Abstract
Background: Food intake fluctuates throughout the menstrual cycle; it is greater during the early follicular and luteal phases than in the late follicular (periovulatory) phase. Ovarian steroids can influence brain areas that process food-related information, but the specific contribution of individual hormones and the importance of the prandial state remain unknown.
Objective: The objective was to examine whether brain activation during food visualization is affected by changes in estradiol concentration in the fasted and fed conditions.
Design: Nine eumenorrheic, lean young women [mean (±SD) age: 26.2 ± 3.2 y; body mass index (in kg/m2): 22.4 ± 1.2] completed 2 visits, one in the early (low estradiol) and one in the late (high estradiol) follicular phase of their menstrual cycle. At each visit, subjects underwent functional magnetic resonance imaging while they viewed food and nonfood images, before and after a standardized meal. Region-of-interest analysis was used to examine the effect of follicular phase and prandial state on brain activation (food > nonfood contrast) and its association with estradiol concentration.
Results: Differences were identified in the inferior frontal and fusiform gyri. In these areas, visualization of food elicited greater activation in the fed state than during fasting but only in the late follicular phase, when estradiol concentration was high. The change in estradiol concentration across the follicular phase (late minus early) was inversely correlated with the change in fusiform gyrus activation in the fasted state but not in the fed state.
Conclusion: Our findings suggest that estradiol may reduce food intake by decreasing sensitivity to food cues in the ventral visual pathway under conditions of energy deprivation. This trial was registered at clinicaltrials.gov as NCT00130117.
INTRODUCTION
Energy intake is regulated by numerous factors in humans (1, 2). At a basic level, food intake is controlled by homeostatic signals that convey information about energy status to the brain (homeostatic control) via neuroendocrine mediators such as leptin, cholecystokinin, peptide YY, glucagon-like peptide 1, ghrelin, and amylin. Complex nonhomeostatic influences (hedonic control) act on those basic physiologic signals and have the potential to override them and ultimately determine what is actually consumed (eg, food attributes such as taste, smell, and texture, but also the social, cognitive, emotional, and rewarding aspects that surround food and eating). Convergence of these multiple influences occurs in the brain through the integration of areas that are involved in the processing of cognitive, reward, and homeostatic information related to food intake.
Among the key regulators of energy intake under normal conditions are gonadal steroid hormones. Evidence from both human and animal studies indicates that food intake fluctuates during the menstrual cycle; it is lower in the periovulatory phase and greater in the early follicular and luteal phases (3–5). The mechanisms that underlie this phenomenon are not fully understood, but have been attributed to the appetite-suppressing effect of estrogen and/or the antagonistic effect of progesterone (6). Direct evidence that supports estrogen's role in the regulation of food intake stems from studies in rodents and nonhuman primates. It has been shown that ovariectomy leads to increased food intake and this effect is prevented by exogenous estrogen administration, but not when progesterone is coadministered (5–7).
Neuroimaging studies that use functional magnetic resonance imaging (fMRI) to estimate neural activity through blood oxygenation level–dependent contrast have shown cyclic changes in brain activation during the normal menstrual cycle with regard to the execution of tasks related to language (8, 9), reward/motivation (10, 11), and emotion/stress (12–14). Changes can also be reflected at the brain structural level (15). Little information is available on the effect of menstrual cycle on brain activation in response to food, and information is limited to the fasting condition (6 h after meal ingestion) (16). In the present study, therefore, we addressed the specific contribution of estradiol to cyclic changes in brain responses to food images and the importance of the prandial state. There may well be differences in the effect of estradiol on brain activation that depend on whether subjects are fasting or fed, the former condition more closely related to homeostatic eating and the latter to hedonic eating.
SUBJECTS AND METHODS
Subjects
Nine young, healthy, nonsmoking, right-handed, English-speaking, normal-weight eumenorrheic women participated in this study. Five of them had prior exposure to magnetic resonance imaging (MRI) scans, whereas 4 were MRI naive. All participants had stable body weight (<2-kg change) for ≥6 mo before enrollment [mean ± SD age: 26.2 ± 3.2 y; weight: 61.1 ± 6.3 kg; body mass index (in kg/m2): 22.4 ± 1.2]. Eumenorrhea was established by medical history; the duration of cycle varied from 24 to 31 d (mean ± SD: 29 ± 1 d) and the duration of menstrual bleeding varied from 3 to 7 d (mean ± SD: 5 ± 1 d). Exclusion criteria included the presence of any type of ferromagnetic bioimplant; diagnosis of anxiety, claustrophobia, depression, or another psychiatric disorder; present or past eating disorder; pregnancy; alcoholism; drug abuse; any known cardiac or circulatory impairment; and neurologic conditions, which included epilepsy or significant motor and sensory impairment. All subjects exhibited normal endocrine function, assessed by medical examination and history, and were not taking medications (which included contraception) at the time of the study. Subjects were recruited through local advertisements and gave written informed consent before participation. The study protocol was approved by the Institutional Review Board of Beth Israel Deaconess Medical Center.
Study design
Each participant completed 2 separate visits: one in the early follicular phase (3–6 d after the onset of menstruation) and one in the late follicular phase (1 wk later). On each occasion, subjects arrived at the General Clinical Research Center of the Beth Israel Deaconess Medical Center after an overnight fast. Body weight and height were measured on a leveled platform scale and a wall-mounted stadiometer to the nearest 0.5 kg and 0.5 cm, respectively. Subjects were asked to complete the Three Factor Eating Questionnaire (17); the individual scales of “Restraint,” “Disinhibition,” and “Hunger” were then calculated. Subjects had an fMRI scanning session in the fasted state, and were then provided with a standardized meal, which consisted of foods of their choice from a predefined list, to meet 20% of their energy requirements for weight maintenance (50% of energy from carbohydrates, 30% from lipids, and 20% from proteins). Approximately 10–15 min after completion of the meal (consumed within 15–30 min), subjects underwent another fMRI session in the fed state, so that a total of 30–45 min elapsed between the end of the first scan and the beginning of the second scan. Before and after each session, subjects were asked to complete a 1–10-point visual analog scale to indicate their subjective feeling of hunger (from 1 = “not hungry at all” to 10 = “very hungry”) and pre/post data for each scan were averaged. A fasting blood sample was taken before the first fMRI scan, serum was separated by centrifugation, and estradiol concentration was measured by Access chemiluminescent immunoassay (Beckman Coulter, Brea, CA) with a sensitivity of 20 pg/mL.
fMRI paradigm
fMRI scans were conducted in the morning (between 0900 and 1200) to minimize the effects of normative circadian rhythms. Each scanning session comprised 3 runs during which participants viewed 15-s blocks of food and nonfood images that alternated (image duration: 3 s), separated by 12-s resting periods (fixation cross). Runs were of 3 types, in accordance with the type of pictures presented during the food blocks: 1) high-calorie or calorie-dense foods (>250 kcal/100 g, both sweet and salty foods); 2) low-calorie foods (<80 kcal/100 g, mostly fruit and vegetables); and 3) general foods (eg, breads, eggs, salads with cheese, casseroles). Blocks of nonfood images consisted of inedible items such as jewelry, soap, candles, shoes, flowers, and plants. The sequence of runs (1, 2, and 3) within a session was randomly assigned across subjects. A total of 60 images (30 food and 30 nonfood) were presented per run. All food and nonfood images were randomized within block and presented only once per subject per session, to avoid habituation. Images were selected carefully by a research psychologist to be similarly neutral to pleasant, not reminiscent of other psychologically salient images (eg, faces), and equivalent in visual complexity (ie, color, clarity, scene or object focus) with the aid of custom-written software in Matlab (The MathWorks Inc, Natick, MA). Adobe Photoshop CS3 (Adobe, San Jose, CA) was used to normalize picture size and pixel density (598 × 441 pixels). Subjects viewed images through a mirror displayed with a projector system. E-Prime software, version 2.0 (Psychology Software Tools Inc, Pittsburgh, PA) was used for stimulus presentation and synchronization with the capture of fMRI images. During the presentation of images, subjects were asked to rate how “appealing” they found each item on a scale from 1 to 4 (1 = “not at all,” 2 = “not really,” 3 = “a little,” and 4 = “a lot”) by the provision of responses with a fiber-optic keypad (Lumina, Cedrus Corporation, San Pedro, CA). Subjects were asked to respond within the time frame of image presentation. Images stayed projected for 3 s with or without subjects’ rating response. Before data acquisition, subjects practiced the use of the keypad response system and the rating procedure while they viewed sample images and were instructed by a technician.
Image acquisition
Images were obtained on a GE Signa 3T MRI scanner equipped with an 8-channel head coil (General Electric, Milwaukee, WI), located at the Department of Radiology, Beth Israel Deaconess Medical Center. Blood oxygenation level–dependent responses were acquired with the use of a T2*-weighted echo-planar imaging sequence with TR = 3000 ms, TE = 40 ms, flip angle = 90°, matrix size = 64 × 64, with an in-plane resolution of 3.91 × 3.91 mm2 over 32 axial slices of 5-mm thickness with no gap. At the beginning of each functional run, the first 5 volumes were discarded to allow for T1 equilibration effects, which left 108 volumes per run. A T1-weighted magnetization prepared rapid gradient echo structural MRI, with 1 × 1 × 1 mm3 resolution, was also acquired.
Data analysis
Images were transferred from the scanner to the workstation in DICOM (Digital Imaging and Communications in Medicine) format and converted into NIfTI (Neuroimaging Informatics Technology Initiative) format with the use of MRIcron software (http://www.cabiatl.com/mricro/mricron/index.html). All analyses were performed with Statistical Parametric Mapping software, version 8 (SPM8, The Wellcome Trust Centre of Neuroimaging, London, United Kingdom). For preprocessing, images were realigned to the mean functional image, normalized to the Montreal Neurological Institute template, resampled to 2-mm isotropic voxels, and spatially smoothed with an 8-mm full-width at half-maximum Gaussian kernel. Initially, we specified a first-level general linear model for each subject on a voxel-wise basis; we modeled the data with a boxcar function convolved with a canonical hemodynamic response function, and added head motion parameters as regressors. Low-frequency noise was removed with a 128-s high-pass filter. Individual statistical maps were then obtained for the food compared with nonfood (food > nonfood) contrast for each condition. We defined a series of regions of interest (ROIs) on the basis of the results from a recent meta-analysis of previous fMRI investigations that used food images as stimuli (18) and from the only study that examined variations over the menstrual cycle in response to food (16) (food > nonfood contrast). Accordingly, ROIs were constructed in the inferior frontal gyrus, fusiform gyrus, insula, hippocampus, amygdala, and nucleus accumbens. All these ROIs were bilateral and were based on anatomic areas from the WFU Pickatlas toolbox (Wake Forest University School of Medicine, Winston-Salem, NC) (19). The nucleus accumbens ROI was built with a 5-mm-radius sphere centered at x = ±14, y = −2, z = −10, based on the results of a previous study (16). Parameter estimates were extracted for each ROI during each subject's 4 conditions (early follicular/fasting, early follicular/fed, late follicular/fasting, and late follicular/fed), with the use of a MarsBar toolbox for SPM8 (20). Subsequent analyses were performed with SPSS software, version 18 (IBM SPSS, Chicago, IL). Data were first tested for normality of distribution with the use of the Shapiro-Milk test, and then modeled as repeated-measures analysis of variance (ANOVA) with 2 within-subject factors (follicular phase and prandial state). The Friedman test was used for variables that were not normally distributed. When significant interactions between follicular phase and prandial state emerged, subsequent paired comparisons were done with Student's paired t test or Wilcoxon signed-rank test. For these analyses, we used a statistical threshold of P < 0.05, because activation was hypothesized for prespecified anatomic ROIs based on independent data (16, 18).
We also conducted whole-brain searches in SPM8, complementary to the ROI analysis. Thus, we performed second-level random-effects analysis; we entered individual food > nonfood contrast images into a 2 (prandial state: fasting or fed) by 2 (follicular phase: early or late) repeated-measures ANOVA, with the use of the full factorial design approach of SPM8. F-contrasts were obtained for main effects and interaction. We then performed post hoc analyses to examine the direction of the effects by using paired t tests. In addition, we examined brain areas that changed activation in association with the elevation in estradiol concentration from the early to the late follicular phase. This was done with paired t tests (separately for fasting and fed conditions), and 2 covariates (added to the models) that corresponded to the concentration of estradiol at the early and late phases of the follicular phase for each subject. To correct for multiple comparisons, we estimated an appropriate cluster size threshold with the use of a Monte Carlo simulation of the brain volume implemented in Matlab (https://www2.bc.edu/~slotnics/scripts/cluster_threshold_beta.m) (21, 22). This method has the advantage of sensitivity to small effect sizes, while it still corrects for multiple comparisons across the whole brain volume. The simulation indicated that for an assumed individual voxel type I error of P < 0.005, uncorrected, a cluster size of 115 contiguous resampled voxels was required to correct for multiple comparisons across the whole brain at P < 0.05 (based on 10,000 iterations for our 64 × 64 × 32 functional image matrix with a full-width at half-maximum kernel of 8 mm). We complemented this analysis with exploratory comparisons for each experimental day and prandial state with a threshold of P < 0.005, uncorrected. Results from these whole-brain analyses are reported in the text and tables, which specify the statistical threshold. Both corrected and uncorrected results are included in the text and tables to facilitate a full description of the data, avoid type II error (ie, missing of true effects), and facilitate comparison with future studies and meta-analyses (23, 24).
All SPM maps were visualized, and anatomically labeled with the use of the xjview visualizing toolbox (http://www.alivelearn.net/xjview8/). Results for other parameters are presented as means ± SDs. For these analyses, differences between the fasted and fed states and between experimental days were evaluated with the use of repeated-measures ANOVA with 2 within-subject factors (prandial state and follicular phase) and simple linear contrasts.
RESULTS
The Three Factor Eating Questionnaire scores averaged 10.8 ± 4.2 for Restraint, 3.7 ± 2.9 for Disinhibition, and 3.7 ± 2.9 for Hunger. By design, estradiol concentration was significantly greater in the late follicular compared with the early follicular phase (126 ± 74 compared with 45 ± 11 pg/mL, respectively; P = 0.008). All subjects but one had greater estradiol concentration in the late follicular than in the early follicular phase; the mean difference was 80 pg/mL (95% CI: 27, 133 pg/mL).
On both days, visual analog scale scores decreased significantly after meal consumption (P = 0.001). Visual analog scale scores were similar (P = 0.276) between the early and late follicular phases, in both the fasting (6.0 ± 2.1 compared with 5.8 ± 2.0, respectively) and the fed (2.3 ± 1.2 compared with 1.7 ± 1.0, respectively) states (P for interaction: 0.519). Food images were rated as more appealing compared with nonfood images (P = 0.002) on both testing days (P = 0.862) and regardless of prandial state (P = 0.212).
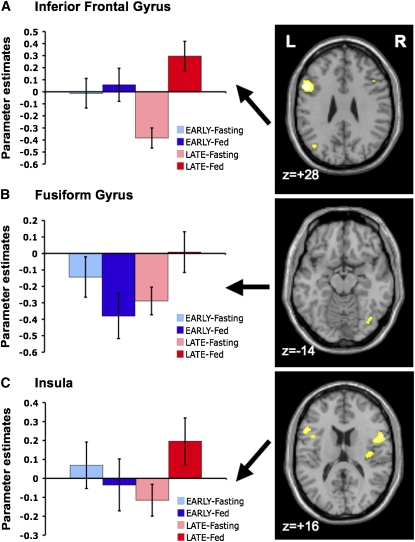
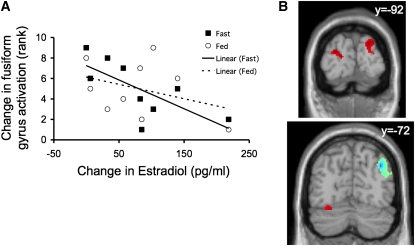
ROI analyses showed a main effect of prandial state in the inferior frontal gyrus (P = 0.030) and an interaction between follicular phase and prandial state in the inferior frontal gyrus and fusiform gyrus (both P = 0.047). Paired comparisons showed that brain responses to food images were significantly greater in the fed state than in the fasting state in these areas but only during the late follicular phase (P = 0.011 and P = 0.015 for inferior frontal gyrus and fusiform, respectively) (Figure 1). In the insula there was an interaction effect of borderline significance (P = 0.076), accounted for by an effect in the same direction (fed state greater than fasted state, restricted to the late follicular phase; P = 0.016) (Figure 1). The change in estradiol concentration across the follicular phase (late minus early) was negatively correlated with the change in fusiform gyrus activation in the fasted (P = 0.013) but not the fed (P = 0.472) state (Figure 2A). No significant effects were identified in the other areas examined (hippocampus, amygdala, and nucleus accumbens).
FIGURE 1.
Region of interest (ROI) analysis. Brain activity in 3 ROIs [inferior frontal gyrus (A), fusiform gyrus (B), and insula (C)] in response to food > nonfood visualization throughout the 4 time points of the study (n = 9). A significant interaction between follicular phase (early/late) and prandial state (fasted/fed) was shown in the inferior frontal gyrus and fusiform gyrus. The insula showed a similar interaction effect of borderline significance. Prandial state affected activation (greater in the fed than in the fasted state) only during the late follicular phase. Columns represent mean parameter estimates (β values) ± SEMs, which are plotted for each ROI/time point. Brain maps (depicted in axial sections) highlight activation within each corresponding ROI for the paired comparison fed minus fasted (fed > fasted) during the late follicular phase (whole-brain analysis: thresholded at P < 0.005, uncorrected; for illustration purposes). L, left; R, right.
FIGURE 2.
Association of brain activation with estradiol concentration. A: Change in estradiol concentration from early to late follicular phase correlated negatively with change in activity in the fusiform gyrus region of interest only during fasting (P = 0.013). Solid and dashed lines indicate the correlation lines for fasting (▪) and fed (○), respectively. B: Brain coronal sections depict the areas that showed a positive (red) and negative (blue) significant correlation with estradiol elevation from the early to the late follicular phase in the whole-brain analysis (contrast food > nonfood; P < 0.05, corrected).
In the whole-brain analysis, repeated-measures ANOVA showed a significant main effect of prandial state on frontal lobe areas in response to food visualization. The most notable effect was shown in the inferior frontal gyrus (peak at x, y, z = 52, 6, 18, respectively), which was overall more activated in the fed than in the fasted state (P < 0.05, corrected) (Table 1). Additional areas in the lateral and medial sectors of the prefrontal cortex were shown to be more activated in association with feeding, but only at a lower, exploratory threshold (P < 0.005, uncorrected) (Table 1). Conversely, there was no effect of follicular phase (early compared with late) on brain activation, but there was an indication of follicular phase × prandial state interaction in the right insula (peak at x, y, z = 40, −16, 16, respectively), also at an exploratory threshold (P < 0.005, uncorrected) (Table 1).
TABLE 1.
Coordinates of peak voxels that showed a significant main effect of prandial state (fasting/fed) and interaction between prandial state and follicular phase (early/late) in response to food visualization (n = 9)1
| Region label | Brodmann area | Cluster size | Peak intensity | P value | MNI coordinates |
||
| x | y | z | |||||
| kE | F | mm | |||||
| Prandial state | |||||||
| R inferior frontal gyrus | 44 | 487 | 22.14 | <0.001* | 52 | 6 | 18 |
| L inferior frontal gyrus | 45 | 131 | 13.00 | 0.001* | −52 | 18 | 20 |
| R cingulate gyrus | 24 | 31 | 12.30 | 0.001 | 16 | 0 | 42 |
| L middle frontal gyrus | 6 | 25 | 11.59 | 0.002 | −20 | 0 | 46 |
| R medial frontal gyrus | 6 | 15 | 11.36 | 0.002 | 8 | −14 | 70 |
| R postcentral gyrus | 3 | 11 | 11.33 | 0.002 | 62 | −14 | 28 |
| R superior frontal gyrus | 8 | 13 | 11.24 | 0.002 | 16 | 40 | 50 |
| L middle frontal gyrus | 8 | 11 | 10.35 | 0.003 | −40 | 18 | 48 |
| L middle frontal gyrus | 9 | 18 | 10.23 | 0.003 | −46 | 30 | 34 |
| Follicular phase | — | — | — | — | — | — | — |
| Interaction | |||||||
| R insula | 13 | 16 | 11.37 | 0.002 | 40 | −16 | 16 |
Results are reported with the use of the Montreal Neurological Institute (MNI) coordinate system. P values represent uncorrected peak-level statistics, unless otherwise specified. See section entitled “Data analysis” for statistical analysis details. L, left; R, right. *P < 0.05, corrected for multiple comparisons with the use of the Monte Carlo simulation.
The effect of estradiol concentration on brain activation was examined in the fasted and fed conditions separately. Remarkably, there was an effect only in the fasted state, with a significant positive correlation identified for 3 clusters located in the occipital cortex (cuneus) and cerebellum (P < 0.05, corrected) (Figure 2B, Table 2). Activation in these areas in response to visual food stimuli was increased from the early to the late follicular phase in parallel with the rise in estradiol concentration. In addition, there was an area in the precuneus adjacent to the angular gyrus that showed a negative correlation with the elevation in estradiol concentration (P < 0.05, corrected) (Figure 2B, Table 2).
TABLE 2.
Coordinates of peak voxels that showed a significant association (positive or negative) with the elevation in estradiol concentration from the early to the late follicular phase in the fasted state (n = 9)1
| Region label | Brodmann area | Cluster size | Peak intensity | P value | MNI coordinates | ||
| x | y | z | |||||
| kE | T | mm | |||||
| Positive association | |||||||
| L precentral gyrus | 6 | 23 | 12.58 | <0.001 | −32 | −10 | 40 |
| L precentral gyrus | 4 | 19 | 8.42 | <0.001 | −56 | −20 | 36 |
| R middle temporal gyrus | 19 | 30 | 6.83 | <0.001 | 42 | −82 | 20 |
| R cerebellum | — | 53 | 6.41 | <0.001 | 26 | −68 | −20 |
| L cerebellum | — | 165 | 6.20 | <0.001* | −28 | −62 | −20 |
| L precentral gyrus | 6 | 46 | 6.18 | <0.001 | −48 | 0 | 30 |
| L occipital cuneus | 18 | 122 | 6.06 | <0.001* | −20 | −90 | 16 |
| L superior occipital gyrus | 19 | 74 | 6.04 | <0.001 | −34 | −84 | 30 |
| R insula | 13 | 20 | 5.89 | 0.001 | 36 | 2 | 0 |
| R occipital cuneus | 19 | 117 | 5.46 | 0.001* | 22 | −92 | 28 |
| R inferior frontal gyrus | 9 | 28 | 5.03 | 0.001 | 56 | 4 | 32 |
| R cerebellum | — | 12 | 4.21 | 0.003 | 30 | −52 | −18 |
| Negative association | |||||||
| L middle temporal gyrus | 39 | 113 | 11.60 | <0.001 | −38 | −70 | 28 |
| L superior frontal gyrus | 8 | 55 | 10.62 | <0.001 | −16 | 48 | 42 |
| R precuneus | 39 | 390 | 9.71 | <0.001* | 38 | −72 | 36 |
| L superior frontal gyrus | 8 | 64 | 8.99 | <0.001 | −20 | 10 | 50 |
| L middle frontal gyrus | 9 | 35 | 7.31 | <0.001 | −34 | 36 | 42 |
| L medial frontal gyrus | 9 | 87 | 7.21 | <0.001 | −2 | 40 | 22 |
| R superior frontal gyrus | 8 | 14 | 5.01 | 0.001 | 18 | 30 | 44 |
| L insula | 13 | 12 | 4.76 | 0.002 | −32 | −28 | 16 |
| L precuneus | 7 | 26 | 4.60 | 0.002 | −16 | −62 | 66 |
| R posterior cingulate | 31 | 13 | 4.45 | 0.002 | 12 | −52 | 24 |
Results are reported with the use of the Montreal Neurological Institute (MNI) coordinate system. In the fed state, no voxels reached statistical significance. P values represent uncorrected peak-level statistics, unless specified. See Data Analysis for details of statistical analysis of data. L, left; R, right. *P < 0.05, corrected for multiple comparisons with the use of the Monte Carlo simulation.
Finally, we performed paired exploratory analyses across prandial state and follicular phase (see under “Supplemental data” in the online issue). First, we examined changes in brain responses to food images associated with satiation, ie, fed compared with fasting (fed > fasting contrast), in the early and late follicular phases. We observed that satiation-related changes in cortical activation were enhanced during the late follicular phase (see under “Supplemental data” in the online issue). Specifically, there was a large area of activation in the left inferior frontal gyrus (peak at x, y, z = −50, 12, 32, respectively) that also extended to the middle frontal gyrus, and covered Brodmann areas 9, 44, and 45, together with additional activation in inferior temporal and parietal areas (see under “Supplemental data” in the online issue). Interestingly, this satiation-related change was not seen in the early follicular phase, when limited activation was shown in right frontal lobe areas (see under “Supplemental data” in the online issue). Subsequently, we performed paired comparisons to examine differences between the late and early follicular phases in the fasting and fed conditions separately, which showed some clusters of activation in the frontal and temporal lobe, but of minor magnitude (see under “Supplemental data” in the online issue).
DISCUSSION
We evaluated brain responses to food images in healthy young women during the early and late follicular phases of the menstrual cycle and under fasting and fed conditions. We observed that brain responses in the fusiform gyrus and the inferior frontal gyrus differed in accordance with prandial state during the late (high estradiol) but not the early (low estradiol) follicular phase. We also showed that the elevation in estradiol concentration from early to late follicular phase was inversely correlated with fusiform gyrus activation in response to food images, but this effect was only evident in the fasted state.
Our findings extend the results from a recent study that examined the modulation of brain responses to food visualization during the menstrual cycle (16) and identify the fusiform gyrus as a potential neural substrate for the anorexigenic effects of estradiol. A previous neuroimaging study reported that this area undergoes fluctuations in gray matter volume throughout the menstrual cycle, and is larger during the early follicular (low estradiol and progesterone) than during the midluteal (high estradiol and progesterone) phase (15). Activation in the fusiform gyrus has been observed consistently in previous fMRI studies that used food images as stimuli (18). Neuroimaging studies also showed variability between men and women (25) and modulation by prandial state (26, 27) in this region, which included a negative correlation with postprandial changes in insulin concentration (28). The specific role of the fusiform gyrus in the perception of food in humans is not fully established. This brain area has been investigated extensively in the cognitive neuroscience of visual processing, especially face perception (29, 30), and belongs to the ventral visual pathway, predominantly involved in object identification. The fusiform gyrus has connections with limbic, temporal, and prefrontal areas that are dynamically adjusted in accordance with the characteristics of visual stimuli and convey information related to attention, memory, emotion, and reward processing (31). Data from studies in nonhuman primates suggest that interactions between the fusiform gyrus and the orbitofrontal cortex allow visual cues to elicit the predicted (long-term) values of food (32). In consideration of all these observations, our findings suggest that high estradiol concentration during the late follicular phase may modulate perceptual processing in the fusiform gyrus/ventral visual pathway to limit the salience of food cues under conditions of energy deprivation (fasting state), and thus cause an anorexigenic effect.
We also identified differences in the inferior frontal gyrus. Activation in this area was greater during the fed state overall, accounted for by a significant increase from fasting to the fed state during the late follicular phase. In a recent meta-analysis of fMRI studies of food visualization, the inferior frontal gyrus was the area that had the densest concentration of activation foci for the food > nonfood contrast (18). The inferior frontal gyrus is involved in executive aspects (ie, top-down regulation) related to visual recognition (33), attention (34, 35), and memory retrieval (36). More broadly, it also plays a role in inhibitory control, cognitive flexibility, and self-regulation (37–39). In the context of food intake, activation of the inferior frontal gyrus is a correlate of satiety (40, 41) and sensory-specific satiation (42). Neuroanatomic studies that used voxel-based morphometry have reported significantly decreased gray matter in the inferior frontal gyrus in association with obesity and a greater body mass index (43, 44). These data suggest that the increased activation of inferior frontal gyrus that we observed with satiation, especially when estradiol concentration was high (late follicular phase), may reflect a higher level of processing of food cues that could ultimately translate into more eating control, consistent with the anorexigenic effect of estradiol during this phase of the menstrual cycle (3, 4). In addition, our findings support the hypothesis that estradiol enhances negative signals during a meal, which facilitates the onset of satiety (7, 45).
We also identified differences in other brain regions in the whole-brain analysis. Areas in the primary visual cortex and cerebellum were positively associated with the elevation in estradiol concentration across the follicular phase, and the precuneus showed a correlation in the opposite direction, in all cases restricted to the fasted state. These results also support a preferential influence of estradiol on brain mechanisms of food intake regulation under conditions of energy deprivation. However, future studies are warranted to replicate and extend these data.
Estradiol concentration that fluctuates is the most likely mediator of our observations. It is unlikely that antagonism by progesterone has confounded our results because, although we did not measure progesterone, its concentration should have been very low during both experimental days of the follicular phase. The exact site of action that underlies the observed effects across the follicular phase is uncertain. Animal studies have shown that the reduction of food intake caused by estradiol requires activation of central, but not peripheral, estrogen receptors (46). Within the brain, estradiol can reach cortical areas directly, via alpha and beta receptors that are present in multiple areas of the corticolimbic network (47–49), or indirectly via additional mediators [eg, by modulation of the gene expression and subsequent secretion of appetite-related neuropeptides in the hypothalamus (48, 50–52)]. In any case, the influences of estradiol on brain activity are relatively fast and can be detected in the form of changes in fMRI connectivity within 24 h (53).
Our study has several limitations. We evaluated responses to food and nonfood images in the fasted and fed states on 2 different days of the normal menstrual cycle. Because the assessments took place within the same follicular phase (ie, with equal scan order for all subjects), we could not control for the influence of habituation and learning effects (54–56). However, we tried to minimize such variability by the inclusion of a systematic period of task practice at the beginning of each scanning session and the presentation of each item only once per session, and also by the correlation of brain responses with estradiol concentration. Furthermore, a previous study that looked at differences in brain responses to food visualization during the fasted and fed states specifically addressed the possibility of habituation effects over testing sessions and concluded that the results were related to the motivational state of the participants and not to habituation (26). Our sample size was relatively small and included only healthy, lean women studied during the first half of the menstrual cycle. Consequently, we could not reliably analyze the effects of high-calorie, low-calorie, and general foods separately, because of a limited number of trials and thus low statistical power for such analysis. As in previous studies, the food > nonfood comparison did not elicit effects in the striatum/brain reward areas, which agrees with the collective of available data (18). Another limitation of our study is that the possible role of progesterone in the mediation of food-related brain activation by estradiol was not specifically addressed. Nor did we measure emotion, state anxiety, or stress, or possible dietary factors (eg, regular breakfast consumption) that may have affected brain responses to visual food stimuli and mediated some of the results. We believe that, despite these limitations, our findings provide the first evidence, in humans in vivo, that brain activation in association with fasting and feeding is affected by estradiol concentration.
In summary, we evaluated brain activation in the fasted and fed states in 2 phases of the menstrual cycle that differed in estradiol concentration, and report, we believe for the first time, differences that can establish a baseline useful for future research in the area. Further studies are required to better understand the role of estradiol in particular, and gonadal steroids in general, in the regulation of appetite and food intake.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—CSM and FM: designed the research; FZ, MB, IB, AR-G, and MY: conducted the research; MA-A, FZ, FM, FAB, MB, and RR: analyzed the data or performed the statistical analysis; MA-A, FZ, FM, AP-L, and CSM: wrote the manuscript; and CSM and MA-A: had primary responsibility for final content. All authors read and approved the final manuscript. None of the authors had a conflict of interest.
REFERENCES
- 1.Shin AC, Zheng H, Berthoud HR. An expanded view of energy homeostasis: neural integration of metabolic, cognitive, and emotional drives to eat. Physiol Behav 2009;97:572–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol 2008;59:55–92 [DOI] [PubMed] [Google Scholar]
- 3.Dye L, Blundell JE. Menstrual cycle and appetite control: implications for weight regulation. Hum Reprod 1997;12:1142–51 [DOI] [PubMed] [Google Scholar]
- 4.Van Vugt DA. Brain imaging studies of appetite in the context of obesity and the menstrual cycle. Hum Reprod Update 2010;16:276–92 [DOI] [PubMed] [Google Scholar]
- 5.Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci 2006;361:1251–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidsen L, Vistisen B, Astrup A. Impact of the menstrual cycle on determinants of energy balance: a putative role in weight loss attempts. Int J Obes (Lond) 2007;31:1777–85 [DOI] [PubMed] [Google Scholar]
- 7.Butera PC. Estradiol and the control of food intake. Physiol Behav 2010;99:175–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez G, Weis S, Stoffel-Wagner B, et al. Menstrual cycle-dependent neural plasticity in the adult human brain is hormone, task, and region specific. J Neurosci 2003;23:3790–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weis S, Hausmann M, Stoffers B, Vohn R, Kellermann T, Sturm W. Estradiol modulates functional brain organization during the menstrual cycle: an analysis of interhemispheric inhibition. J Neurosci 2008;28:13401–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci USA 2007;104:2465–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ossewaarde L, van Wingen GA, Kooijman SC, Backstrom T, Fernandez G, Hermans EJ. Changes in functioning of mesolimbic incentive processing circuits during the premenstrual phase. Soc Cogn Affect Neurosci 2010;Sept 3 (Epub ahead of print; DOI: 10.1093/scan/nsq071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ossewaarde L, Hermans EJ, van Wingen GA, et al. Neural mechanisms underlying changes in stress-sensitivity across the menstrual cycle. Psychoneuroendocrinology 2010;35:47–55 [DOI] [PubMed] [Google Scholar]
- 13.Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. J Neurosci 2010;30:431–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amin Z, Epperson CN, Constable RT, Canli T. Effects of estrogen variation on neural correlates of emotional response inhibition. Neuroimage 2006;32:457–64 [DOI] [PubMed] [Google Scholar]
- 15.Pletzer B, Kronbichler M, Aichhorn M, Bergmann J, Ladurner G, Kerschbaum HH. Menstrual cycle and hormonal contraceptive use modulate human brain structure. Brain Res 2010;1348:55–62 [DOI] [PubMed] [Google Scholar]
- 16.Frank TC, Kim GL, Krzemien A, Van Vugt DA. Effect of menstrual cycle phase on corticolimbic brain activation by visual food cues. Brain Res 2010;1363:81–92 [DOI] [PubMed] [Google Scholar]
- 17.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 1985;29:71–83 [DOI] [PubMed] [Google Scholar]
- 18.van der Laan LN, de Ridder DT, Viergever MA, Smeets PA. The first taste is always with the eyes: a meta-analysis on the neural correlates of processing visual food cues. Neuroimage 2011;55:296–303 [DOI] [PubMed] [Google Scholar]
- 19.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003;19:1233–9 [DOI] [PubMed] [Google Scholar]
- 20.Brett M, Anton J-L, Valbregue R, Poline J-B. Region of interest analysis using an SPM toolbox [abstract 497]. Presented at the 8th International Conference on Functional Mapping of the Human Brain, Sendai, Japan, June 2--6, 2002. Neuroimage 2002;16:372–3 [Google Scholar]
- 21.Slotnick SD, Moo LR, Segal JB, Hart J., Jr Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Brain Res Cogn Brain Res 2003;17:75–82 [DOI] [PubMed] [Google Scholar]
- 22.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med 1995;33:636–47 [DOI] [PubMed] [Google Scholar]
- 23.Poldrack RA, Fletcher PC, Henson RN, Worsley KJ, Brett M, Nichols TE. Guidelines for reporting an fMRI study. Neuroimage 2008;40:409–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lieberman MD, Cunningham WA. Type I and type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci 2009;4:423–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frank S, Laharnar N, Kullmann S, et al. Processing of food pictures: influence of hunger, gender and calorie content. Brain Res 2010;1350:159–66 [DOI] [PubMed] [Google Scholar]
- 26.LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci 2001;115:493–500 [DOI] [PubMed] [Google Scholar]
- 27.Siep N, Roefs A, Roebroeck A, Havermans R, Bonte ML, Jansen A. Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav Brain Res 2009;198:149–58 [DOI] [PubMed] [Google Scholar]
- 28.Del Parigi A, Gautier JF, Chen K, et al. Neuroimaging and obesity: mapping the brain responses to hunger and satiation in humans using positron emission tomography. Ann N Y Acad Sci 2002;967:389–97 [PubMed] [Google Scholar]
- 29.Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci 1997;17:4302–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia 2007;45:174–94 [DOI] [PubMed] [Google Scholar]
- 31.Fairhall SL, Ishai A. Effective connectivity within the distributed cortical network for face perception. Cereb Cortex 2007;17:2400–6 [DOI] [PubMed] [Google Scholar]
- 32.Murray EA, Izquierdo A. Orbitofrontal cortex and amygdala contributions to affect and action in primates. Ann N Y Acad Sci 2007;1121:273–96 [DOI] [PubMed] [Google Scholar]
- 33.Bar M. A cortical mechanism for triggering top-down facilitation in visual object recognition. J Cogn Neurosci 2003;15:600–9 [DOI] [PubMed] [Google Scholar]
- 34.Hampshire A, Owen AM. Fractionating attentional control using event-related fMRI. Cereb Cortex 2006;16:1679–89 [DOI] [PubMed] [Google Scholar]
- 35.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci 2006;9:971–8 [DOI] [PubMed] [Google Scholar]
- 36.Leveroni CL, Seidenberg M, Mayer AR, Mead LA, Binder JR, Rao SM. Neural systems underlying the recognition of familiar and newly learned faces. J Neurosci 2000;20:878–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci 2007;362:917–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biol Psychiatry 2005;57:1285–92 [DOI] [PubMed] [Google Scholar]
- 39.Cohen JR, Lieberman MD. The common neural basis of exerting self-control in multiple domains Hassin RR, Ochsner KN, Trope Y. Self control in society, mind, and brain. New York, NY: Oxford University Press, 2010:141–60 [Google Scholar]
- 40.Del Parigi A, Chen K, Gautier JF, et al. Sex differences in the human brain's response to hunger and satiation. Am J Clin Nutr 2002;75:1017–22 [DOI] [PubMed] [Google Scholar]
- 41.Pannacciulli N, Le DS, Salbe AD, et al. Postprandial glucagon-like peptide-1 (GLP-1) response is positively associated with changes in neuronal activity of brain areas implicated in satiety and food intake regulation in humans. Neuroimage 2007;35:511–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain 2001;124:1720–33 [DOI] [PubMed] [Google Scholar]
- 43.Walther K, Birdsill AC, Glisky EL, Ryan L. Structural brain differences and cognitive functioning related to body mass index in older females. Hum Brain Mapp 2010;31:1052–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pannacciulli N, Del Parigi A, Chen K, Le DS, Reiman EM, Tataranni PA. Brain abnormalities in human obesity: a voxel-based morphometric study. Neuroimage 2006;31:1419–25 [DOI] [PubMed] [Google Scholar]
- 45.Eckel LA. Estradiol: a rhythmic, inhibitory, indirect control of meal size. Physiol Behav 2004;82:35–41 [DOI] [PubMed] [Google Scholar]
- 46.Rivera HM, Eckel LA. Activation of central, but not peripheral, estrogen receptors is necessary for estradiol's anorexigenic effect in ovariectomized rats. Endocrinology 2010;151:5680–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bethea CL, Brown NA, Kohama SG. Steroid regulation of estrogen and progestin receptor messenger ribonucleic acid in monkey hypothalamus and pituitary. Endocrinology 1996;137:4372–83 [DOI] [PubMed] [Google Scholar]
- 48.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev 1999;20:279–307 [DOI] [PubMed] [Google Scholar]
- 49.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol 1997;388:507–25 [DOI] [PubMed] [Google Scholar]
- 50.Bonavera JJ, Dube MG, Kalra PS, Kalra SP. Anorectic effects of estrogen may be mediated by decreased neuropeptide-Y release in the hypothalamic paraventricular nucleus. Endocrinology 1994;134:2367–70 [DOI] [PubMed] [Google Scholar]
- 51.Dhillon SS, Belsham DD. Estrogen inhibits NPY secretion through membrane-associated estrogen receptor (ER)-alpha in clonal, immortalized hypothalamic neurons. Int J Obes (Lond) 2011;35:198–207 [DOI] [PubMed] [Google Scholar]
- 52.Morton GJ, Mystkowski P, Matsumoto AM, Schwartz MW. Increased hypothalamic melanin concentrating hormone gene expression during energy restriction involves a melanocortin-independent, estrogen-sensitive mechanism. Peptides 2004;25:667–74 [DOI] [PubMed] [Google Scholar]
- 53.Ottowitz WE, Siedlecki KL, Lindquist MA, Dougherty DD, Fischman AJ, Hall JE. Evaluation of prefrontal-hippocampal effective connectivity following 24 hours of estrogen infusion: an FDG-PET study. Psychoneuroendocrinology 2008;33:1419–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chapman HA, Bernier D, Rusak B. MRI-related anxiety levels change within and between repeated scanning sessions. Psychiatry Res 2010;182:160–4 [DOI] [PubMed] [Google Scholar]
- 55.Loubinoux I, Carel C, Alary F, et al. Within-session and between-session reproducibility of cerebral sensorimotor activation: a test–retest effect evidenced with functional magnetic resonance imaging. J Cereb Blood Flow Metab 2001;21:592–607 [DOI] [PubMed] [Google Scholar]
- 56.Peters S, Cleare AJ, Papadopoulos A, Fu CH. Cortisol responses to serial MRI scans in healthy adults and in depression. Psychoneuroendocrinology 2010;Nov 10 (Epub ahead of print; DOI: 10.1016/j.psyneuen.2010.10.009) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.