Abstract
Background: The assessment of fat mass and fat-free mass in relation to the symptom-limited maximal exercise duration (Maxdur) of a treadmill test allows for insight into the association of body composition with treadmill performance potential.
Objective: We investigated the complex associations between fat mass and fat-free mass and Maxdur in a population setting.
Design: The Maxdur of a graded exercise treadmill test and body composition by dual-energy X-ray absorptiometry were estimated in 2413 black and white men and women aged 38–50 y from the Coronary Artery Risk Development in Young Adults (CARDIA) cohort.
Results: The mean Maxdur was ≈7.5 s shorter per kilogram of fat mass in both men and women and independent of fat-free mass, height, race, television watching, physical activity, systolic blood pressure, lung function, and education. Fat mass modified the association of fat-free mass with the Maxdur (2-way interaction P < 0.001), and the interaction was stronger in women than in men. In men in the lowest fat-mass quartile, the Maxdur was 1.3 s longer per kilogram of fat-free mass and was 0.5 s shorter per kilogram of fat-free mass in the highest fat-mass quartile. In contrast, in women with the least fat mass, the Maxdur was 2.7 s longer per kilogram of fat-free mass and was 2.8 s shorter per kilogram of fat-free mass in the highest fat-mass quartile.
Conclusions: The Maxdur was negatively related to fat mass. Fat-free mass in obese people contributed little to the treadmill performance potential as assessed by the Maxdur, although the contribution of fat-free mass was positive in thinner people.
INTRODUCTION
Body mass index (BMI; in kg/m2) is inversely associated with cardiovascular fitness (1) and other aspects of treadmill performance. BMI explained ≈10% of the variance in maximal exercise duration (Maxdur) (2) in men who took a Bruce-protocol (3, 4) treadmill test. Furthermore, BMI is importantly involved in the association of cardiorespiratory fitness with risk factors for cardiovascular disease (1). However, a high BMI reflects both high-fat mass and fat-free mass, which may relate differentially to cardiorespiratory fitness. Therefore, additional insight may be gained by specifically assessing fat mass and fat-free mass (5, 6) in relation to treadmill test performance. To our knowledge, no study of this question has previously been undertaken on a large sample of adults.
In a subset of participants from the 20-y follow-up examination from the Coronary Artery Risk Development in Young Adults (CARDIA) Study, we investigated the interaction between fat mass and fat-free mass on the symptom-limited Maxdur on a treadmill in a cross-sectional design. We verified the findings in longitudinal data in a subset of the cohort. We hypothesized that fat mass is inversely associated with the Maxdur, but fat-free mass is differently associated with the Maxdur in individuals with low compared with high fat mass.
SUBJECTS AND METHODS
Study design
The CARDIA Study is a multicenter longitudinal cohort study of cardiovascular risk factors in adults between the ages of 18 and 30 y at study inception in 1985–1986. Recruitment was at random from the general population in Birmingham, AL, Chicago, IL, and Minneapolis, MN, and from members of the Kaiser Permanente Medical Care Plan (Oakland, CA). The full CARDIA Study sample at baseline was balanced by age (45% were aged 18–24 y; 55% were aged 25–30 y), race (52% were African American; 48% were white), sex (46% were men; 54% were women), and educational achievement (40% had completed ≤12 y of education; 60% had completed >12 y of education) (7–9). Six follow-up examinations were conducted at years 2, 5, 7, 10, 15 and 20 with 91%, 86%, 81%, 79%, 74%, and 72% of the surviving cohort returning, respectively. Our analysis included men and women in whom the symptom-limited treadmill exercise tests and dual-energy X-ray absorptiometry (DXA) examinations were performed at year 20 as part of the ancillary CARDIA fitness study. In addition, both DXA and treadmill testing were performed at year 7 in the Oakland clinic sample (10, 11). We used the latter observations to examine the change in Maxdur as a function of the change in fat mass and fat-free mass.
The institutional review boards for the protection of human subjects for the participating study sites provided approval for the study, and written informed consent was obtained from all participants.
Data collection
The graded symptom-limited maximal exercise test followed a modified Balke protocol, which consisted of up to nine 2-min stages of gradually increasing difficulty (12). Stage 1 was a 2% grade at 3 miles/h, stages 2–6 were 6%, 10%, 14%, 18%, and 22% grades at 3.4 miles/h, stages 7 and 8 were 22% and 25% grades at 4.2 miles/h, and stage 9 was a 25% grade at 5.6 miles/h. In the current study, the Maxdur was the primary outcome. The Maxdur is a close approximation of cardiovascular fitness and of the true physiologic maximal oxygen consumption per unit of time (VO2max) (13), but the functioning of other body systems also plays a prominent role in the Maxdur because of the volitional symptom limitation as the stopping criterion. The Maxdur can be seen as a measure of the treadmill performance potential in the face of the maximal physical exertion that the individual can tolerate.
DXA measurements
Body-composition measurements (fat-free mass and fat mass) were obtained by DXA with a Hologic 4500, Hologic Delphi, or Hologic Discovery (Hologic, Bedford, MA) densitometer in the array-scanning mode in 4 centers. To check the calibration of these DXA machines, a whole-body phantom was sent serially to each center and scanned 10 times. The SD of both fat mass and fat-free mass for the phantom reading was ≈0.1 kg within each machine, with similar mean levels across centers.
Analytic sample and exclusion criteria
The exercise test was performed by 2871 participants at year 20 across all clinics. The Maxdur (in s) was the exercise time at symptom-limited cessation of the test. Participants were determined to be ineligible for the treadmill test (n = 224) for the following reasons: a history of ischemic heart disease, the use of cardiovascular medications (except high blood pressure medication), an elevated resting blood pressure (systolic blood pressure >160 mm Hg or diastolic blood pressure >100 mm Hg), or an acute illness with a fever (the test was rescheduled for a later date whenever possible). Criteria for terminating the exercise test included general or leg fatigue, shortness of breath, or participant refusal to continue and certain medical reasons (<2%). For the analysis of the year-20 symptom-limited treadmill tests, we excluded 196 tests of participants with concurrent β-blocker medication use and 36 tests that were terminated for medical reasons because the treadmill test was stopped for a priori safety reasons if a medical condition was encountered. We also excluded all 58 tests of participants who were <18 or >30 y of age at year 0 for conformity with our previous report on treadmill maximal heart rate and age (14). Some participants met more than one exclusion criteria, which left 2677 eligible treadmill tests for data analysis. Participants with an eligible treadmill test but who did not have available (n = 252) or eligible (n = 12) DXA measurements were also excluded from the analysis. A total of 119 tests with missing covariates were also excluded. Therefore, 2294 participants, who had both DXA measurements (fat mass and fat-free mass) and treadmill-test variables at year 20 and no missing covariates were included in the current study analysis. The exercise test and DXA were performed by 437 participants in Oakland at years 7 and 20, and 395 of the participants had all covariates available for the current study analysis.
Measurements of other variables
Sociodemographic variables, including age, race, sex, educational levels, cigarette smoking, dietary behavior, and alcohol consumption, were obtained by self- and interviewer-administered questionnaires at each clinic visit (15–17). The physical activity level was measured at each examination by a separate interviewer-based questionnaire regarding the frequency of 13 different activities during the past 12 mo; the activity level was estimated by exercise units (18, 19). Hours per week of television viewing were assessed by answers to questions about the frequency and duration of watching (20).
Body weight was measured with a balance-beam scale, and height was measured with a vertically mounted metal centimeter ruler and a metal carpenter's square. BMI was calculated as weight in kilograms divided by height in square meters. Seated blood pressure was measured 3 times after 5 min of rest with the average of the last 2 measurements used in the analysis. Concurrent use of β-blocker medications was also obtained from self-reports, with an examination of medication bottles when available, at each time period. Lung function was measured at year 20 with a volume-based spirometer (SensorMedics Inc, Yorba Linda, CA) and following the standard procedures of the combined American Thoracic Society and the European Thoracic Society guidelines (21–23).
Statistical analyses
Analyses were sex specific. A multiple linear regression analysis was used to assess the year-20 cross-sectional association of Maxdur and DXA measurements of fat mass and fat-free mass. Fat mass and fat-free mass were divided into sex-specific quartiles. The interaction between fat mass and fat-free mass was studied by using product variables, first for continuous fat mass within sex-specific quartiles of fat-free mass and then for continuous fat-free mass within sex-specific quartiles of fat mass. Age, ethnicity, height, systolic blood pressure, lung function, television watching, smoking status, physical activity, and educational level at year 20 were included as covariates and BMI as an alternate measure of body size. In a sensitivity analysis that removed the between-person variance, we examined longitudinal associations of change (year 20 – year 7) in the Maxdur with changes in fat mass and in fat-free mass (n = 395). Changes in fat mass and fat-free mass were categorized above and below sex-specific medians in this subset of participants. We hypothesized that the longitudinal analysis would show an interaction between the change in fat mass and change in fat-free mass in predicting the change in Maxdur just as the cross-sectional analysis would find an interaction between levels of fat mass and fat-free mass in predicting the level of Maxdur.
Most of our sample had artifact-free DXA-testing conditions (n = 1561) or had only jewelry (n = 201) or implants (n = 237) that could not be removed during testing (which did not appear to bias DXA values of fat mass and fat-free mass; Table 1). Participants who had a body part out of the X-ray view (n = 367) had clearly biased DXA values. These participants were taller (feet off the DXA table) and had higher BMI (left arm or upper body off the table) than other subjects, so that both the fat mass and fat-free mass were underestimated. We imputed measurements of fat mass and fat-free mass for those having a body part out of the X-ray view by using regression predictions of fat mass and fat-free mass (r2 ≥ 0.9) on weight, height, race, sex, waist, and BMI within the participants with the artifact-free DXA measurement condition with a random Gaussian error added and distributed with a mean of 0 and SD equal to the residual mean-square error in the fat-free mass prediction compared with the fat-mass prediction.
TABLE 1.
Characteristics of men and women under different dual-energy X-ray absorptiometry (DXA)–testing conditions in the Coronary Artery Risk Development in Young Adults (CARDIA) Study, 2005–20061
| DXA condition | Normal | Jewelry | Implants | P2 | Body part off table | P3 |
| Men (total n = 993) | ||||||
| n | 646 | 51 | 85 | — | 211 | — |
| Maximal exercise duration (s) | 525 ± 133.04 | 544 ± 118.0 | 512 ± 172.8 | 0.43 | 519 ± 140.6 | 0.61 |
| DXA measurements (kg) | ||||||
| Fat mass | 20.0 ± 7.7 | 19.9 ± 8.0 | 19.0 ± 7.2 | 0.56 | 25.0 ± 9.4 | <0.0001 |
| Fat-free mass | 63.2 ± 7.9 | 62.7 ± 6.0 | 63.0 ± 8.1 | 0.86 | 71.2 ± 8.4 | <0.0001 |
| BMI (kg/m2) | 28.1 ± 6.1 | 27.6 ± 4.2 | 27.2 ± 4.0 | 0.40 | 29.6 ± 5.1 | 0.0003 |
| Weight (kg) | 86.8 ± 14.4 | 85.8 ± 12.2 | 84.7 ± 13.2 | 0.43 | 100.4 ± 16.1 | <0.0001 |
| Height (cm) | 176.2 ± 7.0 | 176.5 ± 4.9 | 176.5 ± 5.9 | 0.90 | 184.7 ± 6.6 | <0.0001 |
| Age (y) | 45.0 ± 3.5 | 45.2 ± 3.2 | 44.7 ± 3.4 | 0.73 | 45.0 ± 3.4 | 0.97 |
| Race (% black) | 42.4 | 39.2 | 34.1 | 0.33 | 35.6 | 0.13 |
| Women (total n = 1301) | ||||||
| n | 915 | 131 | 99 | — | 156 | — |
| Maximal exercise duration (s) | 385 ± 138.0 | 412 ± 143.1 | 405 ± 153.2 | 0.06 | 270 ± 107.5 | <0.0001 |
| DXA measurements (kg) | ||||||
| Fat mass | 26.1 ± 10.1 | 24.6 ± 9.0 | 24.7 ± 9.8 | 0.14 | 43.4 ± 12.6 | <0.0001 |
| Fat-free mass | 45.8 ± 6.8 | 46.2 ± 5.7 | 45.6 ± 6.5 | 0.72 | 55.8 ± 7.9 | <0.0001 |
| BMI (kg/m2) | 27.7 ± 6.0 | 27.1 ± 6.7 | 27.0 ± 5.7 | 0.41 | 37.0 ± 7.3 | <0.0001 |
| Weight (kg) | 74. 7 ± 15.9 | 73.2 ± 13.7 | 73.0 ± 15.0 | 0.40 | 102.7 ± 19.9 | <0.0001 |
| Height (cm) | 164.6 ± 6.6 | 165.1 ± 8.2 | 164.8 ± 6.3 | 0.71 | 167.0 ± 7.6 | <0.0001 |
| Age (y) | 45.0 ± 3.6 | 44.7 ± 3.8 | 45.6 ± 3.3 | 0.18 | 44.7 ± 3.7 | 0.37 |
| Race (% black) | 43.8 | 52.7 | 25.3 | 0.0001 | 56.4 | 0.002 |
A total of 1561 participants had normal DXA conditions. Three hundred sixty-seven participants had a body part off the table during testing; 53 of these participants also had irremovable implants or jewelry during testing. In participants with no body part off the table, 182 participants had irremovable jewelry; 17 of these participants also had irremovable implants. Of participants with no body part off the table and no irremovable jewelry, 184 participants had irremovable implants.
P values for comparison between participants with normal DXA conditions, those who had jewelry that could not be removed during testing, and those who had implants that could not be removed during testing (df = 2; derived from one-factor ANOVA for continuous variables and the chi-square test for categorical variables).
P values for comparison between body part off the table and the 3 other conditions combined (derived from one-factor ANOVA for continuous variables and the chi-square test for categorical variables).
Mean ± SD (all such values).
All statistical testing was performed by using 2-sided tests with the significance level of type 1 error (α) set at 0.05. Statistical analyses were performed with the SAS 9.2 (SAS Institute Inc, Cary, NC) statistical software package.
RESULTS
Participants included 993 men and 1301 women. The mean (±SD) Maxdur was 439.3 ± 158.2 s for all participants at year 20 and higher for men than for women (523.3 ± 137.7 compared with 375.2 ± 142.1 s, respectively; P < 0.001; Table 2). The Maxdur at year 20 was correlated with that at year 7 (r = 0.79; n = 1456). Compared with men, women had significantly more fat mass (21.0 ± 8.5 compared with 27.7 ± 11.3 kg; P < 0.001) but less fat-free mass (64.7 ± 8.2 compared with 47.1 ± 7.8 kg; P < 0.001). These sex differences were partly explained by height (fat-mass index in men compared with in women: 6.6 ± 2.7 compared with 10.2 ± 4.3 kg/m2; P < 0.001, fat-free mass index: 20.5 ± 3.6 compared with 17.3 ± 2.8 kg/m2; P < 0.001). Men were also more physically active than women (423 ± 297 compared with 298 ± 249 exercise units as defined in the CARDIA Study).
TABLE 2.
Characteristics of the participants in the Coronary Artery Risk Development in Young Adults (CARDIA) Study, 2005–20061
| Variables | Men (n = 993) | Women (n = 1301) | P |
| Exercise-test characteristics | |||
| Maximal exercise duration (s) | 523.3 ± 137.72 | 375.2 ± 142.1 | <0.0001 |
| Resting heart rate (beats/min)3 | 65.1 ± 9.8 | 67.5 ± 10.1 | <0.0001 |
| Peak heart rate (beats/min) | 171.9 ± 15.6 | 168.8 ± 14.5 | <0.0001 |
| DXA measurements (kg)4 | |||
| Fat mass | 21.1 ± 8.5 | 27.7 ± 11.3 | <0.0001 |
| Fat-free mass | 64.7 ± 8.2 | 47.1 ± 7.8 | <0.0001 |
| Age (y) | 45.0 ± 3.4 | 45.0 ± 3.6 | 0.84 |
| Race (% black) | 40.4 | 46.1 | 0.01 |
| BMI (kg/m2) | 28.3 ± 5.7 | 28.7 ± 6.9 | 0.20 |
| Weight status (%) | <0.0001 | ||
| Normal weight | 24.9 | 36.3 | |
| Overweight | 45.5 | 27.9 | |
| Obese | 29.6 | 35.8 | |
| Systolic blood pressure (mm Hg) | 117.4 ± 11.6 | 112.0 ± 14.4 | <0.0001 |
| Lung function | |||
| FVC (L) | 4.71 ± 0.88 | 3.35 ± 0.66 | <0.0001 |
| FEV1 (L) | 3.65 ± 0.71 | 2.64 ± 0.51 | <0.0001 |
| FEV1/FVC | 0.78 ± 0.07 | 0.79 ± 0.06 | <0.0001 |
| Physical activity score (exercise units) | 423.3 ± 296.5 | 298.2 ± 249.1 | <0.0001 |
| Television watching (h/wk) | 8.8 ± 11.0 | 8.7 ± 12.4 | 0.89 |
| Educational level (y in school) | 15.1 ± 2.7 | 15.3 ± 2.4 | 0.10 |
| Alcohol intake (mL/d)5 | 14.7 ± 25.4 | 8.4 ± 16.5 | <0.0001 |
| Smoking status (%) | 0.001 | ||
| Current smoker | 19.9 | 16.5 | |
| Former smoker | 16.0 | 21.6 | |
| Never smoker | 64.1 | 61.9 |
DXA, dual-energy X-ray absorptiometry; FVC, forced vital capacity; FEV1, forced expiratory volume in one second. P values were derived from Student's t test for continuous variables and the chi-square test for categorical variables (df = 1; except for weight and smoking status, df = 2).
Mean ± SD (all such values).
Values were derived from 992 men and 1299 women.
Imputed for participants with a body part out of the X-ray field.
Values were derived from 970 men and 1284 women.
Compared with men, women also a had lower systolic blood pressure, lung function, alcohol intake, and rate of current smoking (Table 2). Fat mass and fat-free mass were positively correlated with each other (0.58 in men and 0.72 in women).
Without other covariates, fat mass and fat-free mass simultaneously included in a model explained 19% of the variance in Maxdur for men and 46% of the variance in Maxdur for women. In contrast, a model that included only BMI explained 10% of the variance for men and 44% of the variance for women. A model that included age, height, race, television watching, physical activity, systolic blood pressure, lung function, educational level, and smoking status, explained 34% of the variance in Maxdur in men and 46% of the variance in Maxdur in women. Adding fat mass and fat-free mass to the above model explained 52% of the variance in Maxdur in men and 67% of the variance in Maxdur in women.
Association of fat mass and fat-free mass with symptom-limited Maxdur
With adjustment for fat-free mass, age, race, height, smoking status, television watching, physical activity score, systolic blood pressure, forced vital capacity, and educational level, each kilogram increase of fat mass was associated with a 7.9-s shorter Maxdur (P < 0.001) in men and a 7.3-s shorter Maxdur (P < 0.001) in women. The strong inverse association between the Maxdur and fat mass was maintained across all quartiles of fat-free mass (Table 3). This inverse association between fat mass and Maxdur was somewhat weaker in women with high fat-free mass (slope of exercise duration on fat mass was −5.4 s/kg for fat-free mass in the fourth quartile of fat mass; P for interaction with 3 df < 0.001). No similar interactive pattern that involved fat mass within quartiles of fat-free mass was seen in men (P for interaction with 3 df = 0.94), although the difference between men and women was not significant (3-way interaction between sex, fat mass, and fat-free mass P = 0.16).
TABLE 3.
Multivariable associations between fat mass and maximal exercise duration in symptom-limited treadmill tests within quartiles of fat-free mass in men and women in the Coronary Artery Risk Development in Young Adults (CARDIA) Study, 2005–20061
| Men (n = 993) |
Women (n = 1301) |
|||
| Values | P | Values | P | |
| Continuous fat slope within fat-free quartile | ||||
| Quartile 0 | −7.7 ± 1.1 | <0.0001 | −8.4 ± 0.8 | <0.0001 |
| Quartile 1 | −7.8 ± 1.0 | <0.0001 | −8.7 ± 0.6 | <0.0001 |
| Quartile 2 | −7.6 ± 0.8 | <0.0001 | −7.8 ± 0.6 | <0.0001 |
| Quartile 3 | −7.2 ± 0.7 | <0.0001 | −5.4 ± 0.5 | <0.0001 |
| P for any difference between slopes (3 df) | — | 0.94 | — | <0.0001 |
All values are means ± SEs and were adjusted for age, race, height, smoking status (categorized as never smoker, former smoker, and current smoker), television watching (h/wk), physical activity scores, systolic blood pressure, lung function (forced vital capacity), and educational level (defined as grades of school completed). Data were analyzed by multivariable linear regression, and significance was assessed by using Student's test for slopes and by using the F test for any difference between slopes. Fat mass and fat-free mass were imputed for participants with a body part out of the X-ray field. P for any difference across fat-free mass categories of the continuous fat-mass slope pattern between men and women was 0.16.
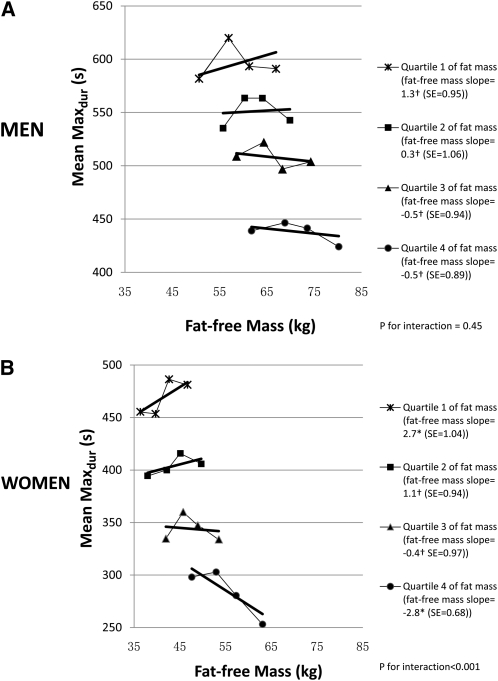
In the analysis adjusted for fat mass, each kilogram increase of fat-free mass was associated with a 1.1-s longer Maxdur (P = 0.04) in men and a 1.7-s longer Maxdur (P = 0.001) in women. However, as hypothesized, fat mass modified the association of fat-free mass with the Maxdur. In women, in the lowest fat mass quartile, the Maxdur was 2.7 s longer per kilogram of fat-free mass in contrast to the highest fat-mass quartile in which the Maxdur was 2.8 s shorter per kilogram of fat-free mass (Figure 1). The interaction tended to be stronger in women (slope difference: 5.5 s/kg fat-free mass for the comparison of lowest to highest fat-mass quartiles; P for interaction <0.001) than in men (slope difference: 1.8 s/kg fat-free mass for the comparison of lowest to highest fat-mass quartiles; P for interaction = 0.45), although the fat-free slope pattern across fat-mass quartiles was not significantly different between men and women (3-way interaction between sex, fat mass, and fat-free mass P = 0.11). A qualitatively similar pattern of interaction was observed if the variable fat mass was replaced by the percentage of body fat (see supplemental material under “Supplemental data” in the online issue).
FIGURE 1.
Mean and fitted symptom-limited maximal exercise duration (Maxdur) of a treadmill test according to fat-free mass within quartiles of fat mass in men (n = 993) (A) and women (n = 1301) (B) in the Coronary Artery Risk Development in Young Adults (CARDIA) Study, 2005–2006. The Maxdur was adjusted for age, race, height, smoking status (categorized as never smoker, former smoker, and current smoker), television watching (h/wk), physical activity scores, systolic blood pressure, lung function (forced vital capacity), and educational level (defined as grades of school completed). Points were plotted on the x axis at the median of the fat-free mass quartile specific to the fat-mass quartile. Data were analyzed by using multivariable linear regression, and significance was assessed by using Student's t test for slopes and the F test for the 2-way interaction between fat mass and fat-free mass and the 3-way interaction (P = 0.11) between sex, fat mass, and fat-free mass. Fat mass and fat-free mass were imputed for participants with a body part out of the X-ray field. †Slopes for the association between fat-free mass expressed as a continuous variable and exercise duration within a fat-mass quartile were not significant (P > 0.05 within each quartile of total fat mass in men and in the second and third quartiles in women); *slopes (s/kg) for the association between fat-free mass expressed as a continuous variable and exercise duration within quartiles of fat mass were significant (P < 0.05 within the first and fourth quartiles of fat mass in women).
Physical activity was related to both fat (r = −0.27, P < 0.0001) and fat-free mass (r = 0.16, P < 0.0001). Physical activity was uniformly positively related to the Maxdur at each level of fat mass but with a differential association in women. Women with a higher level of physical activity had a stronger negative association between fat mass and Maxdur (9.3 s shorter per kilogram of fat mass for those with above-median physical activity compared with 5.9 s shorter per kilogram of fat mass for those with below-median physical activity; P for interaction <0.0001; see supplemental material under “Supplemental data” in the online issue).
Thirteen-year changes in Maxdur, fat mass, and fat-free mass
The magnitude of association of the change of Maxdur on changes in fat mass and fat-free mass from the year-7 exam to the year-20 exam was qualitatively consistent with the cross-sectional findings. Generally, no matter how much fat-free mass was gained, the fat variation within a person over 13 y appeared to be strongly inversely related to the change in Maxdur (5.5 s shorter Maxdur per kilogram of fat mass gained compared with 0.7 s longer per kilogram of fat-free mass gained in both men and women), which was consistent with our main finding that fat mass was strongly associated with the Maxdur. Men with less than the median fat-mass increase (median fat mass gained in this group of 0.7 kg) tended to have a 3.9-s longer Maxdur for each kilogram of fat-free mass gained over 13 y (P = 0.08), whereas men with more than the median fat-mass increase (median fat mass gained in this group of 6.9 kg fat) had a 4.2-s shorter Maxdur for each kilogram of fat-free mass gained over 13 y (P = 0.06, P for interaction = 0.01). There was no significant relation of the Maxdur on changes in fat mass and fat-free mass in women.
DISCUSSION
Our primary finding was that fat mass was strongly and inversely associated with the Maxdur in symptom-limited treadmill testing (≈7.5 s shorter Maxdur per kilogram of fat mass in both sexes) with little dependence on the amount of fat-free mass. In contrast, fat-free mass was more weakly associated with the Maxdur, and the positive association between fat-free mass and the Maxdur was stronger in participants with a lower fat mass than in participants with a higher fat mass, especially in women.
The Maxdur obtained from a symptom-limited treadmill test is often used as a close approximation of the maximal oxygen uptake obtained from a maximal treadmill test to measure cardiorespiratory fitness (1, 13). However, the treadmill duration is influenced by multiple aspects of both physiologic and psychological functioning and could be considered more of a measure of the treadmill performance potential of the tested human being rather than only the capability of a particular organ or system (ie, the cardiorespiratory system) (24). Therefore, the finding of the strong negative association of fat mass with the Maxdur may relate, in part, to the effect of increasing adiposity on cardiorespiratory fitness mediated by skeletal muscle morphologic, metabolic, and contractile functional alterations and heart-function impairment in addition to the mechanical burden imposed on the organism by the excess adiposity (25–31). An increased adiposity affects other factors as well. For instance, it is possible that individuals with a higher fat mass do not perform as close to their true maximum on a treadmill test as do leaner people because individuals with a higher fat mass are not accustomed to exercise or to sweating and exercise-induced fatigue. Also, obesity is related to a high risk of degenerative joint diseases (32), and individuals with more fat mass tend to suffer from more joint problems that may interfere with treadmill performance. In addition, physiologic and psychological inputs before exercise, including previous experiences of participants with exercise and physical exertion may influence exercise performance (24). This could further explain why participants with a higher fat mass performed for a shorter duration on the treadmill. Our finding that physical activity was uniformly positively related to the Maxdur, although increasingly less so in women as fatness increased, was supportive of the concept that the symptom-limited maximal treadmill testing has a major component of general performance potential in addition to its association with cardiorespiratory fitness.
In the current study, we showed that fat-free mass was positively associated with the Maxdur, whereas fat mass was strongly negatively related to the Maxdur. These findings were consistent with findings from intervention studies that involved aerobic and resistance exercise training that showed increases in fat-free mass and decreases in fat mass with improvements in cardiorespiratory fitness in men and women (33–37). The heritability of indicators of cardiorespiratory fitness reaches ≈50% of properly adjusted fitness traits (38, 39). In this context, significant gains in fitness registered with regular exercise or exercise training were strongly influenced by genetic factors (40). As in our data, fitness gains associated with exercise were independent of changes in adiposity and fat-free mass (37).
We showed that fat-free mass in obese people appears to contribute very little to the Maxdur, although the effect was larger in women than in men. Because skeletal muscle is a major component of fat-free mass, it is possible that the different skeletal muscle features between people with high fat mass and with low fat mass contribute to this interaction. Both intermuscular and intramuscular lipid depots increase with growing levels of adiposity (41–43). It is possible that small ectopic fat depots in skeletal muscle that are undetectable by using DXA may be related to fatness and adversely affect muscle function. However, this view may be simplistic; lean persons who were highly trained compared with both lean untrained and obese persons had more intramuscular fat, conceivably for use as an efficient energy source during exercise (44). Because intramuscular fat may be influenced by fitness and adiposity, the influence of intramuscular fat on the decreased contribution of fat-free mass to the Maxdur with increasing adiposity is not clear and needs further study.
Our large, population-based sample of adults who had symptom-limited maximal treadmill tests at 2 examinations and a subset who had 2 DXA measurements available added strength to the current investigation. Our results are more broadly generalizable in young to middle-aged adults than previous studies because of the population-based sampling, the substantial proportion of women and black participants, and the inclusion of smokers and obese persons.
The limitation that our primary findings are cross-sectional, with an unknown extent of residual confounding, was partially mitigated by the analysis in a subset of participants of changes in the Maxdur, fat mass, and fat-free mass over 13 y. The change analysis greatly reduced the residual confounding because of between-person sources. Although the short-term reproducibility of the CARDIA symptom-limited test protocol has not been studied, it is probably higher than the tracking correlation of 0.78 for 2 tests 13 y apart but lower than the reproducibility reported in the literature for true maximal tests, which is generally ≈0.95 for a test-retest situation performed within a few days or, at most, 2 wk (45, 46).
In conclusion, additional insight was gained by specifically assessing fat mass and fat-free mass in relation to treadmill test performance. When using exercise testing for providing an exercise prescription, and in a public health setting as well, one implication from this study was that symptom-limited treadmill performance was more negatively related to the absolute fat mass than positively related to the individual's fat-free mass. Further research is needed to understand the correlates and biological and psychological mechanisms associated with the commonly observed loss of exercise performance potential.
Supplementary Material
Acknowledgments
We thank the staff and participants of the CARDIA Study for their important contributions. We are very grateful to Jack H Wilmore (The University of Texas at Austin) for critical review of the manuscript.
The authors’ responsibilities were as follows—NZ and DRJ: initially posed the question addressed in this manuscript, analyzed data, and wrote the manuscript; DRJ: provided supervision; and SS, BS, MC, CEL, CMS, AS, and CB: discussed findings and edited the draft of the manuscript. None of the authors had a conflict of interest.
REFERENCES
- 1.Carnethon MR, Gidding SS, Nehgme R, Sidney S, Jacobs DR, Jr, Liu K. Cardiorespiratory fitness in young adulthood and the development of cardiovascular disease risk factors. JAMA 2003;290:3092–100 [DOI] [PubMed] [Google Scholar]
- 2.Leon AS, Jacobs DR, Jr, DeBacker G, Taylor HL. Relationship of physical characteristics and life habits to treadmill exercise capacity. Am J Epidemiol 1981;113:653–60 [DOI] [PubMed] [Google Scholar]
- 3.Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J 1973;85:546–62 [DOI] [PubMed] [Google Scholar]
- 4.Bruce RA. Exercise testing of patients with coronary heart disease. Principles and normal standards for evaluation. Ann Clin Res 1971;3:323–32 [PubMed] [Google Scholar]
- 5.Pietrobelli A, Formica C, Wang Z, Heymsfield SB. Dual-energy X-ray absorptiometry body composition model: review of physical concepts. Am J Physiol 1996;271:E941–51 [DOI] [PubMed] [Google Scholar]
- 6.Bachrach LK. Dual energy X-ray absorptiometry (DEXA) measurements of bone density and body composition: promise and pitfalls. J Pediatr Endocrinol Metab 2000;13:983–8 [PubMed] [Google Scholar]
- 7.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988;41:1105–16 [DOI] [PubMed] [Google Scholar]
- 8.Hughes GH, Cutter G, Donahue R, et al. Recruitment in the Coronary Artery Disease Risk Development in Young Adults (CARDIA) Study. Control Clin Trials 1987;8(suppl):68S–73S [DOI] [PubMed] [Google Scholar]
- 9.Cutter GR, Burke GL, Dyer AR, et al. Cardiovascular risk factors in young adults: The CARDIA baseline monograph. Control Clin Trials 1991;12(suppl):1S–77S [DOI] [PubMed] [Google Scholar]
- 10.Cobb KL, Kelsey JL, Sidney S, Ettinger B, Lewis CE. Oral contraceptives and bone mineral density in white and black women in CARDIA. Coronary Risk Development in Young Adults. Osteoporos Int 2002;13:893–900 [DOI] [PubMed] [Google Scholar]
- 11.Sidney S, Lewis CE, Hill JO, et al. Association of total and central adiposity measures with fasting insulin in a biracial population of young adults with normal glucose tolerance: the CARDIA study. Obes Res 1999;7:265–72 [DOI] [PubMed] [Google Scholar]
- 12.Sidney S, Haskell WL, Crow R, et al. Symptom-limited graded treadmill exercise testing in young adults in the CARDIA study. Med Sci Sports Exerc 1992;24:177–83 [PubMed] [Google Scholar]
- 13.Pollock ML, Bohannon RL, Cooper KH, et al. A comparative analysis of four protocols for maximal treadmill stress testing. Am Heart J 1976;92:39–46 [DOI] [PubMed] [Google Scholar]
- 14.Zhu N, Suarez J, Sidney S, et al. Longitudinal examination of age-predicted symptom-limited exercise maximum heart rate. Med Sci Sports Exerc 2010;42:1519–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagenknecht LE, Perkins LL, Cutter GR. Cigarette smoking behavior is strongly related to educational status: the CARDIA study. Prev Med 1990;19:158–69 [DOI] [PubMed] [Google Scholar]
- 16.McDonald A, Van Horn L, Slattery M, et al. The CARDIA dietary history: development, implementation, and evaluation. J Am Diet Assoc 1991;91:1104–12 [PubMed] [Google Scholar]
- 17.Dyer AR, Cutter GR, Liu K, et al. Alcohol intake and blood pressure in young adults: the CARDIA study. J Clin Epidemiol 1990;43:1–13 [DOI] [PubMed] [Google Scholar]
- 18.Jacobs DR, Hahn L, Haskell WL, Pirie P, Sidney S. Validity and reliability of short physical activity history: CARDIA study and the Minnesota Heart Health Program. J Cardiopulm Rehabil 1989;9:448–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sidney S, Jacobs DR, Jr, Haskell WL, et al. Comparison of two methods of assessing physical activity in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol 1991;133:1231–45 [DOI] [PubMed] [Google Scholar]
- 20.Sidney S, Sternfeld B, Haskell WL, Jacobs DR, Jr, Chesney MA, Hulley SB. Television viewing and cardiovascular risk factors in young adults: the CARDIA study. Ann Epidemiol 1996;6:154–9 [DOI] [PubMed] [Google Scholar]
- 21.American Thoracic Society Standardization of spirometry, 1994 update. Am J Respir Crit Care Med 1995;152:1107–36 [DOI] [PubMed] [Google Scholar]
- 22.Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Respir J 2005;26:153–61 [DOI] [PubMed] [Google Scholar]
- 23.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319–38 [DOI] [PubMed] [Google Scholar]
- 24.Noakes TD. Time to move beyond a brainless exercise physiology: the evidence for complex regulation of human exercise performance. Appl Physiol Nutr Metab 2011;36:23–35 [DOI] [PubMed] [Google Scholar]
- 25.Shephard RJ. Physiological determinants of cardiorespiratory fitness. J Sports Med Phys Fitness 1967;7:111–34 [PubMed] [Google Scholar]
- 26.Mac Kenzie R. Editorial: cardiorespiratory fitness. Can Med Assoc J 1974;111:7– passim [PMC free article] [PubMed] [Google Scholar]
- 27.Toledo FG, Watkins S, Kelley DE. Changes induced by physical activity and weight loss in the morphology of intermyofibrillar mitochondria in obese men and women. J Clin Endocrinol Metab 2006;91:3224–7 [DOI] [PubMed] [Google Scholar]
- 28.Toledo FGS, Kelley DE. Mitochondrial dysfunction in the pathogenesis of insulin resistance associated with obesity, diabetes, and aging. Curr Opin Endocrinol Diabetes Obes 2005;12:157–62 [Google Scholar]
- 29.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 2002;51:2944–50 [DOI] [PubMed] [Google Scholar]
- 30.Harber M, Trappe S. Single muscle fiber contractile properties of young competitive distance runners. J Appl Physiol 2008;105:629–36 [DOI] [PubMed] [Google Scholar]
- 31.Alpert MA, Hashimi MW. Obesity and the heart. Am J Med Sci 1993;306:117–23 [DOI] [PubMed] [Google Scholar]
- 32.Pi-Sunyer FX. Medical hazards of obesity. Ann Intern Med 1993;119:655–60 [DOI] [PubMed] [Google Scholar]
- 33.Sisson SB, Katzmarzyk PT, Earnest CP, Bouchard C, Blair SN, Church TS. Volume of exercise and fitness nonresponse in sedentary, postmenopausal women. Med Sci Sports Exerc 2009;41:539–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saavedra JM, De La Cruz E, Escalante Y, Rodríguez FA. Influence of a medium-impact aquaerobic program on health-related quality of life and fitness level in healthy adult females. J Sports Med Phys Fitness 2007;47:468–74 [PubMed] [Google Scholar]
- 35.Roberts MA, O'Dea J, Boyce A, Mannix ET. Fitness levels of firefighter recruits before and after a supervised exercise training program. J Strength Cond Res 2002;16:271–7 [PubMed] [Google Scholar]
- 36.Slentz CA, Duscha BD, Johnson JL, et al. Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE–a randomized controlled study. Arch Intern Med 2004;164:31–9 [DOI] [PubMed] [Google Scholar]
- 37.Wilmore JH, Després JP, Stanforth PR, et al. Alterations in body weight and composition consequent to 20 wk of endurance training: the HERITAGE Family Study. Am J Clin Nutr 1999;70:346–52 [DOI] [PubMed] [Google Scholar]
- 38.Bouchard C, Lesage R, Lortie G, et al. Aerobic performance in brothers, dizygotic and monozygotic twins. Med Sci Sports Exerc 1986;18:639–46 [PubMed] [Google Scholar]
- 39.Bouchard C, Daw EW, Rice T, et al. Familial resemblance for VO2max in the sedentary state: the HERITAGE family study. Med Sci Sports Exerc 1998;30:252–8 [DOI] [PubMed] [Google Scholar]
- 40.Bouchard C, An P, Rice T, et al. Familial aggregation of VO(2max) response to exercise training: results from the HERITAGE Family Study. J Appl Physiol 1999;87:1003–8 [DOI] [PubMed] [Google Scholar]
- 41.Gallagher D, Kuznia P, Heshka S, et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr 2005;81:903–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boettcher M, Machann J, Stefan N, et al. Intermuscular adipose tissue (IMAT): association with other adipose tissue compartments and insulin sensitivity. J Magn Reson Imaging 2009;29:1340–5 [DOI] [PubMed] [Google Scholar]
- 43.Malenfant P, Joanisse DR, Thériault R, Goodpaster BH, Kelley DE, Simoneau JA. Fat content in individual muscle fibers of lean and obese subjects. Int J Obes Relat Metab Disord 2001;25:1316–21 [DOI] [PubMed] [Google Scholar]
- 44.Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 2001;86:5755–61 [DOI] [PubMed] [Google Scholar]
- 45.Skinner JS, Wilmore KM, Jaskolska A, et al. Reproducibility of maximal exercise test data in the HERITAGE family study. Med Sci Sports Exerc 1999;31:1623–8 [DOI] [PubMed] [Google Scholar]
- 46.Daw EW, Province MA, Gagnon J, et al. Reproducibility of the HERITAGE Family Study intervention protocol: drift over time. Ann Epidemiol 1997;7:452–62 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.