signaling events that either increase or inhibit pro-inflammatory cytokines release by different signal pathways [12]. Although the role of free zinc on signal transduction in monocytes has been observed, such effect of free zinc in T helper lymphocytes has not been reported. In this study, we have demonstrated that free zinc is released following stimulation of HUT-78 cells with Con-A within 30 to 60 min. Con-A triggers T-cells by directly interacting with TCR receptors for activation [13]. Free zinc can increase the activity of cytosolic PKC [11, 13]. Zinc reversibly binds to PKC in the plasma membrane of T cells stimulated by phorbol ester or antigen [11, 13].
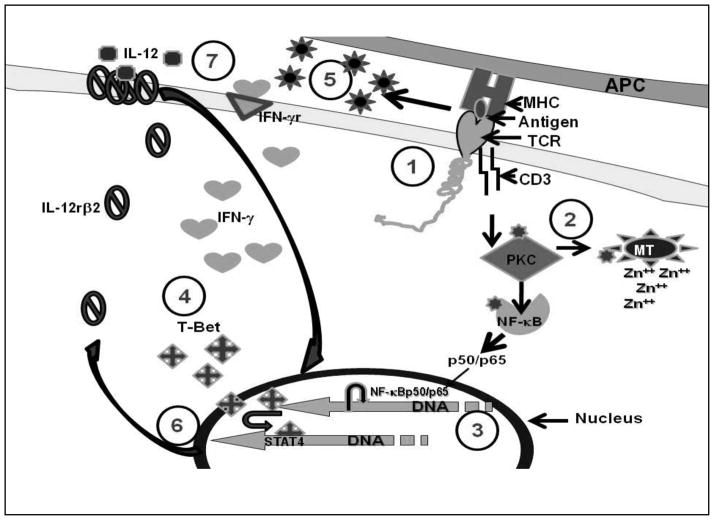
In summary, our present study demonstrates that zinc modulates Th1 differentiation by up-regulation of INF-γ, IL-12Rβ2, and T-bet in HUT-78 cells and that an increase in the levels of intracellular free zinc accompanies Con-A-induced TCR-initiated Th1 differentiation. Figure 2 shows our concept of the mechanism by which intracellular free zinc as a signal messenger may play an important role in Th1 differentiation and activation via PKC pathway. We hypothesize that following TCR activation by Con-A, PKC-θ is activated. PKC-θ releases free zinc from metallothionein. PKC-θ transports free zinc to nucleus where zinc is involved in NF-κB binding to DNA and gene expression of IFN-γ as a first-step in Th1 cell differentiation. IFN-γ is involved in generation of T-bet the master gene for Th1 differentiation. IFN-γ and T-bet then function as autocrine/paracrine models and after TCR is disengaged, T-bet in association with STAT4, enhances generation of IL-12Rβ2. Once the expression of IL-12Rβ2 is established, sustained T-bet expression is then dependent on IL-12. By this mechanism, Th1 cell differentiation is maintained.
Figure 2. Role of zinc in Differentiating Th0 T cells to the Th1 subtype.
The differentiation of Th0 or CD4+ naïve T-cells to the Th1 subtype is a two-stage process involving many transcription factors and events which are zinc-dependent. 1). Engagement of TCR by CD3 antibodies or Con-A or an antigen by APC is the first step in the differentiation process. Here, zinc acts as a bridge between the CD4 or CD8 receptor and Lck, a tyrosine kinase activated during differentiation. 2). Once the initial process begins, zinc-dependent PKC-θ is phosphorylated and able to activate the release of free zinc from metallothionein, the endoplasmic reticulum or the Golgi. 3). An increase in free zinc is then used for binding of activated NF-κB-p50/p65 to DNA, which initiates the transcription and production of IFN-γ. 4). IFN-γ enhances the expression of T-bet. IFN-γ and T-bet expression then becomes autocrine/paracrine. During a second stage, after TCR is disengaged. 5). T-bet associates with STAT4 for transcription and expression of IL-12Rβ2 which is a zinc dependent process. 6). Once the expression of IL-12Rβ2 is enhanced, T-bet expression then depends upon IL-12. 7). Once the Th1 cells is differentiated and stabilized, it functions in the absence of IFN-γ. Full expression of T-bet also inhibits the expression of GATA3 which is responsible for Th2 differentiation, thus, locking in the Th1 phenotype. Results from our previous data demonstrated that activation of NF-κB, transcription of IFN-γ, T-bet and IL-12Rβ2 are all zinc-dependent processes [14–16].
Zinc is known to up-regulate IKK and NF-κB activation in stimulated HUT-78 cells, which is essential for generation of mRNAs of Th1 cytokines IL-2 and IFN-γ in differentiated cells [4–6]. In this study, we show that Con-A stimulation of TCR in HUT-78 cells leads to an early increase in intracellular free zinc which probably activates PKC-θ and leads to early generation of IFN-γ and T-bet which are required for Th1 cell differentiation (see Figure 2). Inasmuch as we did not observe a sustained increase in intracellular free zinc following PHA, and PMA/ionomycin stimulation of HUT-78 cells which utilize PKC-α activation pathway, we hypothesize that PKC-α activation is not involved in Th1 cell differentiation in HUT-78 cells.
Research Highlights.
Zinc deficiency impairs cell-mediated immunity in humans.
We find zinc increases the expression of IL-2, IFN-γ, IL-12Rβ2, and T-bet, essential for Th1-cell differentiation in Th0 cells.
Zinc-specific chelator, TPEN, decreases the mRNA expression of IFN-γ and T-bet in Con-A-stimulated cells.
High level of zinc increases intracellular free zinc in Con-A-stimulated cells.
We hypothesize that free zinc is a molecular signal involved in expression of cytokines and transcription factors.
Acknowledgments
This work was supported by NIH grant no.5RO1A150698-04 and Labcatal Laboratories, Paris, France. We gratefully acknowledge the help and support of Chris Fredrickson, Neurobiotek, Galveston, TX in setting up the procedure for free zinc determination. Grateful appreciation is extended to the National Institute of AIDS for the gracious gift of HUT-78 cells. All authors have confirmed that no competing financial interests exist. B.B. designed and conducted experiments; A.S.P. designed and provided funding; F.W.B. conducted zinc assay; G.W.B and T.S. conducted cytokine assays; S.A. and F.H.S. revised the manuscript.
Abbreviations
- IFN-γ
interferon-γ
- IL-12Rβ2
IL-12 receptor β2
- PKC
protein kinase C
- Con-A
concanavalin-A
- TCR
T-cell receptor
- PMA
phorbol-12 myristate-13-acetate
- PHA
phytohemagglutinin-p
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prasad AS. In: Biochemistry of zinc. , editor. Plenum Press; New York, New York: 1993. [Google Scholar]
- 2.Prasad AS, Meftah S, Abdallah J, Kaplan J, Brewer GJ, Bach JF, Dardenne M. Serum thymulin in human zinc deficiency. J Clin Invest. 1988;82:1202–1210. doi: 10.1172/JCI113717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dardenne M, Pleau JM, Nabarra B, Lefrancier P, Derrien M, Choay J, Bach JF. Contribution of zinc and other metals to the biological activity of the serum thymic factor. Proc Natl Acad Sci USA. 1982;79:5370–5373. doi: 10.1073/pnas.79.17.5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao B, Prasad AS, Beck FW, Godmere M. Zinc modulates mRNA levels of cytokines. Am J Physiol Endocrinol Metab. 2003;285:E1095–E1102. doi: 10.1152/ajpendo.00545.2002. [DOI] [PubMed] [Google Scholar]
- 5.Prasad AS, Beck FW, Bao B, Fitzgerald JT, Snell DC, Steinberg JD, Cardozo LJ. Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr. 2007;85:837–844. doi: 10.1093/ajcn/85.3.837. [DOI] [PubMed] [Google Scholar]
- 6.Beck FW, Li Y, Bao B, Prasad AS, Sarkar FH. Evidence for reprogramming global gene expression during zinc deficiency in the HUT-78 cell line. Nutrition. 2006;22:1045–1056. doi: 10.1016/j.nut.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Malavolta M, Costarelli L, Giacconi R, Muti E, Bernardini G, Tesei S, Cipriano C, Mocchegiani E. Single and three-color flow cytometry assay for intracellular zinc ion availability in human lymphocytes with Zinpyr-1 and double immunofluorescence: relationship with metallothioneins. Cytometry A. 2006;69:1043–1053. doi: 10.1002/cyto.a.20335. [DOI] [PubMed] [Google Scholar]
- 8.Agnello D, Lankford CS, Bream J, Morinobu A, Gadina M, O’Shea JJ, Frucht DM. Cytokines and transcription factors that regulate T helper cell differentiation: new players and new insights. J Clin Immunol. 2003;23:147–161. doi: 10.1023/a:1023381027062. [DOI] [PubMed] [Google Scholar]
- 9.Boothby M. The calculus of integrating differentiation: timing control of T-bet Immunity. 2009;30:666–668. doi: 10.1016/j.immuni.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Sinigaglia F, D’Ambrosio D, Panina-Bordignon P, Rogge L. Regulation of the IL-12/IL-12R axis: a critical step in T-helper cell differentiation and effector function. Immunol Rev. 1999;170:65–72. doi: 10.1111/j.1600-065x.1999.tb01329.x. [DOI] [PubMed] [Google Scholar]
- 11.Beyersmann D, Haase H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals. 2001;14:331–341. doi: 10.1023/a:1012905406548. [DOI] [PubMed] [Google Scholar]
- 12.Haase H, Rink L. Signal transduction in monocytes: the role of zinc ions. Biometals. 2007;20:579–585. doi: 10.1007/s10534-006-9029-8. [DOI] [PubMed] [Google Scholar]
- 13.Palacios R. Concanavalin A triggers T lymphocytes by directly interacting with their receptors for activation. J Immunol. 1982;128:337–342. [PubMed] [Google Scholar]
- 14.Bao B, Prasad AS, Beck FW, Sarkar FH. Zinc up-regulates NF-kappaB activation via phosphorylation of IkappaB in HUT-78 (Th0) cells. FEBS Lett. 2007;581:4507–4511. doi: 10.1016/j.febslet.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 15.Prasad AS, Bao B, Beck FW, Sarkar FH. Zinc activates NF-kappaB in HUT-78 cells. J Lab Clin Med. 2001;138:250–256. doi: 10.1067/mlc.2001.118108. [DOI] [PubMed] [Google Scholar]
- 16.Prasad AS, Bao B, Beck FW, Sarkar FH. Zinc enhances the expression of interleukin-2 and interleukin-2 receptors in HUT-78 cells by way of NF-kappaB activation. J Lab Clin Med. 2002;140:272–289. doi: 10.1067/mlc.2002.127908. [DOI] [PubMed] [Google Scholar]